Increased Circulating CD14+ Monocytes in Patients with Psoriatic Arthritis Presenting Impaired Apoptosis Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Enrollment
2.2. Monocyte Enrichment
2.3. Gallium-67 Whole-Body Scans and Interpretation
2.4. Microarray Assay and Analysis
2.5. Real-Time qPCR Assay
2.6. Flow Cytometry Assay
2.7. Statistical Analysis
3. Results
3.1. Subject Information
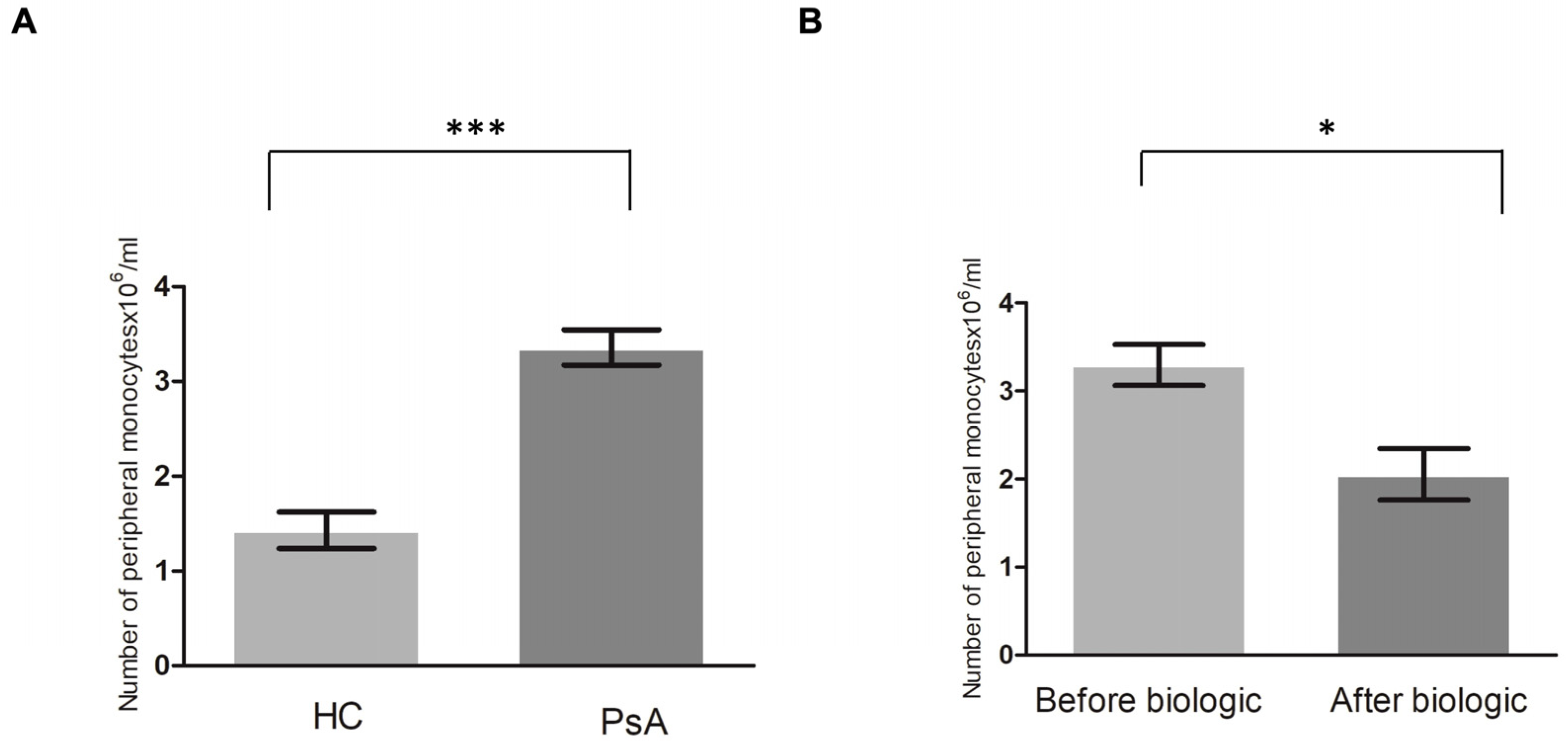
3.2. Higher Level of Monocytes in Patients with PsA
3.3. Increased 67Ga Activity in the Joints of Patients with PsA
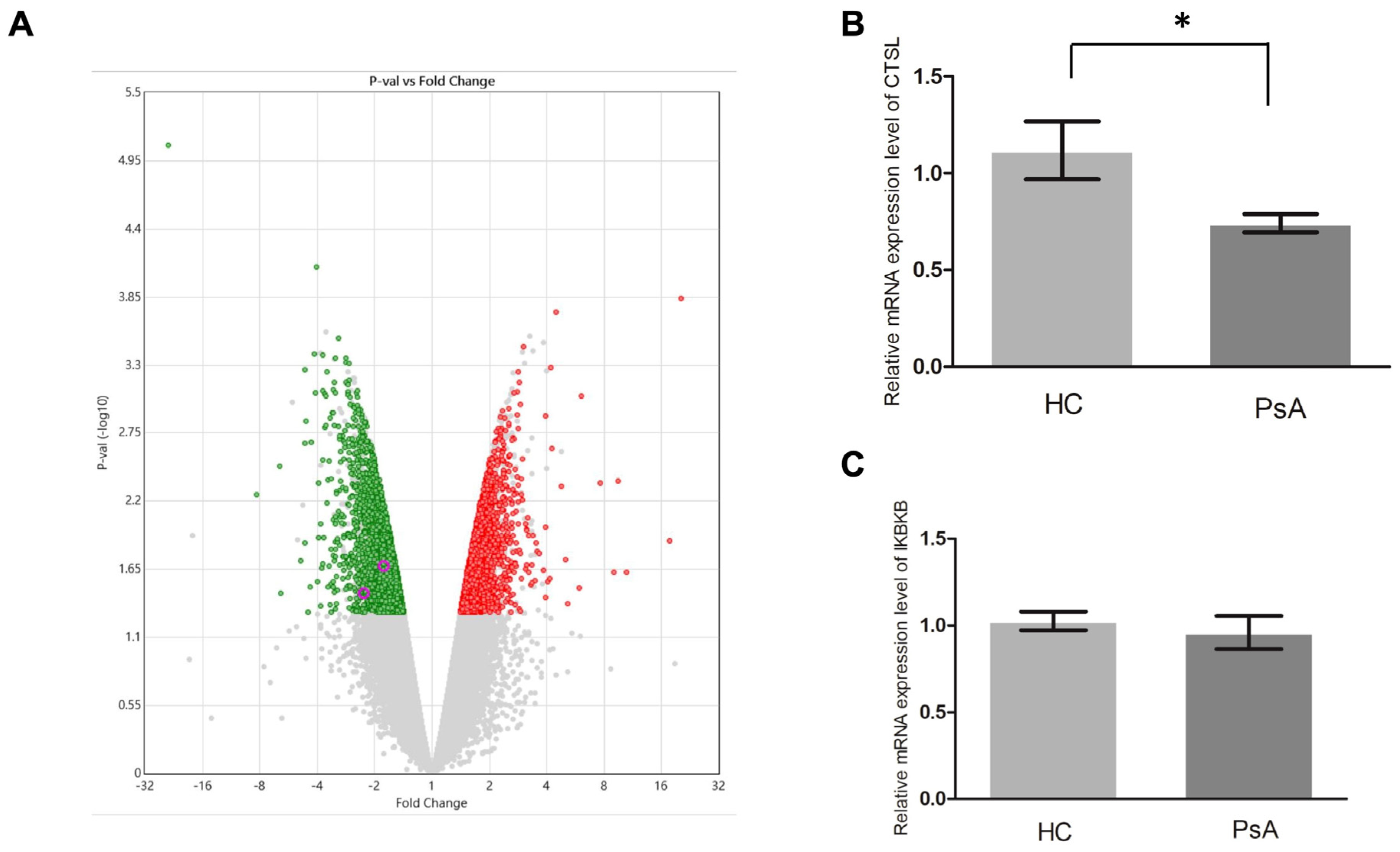
3.4. Repressed Apoptosis Pathway in the Monocytes from Patients with PsA
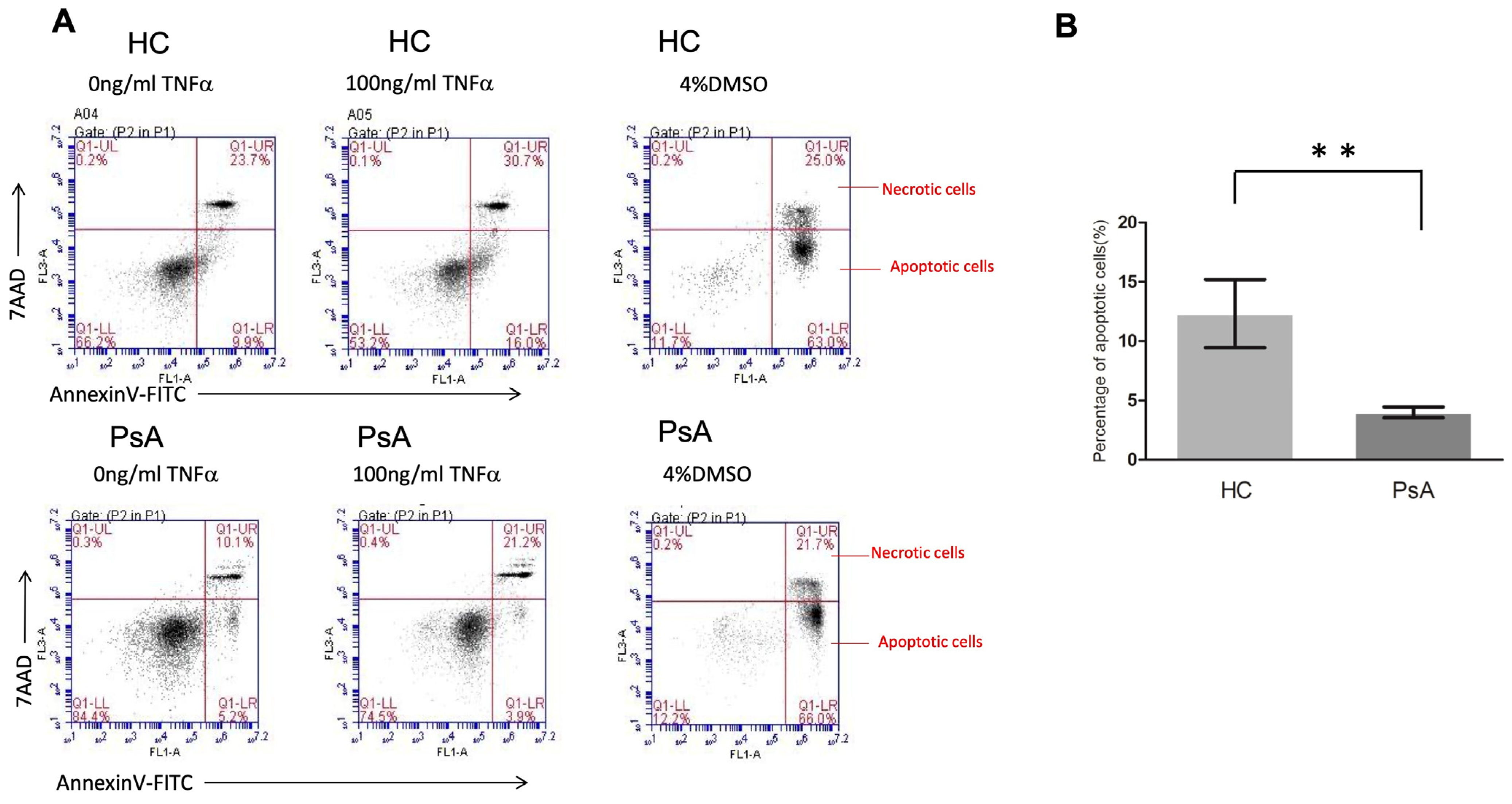
3.5. Impaired Apoptosis Activity in PsA Monocytes with Flow Cytometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson-Huang, L.M.; Lowes, M.A.; Krueger, J.G. Putting together the psoriasis puzzle: An update on developing targeted therapies. Dis. Model. Mech. 2012, 5, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Antoni, C.; Mease, P.; Clegg, D.O.; Nash, P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 2005, 64 (Suppl. S2), ii14–ii17. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef]
- Schett, G.; Rahman, P.; Ritchlin, C.; McInnes, I.B.; Elewaut, D.; Scher, J.U. Psoriatic arthritis from a mechanistic perspective. Nature reviews. Rheumatology 2022, 18, 311–325. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.F.; Wang, H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- McGonagle, D.; Marzo-Ortega, H.; O’Connor, P.; Gibbon, W.; Hawkey, P.; Henshaw, K.; Emery, P. Histological assessment of the early enthesitis lesion in spondyloarthropathy. Ann. Rheum. Dis. 2002, 61, 534–537. [Google Scholar] [CrossRef]
- De Beaucoudrey, L.; Puel, A.; Filipe-Santos, O.; Cobat, A.; Ghandil, P.; Chrabieh, M.; Feinberg, J.; von Bernuth, H.; Samarina, A.; Janniere, L.; et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 2008, 205, 1543–1550. [Google Scholar] [CrossRef]
