Coronary Microvascular Dysfunction in Acute Cholestasis-Induced Liver Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Bile Duct Ligation Surgery and Echocardiography
2.3. Final Surgery and Echocardiography
2.4. Analysis of Echocardiographic Examination
2.5. Histological Staining
2.5.1. Immunofluorescence Staining
2.5.2. Hypoxyprobe™
2.5.3. Fluorescence Imaging
2.6. Blood and Serum Analysis
2.7. Statistics
3. Results
3.1. Serum Parameters
3.2. Transthoracic Echocardiography
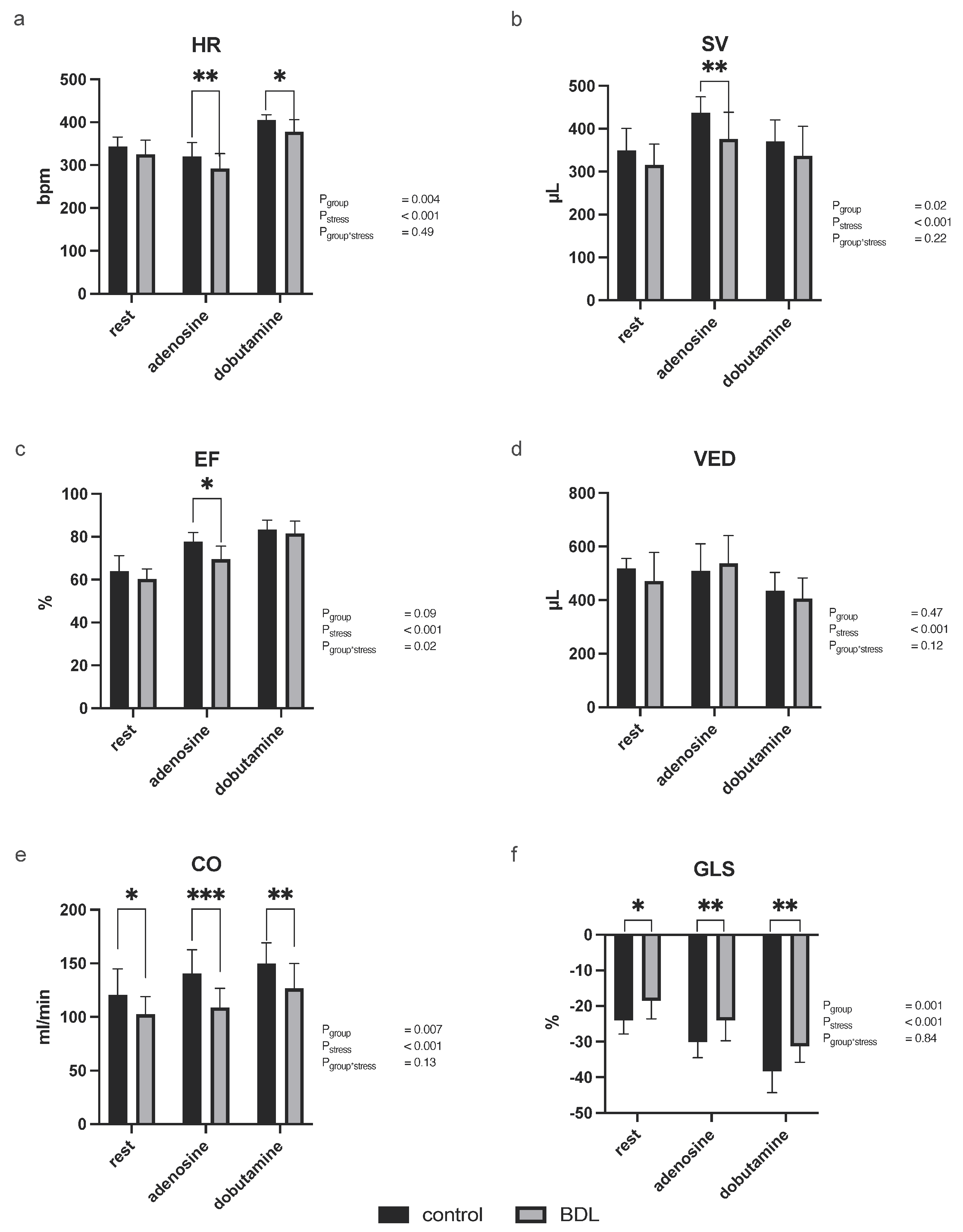
3.2.1. Myocardial Function