- Bridgewood, C.; Sharif, K.; Sherlock, J.; Watad, A.; McGonagle, D. Interleukin-23 pathway at the enthesis: The emerging story of enthesitis in spondyloarthropathy. Immunol. Rev. 2020, 294, 27–47. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Massey, H.M.; Flanagan, A.M. Human osteoclasts derive from CD14-positive monocytes. Br. J. Haematol. 1999, 106, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Mensah, K.A.; Schwarz, E.M.; Ju, Y.; Takahata, M.; Feng, C.; McMahon, L.A.; Hicks, D.G.; Panepento, B.; Keng, P.C.; et al. Regulation of human osteoclast development by dendritic cell-specific transmembrane protein (DC-STAMP). J. Bone Miner. Res. 2012, 27, 79–92. [Google Scholar] [CrossRef]
- Savnik, A.; Malmskov, H.; Thomsen, H.S.; Graff, L.B.; Nielsen, H.; Danneskiold-Samsoe, B.; Boesen, J.; Bliddal, H. MRI of the wrist and finger joints in inflammatory joint diseases at 1-year interval: MRI features to predict bone erosions. Eur. Radiol. 2002, 12, 1203–1210. [Google Scholar] [CrossRef]
- Ritchlin, C.T.; Haas-Smith, S.A.; Li, P.; Hicks, D.G.; Schwarz, E.M. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J. Clin. Investig. 2003, 111, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Ho, J.C.; Li, S.C.; Chen, J.F.; Hsiao, C.C.; Lee, C.H. MiR-146a-5p Expression in Peripheral CD14+ Monocytes from Patients with Psoriatic Arthritis Induces Osteoclast Activation, Bone Resorption, and Correlates with Clinical Response. J. Clin. Med. 2019, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J. Tumour necrosis factor (TNF) in psoriatic arthritis: Pathophysiology and treatment with TNF inhibitors. Ann. Rheum. Dis. 2002, 61, 298–304. [Google Scholar] [CrossRef]
- Lin, S.H.; Ho, J.C.; Li, S.C.; Cheng, Y.W.; Hsu, C.Y.; Chou, W.Y.; Hsiao, C.C.; Lee, C.H. TNF-alpha Activating Osteoclasts in Patients with Psoriatic Arthritis Enhances the Recruitment of Osteoclast Precursors: A Plausible Role of WNT5A-MCP-1 in Osteoclast Engagement in Psoriatic Arthritis. Int. J. Mol. Sci. 2022, 23, 921. [Google Scholar] [CrossRef]
- Krammer, P.H. CD95’s deadly mission in the immune system. Nature 2000, 407, 789–795. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Meusch, U.; Rossol, M.; Baerwald, C.; Hauschildt, S.; Wagner, U. Outside-to-inside signaling through transmembrane tumor necrosis factor reverses pathologic interleukin-1beta production and deficient apoptosis of rheumatoid arthritis monocytes. Arthritis Rheum. 2009, 60, 2612–2621. [Google Scholar] [CrossRef]
- Srivastava, S.; Macaubas, C.; Deshpande, C.; Alexander, H.C.; Chang, S.Y.; Sun, Y.; Park, J.L.; Lee, T.; Begovich, A.; Mellins, E.D. Monocytes are resistant to apoptosis in systemic juvenile idiopathic arthritis. Clin. Immunol. 2010, 136, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Gulino, G.R.; Van Mechelen, M.; Lories, R. Cellular and molecular diversity in spondyloarthritis. Semin. Immunol. 2021, 58, 101521. [Google Scholar] [CrossRef]
- Munoz, L.E.; van Bavel, C.; Franz, S.; Berden, J.; Herrmann, M.; van der Vlag, J. Apoptosis in the pathogenesis of systemic lupus erythematosus. Lupus 2008, 17, 371–375. [Google Scholar] [CrossRef]
- Korb, A.; Pavenstadt, H.; Pap, T. Cell death in rheumatoid arthritis. Apoptosis 2009, 14, 447–454. [Google Scholar] [CrossRef]
- Lin, S.H.; Chuang, H.Y.; Ho, J.C.; Lee, C.H.; Hsiao, C.C. Treatment with TNF-alpha inhibitor rectifies M1 macrophage polarization from blood CD14+ monocytes in patients with psoriasis independent of STAT1 and IRF-1 activation. J. Dermatol. Sci. 2018, 91, 276–284. [Google Scholar] [CrossRef]
- Gallardo-Villagran, M.; Paulus, L.; Leger, D.Y.; Therrien, B.; Liagre, B. Dimethyl Sulfoxide: A Bio-Friendly or Bio-Hazard Chemical? The Effect of DMSO in Human Fibroblast-like Synoviocytes. Molecules 2022, 27, 4472. [Google Scholar] [CrossRef]