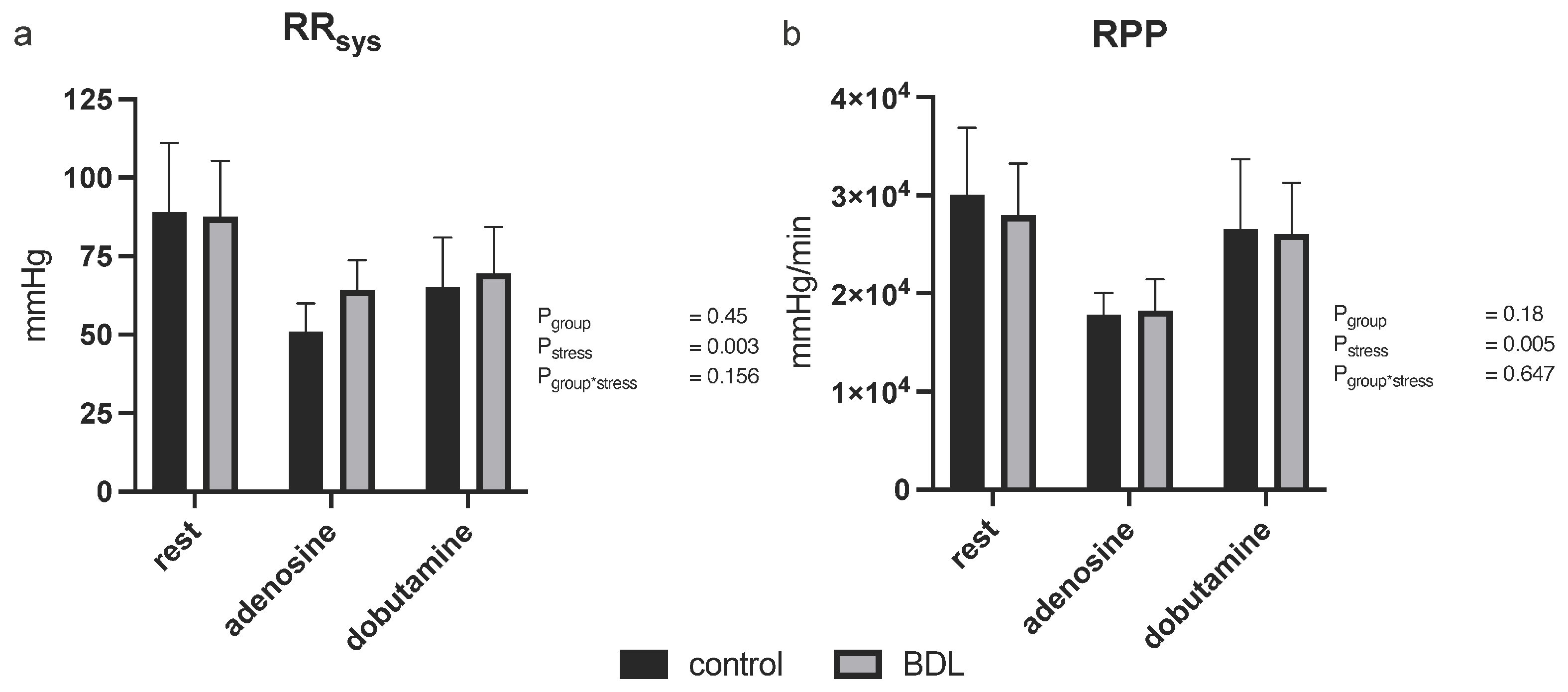
3.2.2. Blood Pressure
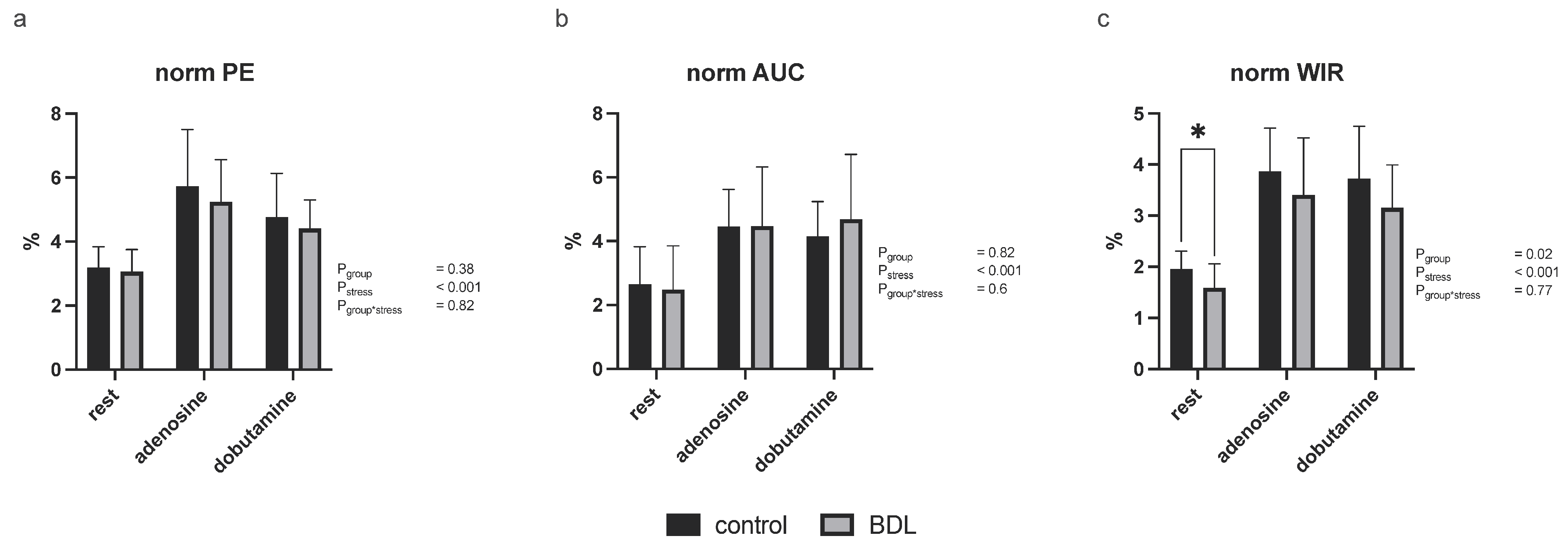
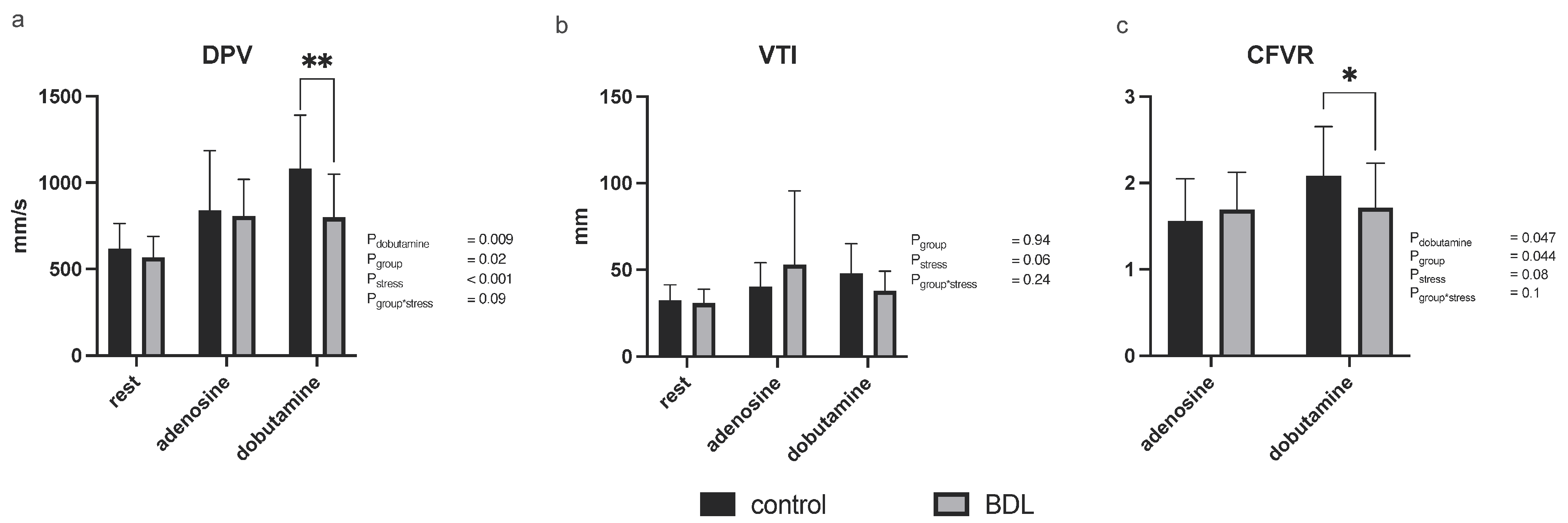
3.2.3. Myocardial Contrast Echocardiography and Pulsed-Wave Doppler
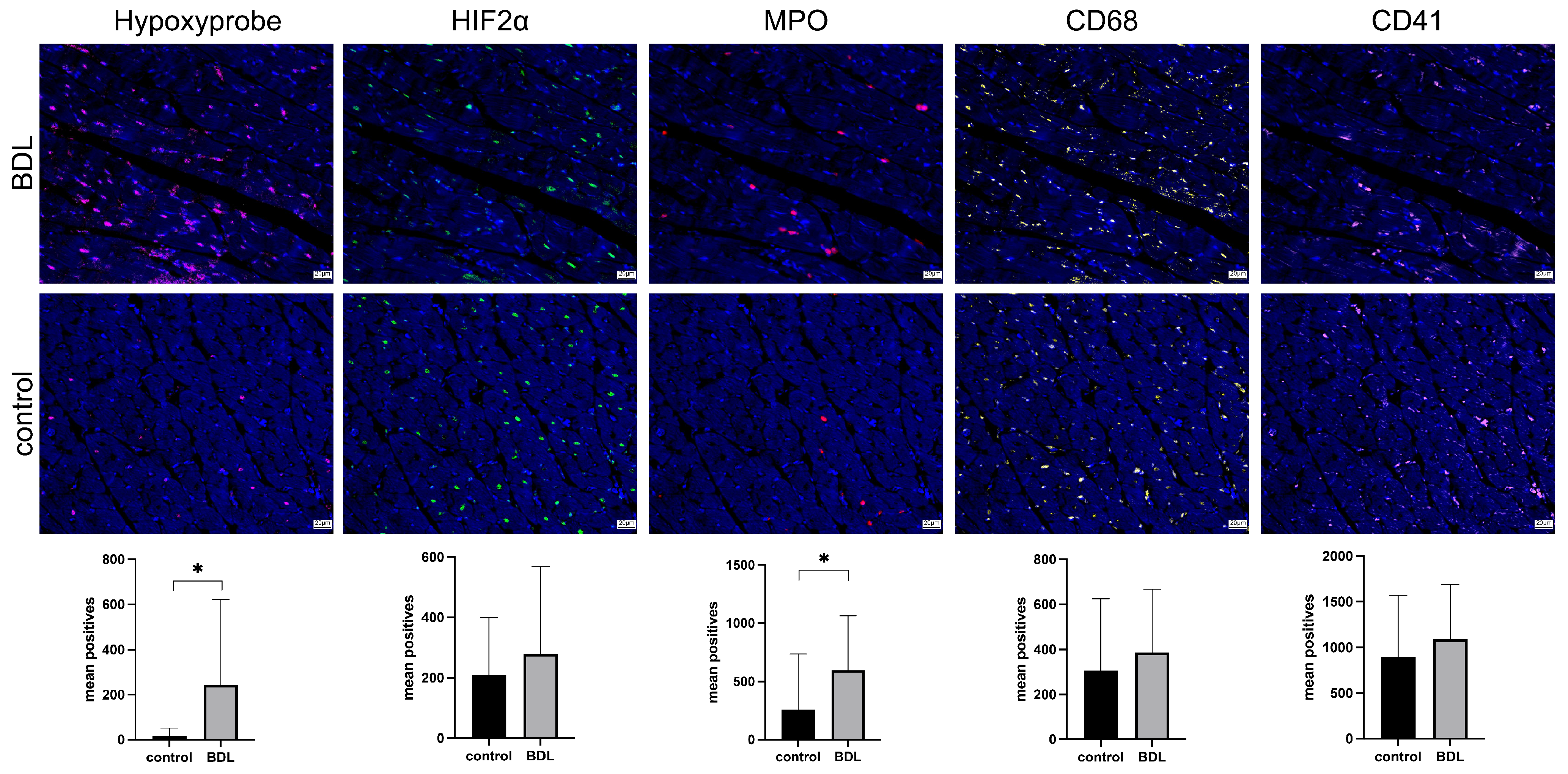
3.3. Immunohistology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pievsky, D.; Rustgi, N.; Pyrsopoulos, N.T. Classification and Epidemiologic Aspects of Acute Liver Failure. Clin. Liver Dis. 2018, 22, 229–241. [Google Scholar] [CrossRef]