- Maciel, E.; Neves, B.M.; Santinha, D.; Reis, A.; Domingues, P.; Teresa Cruz, M.; Pitt, A.R.; Spickett, C.M.; Domingues, M.R. Detection of phosphatidylserine with a modified polar head group in human keratinocytes exposed to the radical generator AAPH. Arch. Biochem. Biophys. 2014, 548, 38–45. [Google Scholar] [CrossRef]
- Martinez-Ramos, S.; Rafael-Vidal, C.; Pego-Reigosa, J.M.; Garcia, S. Monocytes and Macrophages in Spondyloarthritis: Functional Roles and Effects of Current Therapies. Cells 2022, 11, 515. [Google Scholar] [CrossRef]
- Anandarajah, A.P.; Schwarz, E.M.; Totterman, S.; Monu, J.; Feng, C.Y.; Shao, T.; Haas-Smith, S.A.; Ritchlin, C.T. The effect of etanercept on osteoclast precursor frequency and enhancing bone marrow oedema in patients with psoriatic arthritis. Ann. Rheum. Dis. 2008, 67, 296–301. [Google Scholar] [CrossRef]
- Kanekura, T.; Seishima, M.; Honma, M.; Etou, T.; Eto, H.; Okuma, K.; Okubo, Y.; Yamaguchi, Y.; Kambara, T.; Mabuchi, T.; et al. Therapeutic depletion of myeloid lineage leukocytes by adsorptive apheresis for psoriatic arthritis: Efficacy of a non-drug intervention for patients refractory to pharmacologics. J. Dermatol. 2017, 44, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Gandjbakhch, F.; Terslev, L.; Joshua, F.; Wakefield, R.J.; Naredo, E.; D’Agostino, M.A.; Force, O.U.T. Ultrasound in the evaluation of enthesitis: Status and perspectives. Arthritis Res. Ther. 2011, 13, R188. [Google Scholar] [CrossRef] [PubMed]
- Elalouf, O.; Bakirci Ureyen, S.; Touma, Z.; Anderson, M.; Kaeley, G.S.; Aydin, S.Z.; Eder, L. Psoriatic Arthritis Sonographic Enthesitis Instruments: A Systematic Review of the Literature. J. Rheumatol. 2019, 46, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.Z.; Mathew, A.J.; Koppikar, S.; Eder, L.; Ostergaard, M. Imaging in the diagnosis and management of peripheral psoriatic arthritis. Best. Pract. Res. Clin. Rheumatol. 2020, 34, 101594. [Google Scholar] [CrossRef] [PubMed]