- Rajaram, P.; Subramanian, R. Management of Acute Liver Failure in the Intensive Care Unit Setting. Clin. Liver. Dis. 2018, 22, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Bower, W.; Johns, M.; Margolis, H.S.; Williams, I.T.; Bell, B.P. Population-Based Surveillance for Acute Liver Failure. Am. J. Gastroenterol. 2007, 102, 2459–2463. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M. Acute Liver Failure. Semin. Respir. Crit. Care. Med. 2012, 33, 36–45. [Google Scholar] [CrossRef]
- Weston, M.J.; Talbot, I.C.; Horoworth, P.J.; Mant, A.K.; Capildeo, R.; Williams, R. Frequency of Arrhythmias and Other Cardiac Abnormalities in Fulminant Hepatic Failure. Br. Heart. J. 1976, 38, 1179–1188. [Google Scholar] [CrossRef]
- Parekh, N.K.; Hynan, L.S.; De Lemos, J.; Lee, W.M.; Group Acute Liver Failure Study. Elevated Troponin I Levels in Acute Liver Failure: Is Myocardial Injury an Integral Part of Acute Liver Failure? Hepatology 2007, 45, 1489–1495. [Google Scholar] [CrossRef]
- ALFSG. Acute Liver Failure Study Group (ALFSG). Available online: https://www.utsouthwestern.edu/labs/acute-liver/overview/ (accessed on 5 February 2019).
- Audimooolam, V.K.; McPhail, M.J.; Sherwood, R.; Willars, C.; Bernal, W.; Wendon, J.A.; Auzinger, G. Elevated Troponin I and Its Prognostic Significance in Acute Liver Failure. Crit. Care 2012, 16, R228. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef]
- Chilian, W.M. Coronary Microcirculation in Health and Disease. Summary of an NHLBI Workshop. Circulation 1997, 95, 522–528. [Google Scholar] [CrossRef]
- Uhlig, M.; Hein, M.; Habigt, M.A.; Tolba, R.H.; Braunschweig, T.; Helmedag, M.J.; Klinge, U.; Koch, A.; Trautwein, C.; Mechelinck, M. Acute Myocardial Injury Secondary to Severe Acute Liver Failure: A Retrospective Analysis Supported by Animal Data. PLoS ONE 2021, 16, e0256790. [Google Scholar] [CrossRef]
- Georgiev, P.; Jochum, W.; Heinrich, S.; Jang, J.H.; Nocito, A.; Dahm, F.; Clavien, P.A. Characterization of Time-Related Changes after Experimental Bile Duct Ligation. Br. J. Surg. 2008, 95, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Vohra, B.P.; Zhang, Y.; Heuckeroth, R.O. Transcriptional Profiling after Bile Duct Ligation Identifies PAI-1 as a Contributor to Cholestatic Injury in Mice. Hepatology 2005, 42, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, I.; Guo, L.; Davis, M.A.; Duveau, I.; Arteel, G.E. Critical Role of Plasminogen Activator Inhibitor-1 in Cholestatic Liver Injury and Fibrosis. J. Pharmacol. Exp. Ther. 2006, 316, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Tag, C.G.; Sauer-Lehnen, S.; Weiskirchen, S.; Borkham-Kamphorst, E.; Tolba, R.H.; Tacke, F.; Weiskirchen, R. Bile Duct Ligation in Mice: Induction of Inflammatory Liver Injury and Fibrosis by Obstructive Cholestasis. J. Vis. Exp. 2015, e52438. [Google Scholar] [CrossRef]
- Bundesamt für Justiz (Ed.) Tierschutzgesetz, Neugefasst durch Bek. v. 18.5.2006 I 1206, 1313 ed.; Bundesamt für Justiz, Adenauerallee 99–103; Bundesamt für Justiz: Bonn, Deutschland, 24 July 1972; Volume 2019. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. Exp. Physiol. 2020, 105, 1459–1466. [Google Scholar] [CrossRef]
- Krueger, J.C.; Habigt, M.A.; Helmedag, M.J.; Uhlig, M.; Moss, M.; Bleich, A.; Tolba, R.H.; Rossaint, R.; Hein, M.; Mechelinck, M. Evaluation of Score Parameters for Severity Assessment of Surgery and Liver Cirrhosis in Rats. Anim. Welf. 2023, 32, e29. [Google Scholar] [CrossRef]
- Billig, S.; Hein, M.; Mechelinck, M.; Schumacher, D.; Roehl, A.B.; Fuchs, D.; Kramann, R.; Uhlig, M. Comparative Assessment of Coronary Physiology Using Transthoracic Pulsed-Wave Doppler and Myocardial Contrast Echocardiography in Rats. Eur. Radiol. Exp. 2023, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Kelm, N.Q.; Beare, J.E.; LeBlanc, A.J. Evaluation of Coronary Flow Reserve after Myocardial Ischemia Reperfusion in Rats. J. Vis. Exp. 2019, e59406. [Google Scholar] [CrossRef]
- Alvarez, E.; Dalton, N.D.; Gu, Y.; Smith, D.; Luong, A.; Hoshijima, M.; Peterson, K.L.; Rychak, J. A Novel Method for Quantitative Myocardial Contrast Echocardiography in Mice. Am. J. Physiol. Heart. Circ. Physiol. 2018, 314, H370–H379. [Google Scholar] [CrossRef]
- Klinge, U.; Dievernich, A.; Stegmaier, J. Quantitative Characterization of Macrophage, Lymphocyte, and Neutrophil Subtypes within the Foreign Body Granuloma of Human Mesh Explants by 5-Marker Multiplex Fluorescence Microscopy. Front. Med. 2022, 9, 777439. [Google Scholar] [CrossRef]