- McCall, I.W.; Sheppard, H.; Haddaway, M.; Park, W.M.; Ward, D.J. Gallium 67 scanning in rheumatoid arthritis. Br. J. Radiol. 1983, 56, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog. Neurobiol. 2000, 62, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Perera, L.P.; Waldmann, T.A. Activation of human monocytes induces differential resistance to apoptosis with rapid down regulation of caspase-8/FLICE. Proc. Natl. Acad. Sci. USA 1998, 95, 14308–14313. [Google Scholar] [CrossRef]
- Perlman, H.; Pagliari, L.J.; Liu, H.; Koch, A.E.; Haines, G.K., 3rd; Pope, R.M. Rheumatoid arthritis synovial macrophages express the Fas-associated death domain-like interleukin-1beta-converting enzyme-inhibitory protein and are refractory to Fas-mediated apoptosis. Arthritis Rheum. 2001, 44, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.L.; Chikura, B.; Bucknall, R.C.; Moots, R.J.; Edwards, S.W. Changes in expression of membrane TNF, NF-kappaB activation and neutrophil apoptosis during active and resolved inflammation. Ann. Rheum. Dis. 2011, 70, 537–543. [Google Scholar] [CrossRef]
- Cirman, T.; Oresic, K.; Mazovec, G.D.; Turk, V.; Reed, J.C.; Myers, R.M.; Salvesen, G.S.; Turk, B. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J. Biol. Chem. 2004, 279, 3578–3587. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, Y.F.; Xu, S.Q.; Wang, L.; Cao, H.M.; Cao, Y.; Zhu, Y.; Wang, Y.; Liang, Z.Q. Cathepsin L induced PC-12 cell apoptosis via activation of B-Myb and regulation of cell cycle proteins. Acta Pharmacol. Sin. 2019, 40, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kornmark, L.; Jonasson, L.; Forssell, C.; Yuan, X.M. Cathepsin L is significantly associated with apoptosis and plaque destabilization in human atherosclerosis. Atherosclerosis 2009, 202, 92–102. [Google Scholar] [CrossRef]