- Curran-Everett, D. Multiple Comparisons: Philosophies and Illustrations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1–R8. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, M.; Hein, M.; Habigt, M.A.; Tolba, R.H.; Braunschweig, T.; Helmedag, M.J.; Arici, M.; Theissen, A.; Klinkenberg, A.; Klinge, U.; et al. Cirrhotic Cardiomyopathy Following Bile Duct Ligation in Rats-a Matter of Time? Int. J. Mol. Sci. 2023, 24, 8147. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Marty, J.; Mantz, J.; Samain, E.; Braillon, A.; Lebrec, D. Desensitization of Myocardial Beta-Adrenergic Receptors in Cirrhotic Rats. Hepatology 1990, 12, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Gazawi, H.; Ljubuncic, P.; Cogan, U.; Hochgraff, E.; Ben-Shachar, D.; Bomzon, A. The Effects of Bile Acids on Beta-Adrenoceptors, Fluidity, and the Extent of Lipid Peroxidation in Rat Cardiac Membranes. Biochem. Pharmacol. 2000, 59, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Penny, D.J. Bile Acids Induce Arrhythmias: Old Metabolite, New Tricks. Heart 2013, 99, 1629–1630. [Google Scholar] [CrossRef] [PubMed]
- Joubert, P. Cholic Acid and the Heart: In Vitro Studies of the Effect on Heart Rate and Myocardial Contractility in the Rat. Clin. Exp. Pharmacol. Physiol. 1978, 5, 9–16. [Google Scholar] [CrossRef]
- Mathur, S.; Shah, A.R.; Ahlberg, A.W.; Katten, D.M.; Heller, G.V. Blunted Heart Rate Response as a Predictor of Cardiac Death in Patients Undergoing Vasodilator Stress Technetium-99m Sestamibi Gated SPECT Myocardial Perfusion Imaging. J. Nucl. Cardiol. 2010, 17, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction across the Spectrum of Cardiovascular Diseases: Jacc State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Hall, C. Nt-ProBNP: The Mechanism Behind the Marker. J. Card. Fail. 2005, 11, S81–S83. [Google Scholar] [CrossRef]
- Hinschen, A.K.; Rose, R.B.; Headrick, J.P. Adenosine Receptor Subtypes Mediating Coronary Vasodilation in Rat Hearts. J. Cardiovasc. Pharmacol. 2003, 41, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, R.R., Jr. The Pharmacology of Dobutamine. Am. J. Med. Sci. 1987, 294, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Jorgensen, C.R.; Gobel, F.L.; Taylor, H.L.; Wang, Y. Hemodynamic Correlates of Myocardial Oxygen Consumption During Upright Exercise. J. Appl. Physiol. 1972, 32, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Laclau, M.N.; Tariosse, L.; Daret, D.; Gouverneur, G.; Bonoron-Adèle, S.; Saks, V.A.; Dos Santos, P. Alteration of Mitochondrial Function in a Model of Chronic Ischemia in Vivo in Rat Heart. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H821–H831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gamez-Mendez, A.M.; Vargas-Robles, H.; Rios, A.; Escalante, B. Oxidative Stress-Dependent Coronary Endothelial Dysfunction in Obese Mice. PLoS ONE 2015, 10, e0138609. [Google Scholar] [CrossRef] [PubMed]
- Atochin, D.N.; Huang, P.L. Endothelial Nitric Oxide Synthase Transgenic Models of Endothelial Dysfunction. Pflugers Arch. 2010, 460, 965–974. [Google Scholar] [CrossRef]
- Ljubuncic, P.; Tanne, Z.; Bomzon, A. Evidence of a Systemic Phenomenon for Oxidative Stress in Cholestatic Liver Disease. Gut 2000, 47, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Zhang, H.; Hill, M.A.; Zhang, C.; Park, Y. Interaction of Il-6 and Tnf-Alpha Contributes to Endothelial Dysfunction in Type 2 Diabetic Mouse Hearts. PLoS ONE 2017, 12, e0187189. [Google Scholar]
- Ross, R. Atherosclerosis--an Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Shaul, P.W. Regulation of Endothelial Nitric Oxide Synthase: Location, Location, Location. Annu. Rev. Physiol. 2002, 64, 749–774. [Google Scholar] [CrossRef]
- Tajuddin, M.; Tariq, M.; Bilgrami, N.L.; Kumar, S. Biochemical and Pathological Changes in the Heart Following Bile Duct Ligation. Adv. Myocardiol. 1980, 2, 209–212. [Google Scholar] [PubMed]
- Binah, O.; Rubinstein, I.; Bomzon, A.; Better, O.S. Effects of Bile Acids on Ventricular Muscle Contraction and Electrophysiological Properties: Studies in Rat Papillary Muscle and Isolated Ventricular Myocytes. Naunyn. Schmiedebergs. Arch. Pharmacol. 1987, 335, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a Nuclear Receptor for Bile Acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G Protein-Coupled Receptor Responsive to Bile Acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Yuan, A.; Shan, P.; Gao, E.; Wang, X.; Wang, Y.; Lau, W.B.; Koch, W.; Ma, X.L.; He, B. Cardiomyocyte-Expressed Farnesoid-X-Receptor Is a Novel Apoptosis Mediator and Contributes to Myocardial Ischaemia/Reperfusion Injury. Eur. Heart J. 2013, 34, 1834–1845. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, F.; Zhao, S.; Li, Y.; Chen, X.; Gao, E.; Xu, X.; Xiong, Z.; Zhang, X.; Zhang, J.; et al. Adiponectin Determines Farnesoid X Receptor Agonism-Mediated Cardioprotection against Post-Infarction Remodelling and Dysfunction. Cardiovasc. Res. 2018, 114, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Eblimit, Z.; Thevananther, S.; Karpen, S.J.; Taegtmeyer, H.; Moore, D.D.; Adorini, L.; Penny, D.J.; Desai, M.S. TGR5 Activation Induces Cytoprotective Changes in the Heart and Improves Myocardial Adaptability to Physiologic, Inotropic, and Pressure-Induced Stress in Mice. Cardiovasc. Ther. 2018, 36, e12462. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, R.; Wan, H. Overexpression of Tgr5 Alleviates Myocardial Ischemia/Reperfusion Injury Via AKT/GSK-3beta Mediated Inflammation and Mitochondrial Pathway. Biosci. Rep. 2020, 40, BSR20193482. [Google Scholar]
- Wu, H.; Liu, G.; He, Y.; Da, J.; Xie, B. Obeticholic Acid Protects against Diabetic Cardiomyopathy by Activation of FXR/Nrf2 Signaling in db/db Mice. Eur. J. Pharmacol. 2019, 858, 172393. [Google Scholar] [CrossRef]
- Haga, S.; Yimin; Ozaki, M. Relevance of FXR-p62/SQSTM1 Pathway for Survival and Protection of Mouse Hepatocytes and Liver, Especially with Steatosis. BMC Gastroenterol. 2017, 17, 9. [Google Scholar] [CrossRef]
- Bayat, G.; Hashemi, S.A.; Karim, H.; Fallah, P.; Hedayatyanfard, K.; Bayat, M.; Khalili, A. Biliary Cirrhosis-Induced Cardiac Abnormality in Rats: Interaction between Farnesoid-X-Activated Receptors and the Cardiac Uncoupling Proteins 2 and 3. Iran. J. Basic. Med. Sci. 2022, 25, 126–133. [Google Scholar] [PubMed]
- Zhang, Y.; Hong, J.Y.; Rockwell, C.E.; Copple, B.L.; Jaeschke, H.; Klaassen, C.D. Effect of Bile Duct Ligation on Bile Acid Composition in Mouse Serum and Liver. Liver. Int. 2012, 32, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, T.; Uchida, K.; Kadowaki, M.; Takase, H.; Nomura, Y.; Saito, Y. Effect of Bile Duct Ligation on Bile Acid Metabolism in Rats. J. Lipid Res. 1981, 22, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Mathur, B.; Eblimit, Z.; Vasquez, H.; Taegtmeyer, H.; Karpen, S.J.; Penny, D.J.; Moore, D.D.; Anakk, S. Bile Acid Excess Induces Cardiomyopathy and Metabolic Dysfunctions in the Heart. Hepatology 2017, 65, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Goethals, L.; Debucquoy, A.; Perneel, C.; Geboes, K.; Ectors, N.; De Schutter, H.; Penninckx, F.; McBride, W.H.; Begg, A.C.; Haustermans, K.M. Hypoxia in Human Colorectal Adenocarcinoma: Comparison between Extrinsic and Potential Intrinsic Hypoxia Markers. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Bussink, J.; Kaanders, J.H.; Strik, A.M.; van der Kogel, A.J. Effects of Nicotinamide and Carbogen on Oxygenation in Human Tumor Xenografts Measured with Luminescense Based Fiber-Optic Probes. Radiother. Oncol. 2000, 57, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, J.A.; Chou, S.C.; Arteel, G.E.; Horsman, M.R. Comparisons among Pimonidazole Binding, Oxygen Electrode Measurements, and Radiation Response in C3H Mouse Tumors. Radiat. Res. 1999, 151, 580–589. [Google Scholar] [CrossRef]