- Mahmood, D.F.D.; Jguirim-Souissi, I.; Khadija, E.H.; Blondeau, N.; Diderot, V.; Amrani, S.; Slimane, M.N.; Syrovets, T.; Simmet, T.; Rouis, M. Peroxisome proliferator-activated receptor gamma induces apoptosis and inhibits autophagy of human monocyte-derived macrophages via induction of cathepsin L: Potential role in atherosclerosis. J. Biol. Chem. 2011, 286, 28858–28866. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemund, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]

| PsA (n= 15) | Healthy Control (n= 15) | |
|---|---|---|
| Age (years) | 43.7 ± 14.5 | 44.9 ± 12.6 |
| Female sex no. (%) | 7 (46.7) | 7 (46.7) |
| Psoriasis (years) | 17.5 ± 9.7 | |
| Psoriatic arthritis (years) | 8.9 ± 6.9 | |
| PASI | 17.6 ± 9.3 | |
| Peripheral arthritis | 15 (100) | |
| Peripheral and axil arthritis | 7 (46.7) | |
| Dactylitis | 5 (33.3) | |
| Enthesitis | 10 (66.7) | |
| Tender joint count (of 68 joints) | 16.9 ± 11.9 | |
| Swollen joint count (of 66 joints) | 9.7 ± 6.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-H.; Hsu, C.-Y.; Li, S.-C. Increased Circulating CD14+ Monocytes in Patients with Psoriatic Arthritis Presenting Impaired Apoptosis Activity. Biomedicines 2024, 12, 775. https://doi.org/10.3390/biomedicines12040775
Lin S-H, Hsu C-Y, Li S-C. Increased Circulating CD14+ Monocytes in Patients with Psoriatic Arthritis Presenting Impaired Apoptosis Activity. Biomedicines. 2024; 12(4):775. https://doi.org/10.3390/biomedicines12040775
Chicago/Turabian StyleLin, Shang-Hung, Chung-Yuan Hsu, and Sung-Chou Li. 2024. "Increased Circulating CD14+ Monocytes in Patients with Psoriatic Arthritis Presenting Impaired Apoptosis Activity" Biomedicines 12, no. 4: 775. https://doi.org/10.3390/biomedicines12040775
APA StyleLin, S.-H., Hsu, C.-Y., & Li, S.-C. (2024). Increased Circulating CD14+ Monocytes in Patients with Psoriatic Arthritis Presenting Impaired Apoptosis Activity. Biomedicines, 12(4), 775. https://doi.org/10.3390/biomedicines12040775