- McCutcheon, J.E.; Marinelli, M. Age Matters. Eur. J. Neurosci. 2009, 29, 997–1014. [Google Scholar] [CrossRef]
| Parameter | Control (Mean ± SD) | BDL (Mean ± SD) | p-Value | |
|---|---|---|---|---|
| alkaline phosphatase (U/L) n | 166.78 ± 36.82 | 275.31 ± 66.58 | <0.001 | *** |
| 9 | 23 | |||
| aspartate transaminase (U/L) n | 55.33 ± 6.87 | 338.61 ± 204.68 | <0.001 | *** |
| 9 | 23 | |||
| alanine transaminase (U/L) n | 95.33 ± 86.46 | 160.65 ± 109.16 | 0.06 | ns |
| 9 | 23 | |||
| gamma-glutamyl transferase (U/L) n | 10.38 ± 2.56 | 35.48 ± 10.91 | <0.001 | *** |
| 8 | 23 | |||
| albumin (g/dL) n | 4.14 ± 0.4 | 4.67 ± 0.66 | 0.013 | * |
| 8 | 23 | |||
| creatinine (µmol/L) n | 48.33 ± 5.64 | 60.91 ± 35.97 | 0.48 | ns |
| 9 | 21 | |||
| total bile acids (µmol/L) n | 0.81 ± 2.54 | 127.52 ± 57.03 | <0.001 | *** |
| 9 | 22 | |||
| bilirubin (µmol/L) n | 2 ± 0 | 169.44 ± 44.55 | <0.001 | *** |
| 6 | 23 | |||
| creatine kinase (U/L) n | 92.56 ± 36.16 | 116.52 ± 66.05 | 0.31 | ns |
| 9 | 23 | |||
| creatine kinase MB isoenzyme (U/L) n | 178.22 ± 87.82 | 139.83 ± 72.35 | 0.21 | ns |
| 9 | 23 | |||
| troponin I (pg/mL) n | 15.66 ± 13.69 | 7.64 ± 9.06 | 0.07 | ns |
| 9 | 20 | |||
| brain natriuretic peptide (pg/mL) n | 218.02 ± 207.96 | 335.66 ± 557.62 | 0.21 | ns |
| 4 | 17 | |||
| white blood cells (103/µL) n | 11.11 ± 3.86 | 11.57 ± 4.62 | 0.81 | ns |
| 8 | 21 | |||
| tumor necrosis factor-α (pg/mL) n | 8.16 ± 0.35 | 8.54 ± 0.47 | 0.047 | * |
| 8 | 25 | |||
| platelets (103/µL) n | 305.4 ± 180.71 | 407.8 ± 202.82 | 0.16 | ns |
| 7 | 20 | |||
| hemoglobin (g/dL) n | 13.87 ± 0.62 | 13.12 ± 1.98 | 0.2 | ns |
| 6 | 22 | |||
| lactate (mmol/L) n | 3.31 ± 2.3 | 2.81 ± 1.55 | 0.36 | ns |
| 8 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billig, S.; Hein, M.; Kirchner, C.; Schumacher, D.; Habigt, M.A.; Mechelinck, M.; Fuchs, D.; Klinge, U.; Theißen, A.; Beckers, C.; et al. Coronary Microvascular Dysfunction in Acute Cholestasis-Induced Liver Injury. Biomedicines 2024, 12, 876. https://doi.org/10.3390/biomedicines12040876
Billig S, Hein M, Kirchner C, Schumacher D, Habigt MA, Mechelinck M, Fuchs D, Klinge U, Theißen A, Beckers C, et al. Coronary Microvascular Dysfunction in Acute Cholestasis-Induced Liver Injury. Biomedicines. 2024; 12(4):876. https://doi.org/10.3390/biomedicines12040876
Chicago/Turabian StyleBillig, Sebastian, Marc Hein, Celine Kirchner, David Schumacher, Moriz Aljoscha Habigt, Mare Mechelinck, Dieter Fuchs, Uwe Klinge, Alexander Theißen, Christian Beckers, and et al. 2024. "Coronary Microvascular Dysfunction in Acute Cholestasis-Induced Liver Injury" Biomedicines 12, no. 4: 876. https://doi.org/10.3390/biomedicines12040876
APA StyleBillig, S., Hein, M., Kirchner, C., Schumacher, D., Habigt, M. A., Mechelinck, M., Fuchs, D., Klinge, U., Theißen, A., Beckers, C., Bleilevens, C., Kramann, R., & Uhlig, M. (2024). Coronary Microvascular Dysfunction in Acute Cholestasis-Induced Liver Injury. Biomedicines, 12(4), 876. https://doi.org/10.3390/biomedicines12040876






