Abstract
Mesenchymal stem cells (MSCs) have been recognized as a cell therapy with the potential to promote skin healing. MSCs, with their multipotent differentiation ability, can generate various cells related to wound healing, such as dermal fibroblasts (DFs), endothelial cells, and keratinocytes. In addition, MSCs promote neovascularization, cellular regeneration, and tissue healing through mechanisms including paracrine and autocrine signaling. Due to these characteristics, MSCs have been extensively studied in the context of burn healing and chronic wound repair. Furthermore, during the investigation of MSCs, their unique roles in skin aging and scarless healing have also been discovered. In this review, we summarize the mechanisms by which MSCs promote wound healing and discuss the recent findings from preclinical and clinical studies. We also explore strategies to enhance the therapeutic effects of MSCs. Moreover, we discuss the emerging trend of combining MSCs with tissue engineering techniques, leveraging the advantages of MSCs and tissue engineering materials, such as biodegradable scaffolds and hydrogels, to enhance the skin repair capacity of MSCs. Additionally, we highlight the potential of using paracrine and autocrine characteristics of MSCs to explore cell-free therapies as a future direction in stem cell-based treatments, further demonstrating the clinical and regenerative aesthetic applications of MSCs in skin repair and regeneration.
1. Introduction
MSCs are a multipotent type of stem cells that originate from the mesoderm and ectoderm [1] in early development and can differentiate into a wide range of tissue cells, such as bone, cartilage, fat, muscle, and nerve cells. MSCs were first discovered in bone marrow, but have since been found in many other tissues of the body, such as adipose tissue, synovial tissue bone, muscle, lung, liver, pancreas, amniotic fluid, and umbilical cord blood [2,3]. MSCs from different sources differ in their accessibility, content, proliferation ability, immunomodulatory capacity, and the cytokines they secrete, and have different therapeutic potentials for different diseases.
Since MSCs were first discovered in bone marrow by Cohnheim in 1867 [4], they have been considered to play an important role in skin regeneration and wound healing. In 1991, Professor Caplan first introduced the concept of mesenchymal stem cells and emphasized their potential for multidirectional differentiation [5]. Subsequently, many researchers have isolated MSCs from different tissues and have demonstrated that they can differentiate into a wide range of cell types such as osteoblasts, adipocytes, chondrocytes, tenocytes, cardiomyocytes, keratinocytes, hepatocytes, and neural cells [6,7]. For example, dental-derived MSCs can be obtained from different parts of the teeth, such as the pulp, the ligament, the follicle, and the gingiva [8]. These stem cells have the ability to differentiate into various tissue types, such as bone, cartilage, fat, nerve, and skin [8]. Dental-derived MSCs can be used to repair damaged tissues and organs, and to treat diseases that; affect the immune system, the nervous system, the liver, and the skin [9]. In addition, MSCs have been found to have various biological functions such as immunomodulation, anti-inflammation, anti-apoptosis, and pro-angiogenesis [10,11]. In 1995, Professor Caplan extracted and isolated cultured MSCs from the bone marrow of patients with malignant hematological disorders and then infused them back into the patients to observe the clinical effects and to demonstrate the safety of these matrices [12]. Since then, the clinical application of MSCs has gradually expanded to a wide range of diseases, such as cardiovascular diseases, neurological diseases, bone and joint diseases, autoimmune diseases, liver diseases, diabetes, and so on [13].
Currently, several MSC-based therapies have received FDA approval for the treatment of acute graft-versus-host disease, bone defects, osteoporotic vertebral compression fractures, and ischemic heart failure [14]. According to ClinicalTrials.gov clinical registry data, there are currently more than 1300 clinical trials related to MSCs around the world, covering more than 300 diseases. Most of these clinical trials are being carried out for musculoskeletal disorders, central nervous system disorders, immune system disorders, wounds and traumas, rheumatic disorders, joint disorders, arthritis, vascular disorders, respiratory disorders, digestive disorders, and gastrointestinal disorders. These clinical studies have demonstrated that MSCs are a safe and effective therapeutic tool, providing a new treatment strategy for difficult multi-system diseases [15].
The clinical value of MSCs is not only reflected in their therapeutic efficacy, but also in their advantages in terms of high proliferation ability, immunogenicity, and differentiation capacity [16]. Currently, the most used stem cells are bone marrow-derived mesenchymal stem cells, but they have some limitations, such as a decline in the number and activity of the stem cells with age [17]. Therefore, the search for other alternative sources of MSCs is an important research direction. Among them, umbilical cord-derived MSCs have some advantages, such as abundant number of stem cells, easy collection, no harm to donors, low immunogenicity, high differentiation capacity, and no ethical controversy [18]. Umbilical cord-derived MSCs have been used to treat a wide range of diseases, such as cardiovascular diseases, liver diseases, bone and muscle degenerative diseases, neurological injuries, and autoimmune diseases [19,20,21].
In conclusion, MSCs are stem cells with a wide range of clinical applications, and they can promote tissue and organ regeneration and repair through a variety of mechanisms, providing new possibilities for the treatment of a wide range of diseases [13]. However, the clinical application of MSCs also faces some challenges and problems, such as cell quality control, immunological rejection, carcinogenic risk, standardized management, etc., which require further research and optimization [22].
2. The Wound Healing-Promoting Mechanisms of Mesenchymal Stem Cells
Wound healing is a complex biological process involving the coordinated action of multiple cellular, molecular, and signaling pathways. The process of wound healing can be divided into four phases: hemostasis, inflammation, proliferation, and remodeling (Figure 1) [23,24]. Under normal circumstances, the wound healing process is orderly and can restore the structure and function of the tissue. However, in some cases of disease or injury, the process of wound healing may be disrupted, resulting in difficulty in healing or the formation of pathological scars [25]. Therefore, exploring effective ways to promote wound healing and improve scar quality is an important clinical issue.
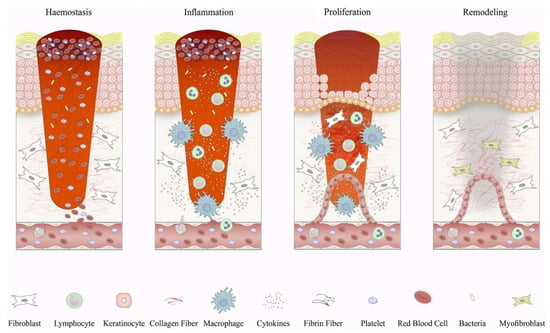
Figure 1.
Schematic depiction of wound healing phases and the corresponding cellular responses. In the initial phase of wound healing, when blood clotting occurs, platelets release signaling molecules and chemical messengers that attract inflammatory cells. Inflammation begins with the influx of neutrophils, facilitated by the release of histamine from mast cells. Subsequently, monocytes arrive and differentiate into tissue macrophages, which are responsible for clearing residual cell debris and neutrophils. In the proliferative phase, keratinocytes migrate to bridge the wound, new blood vessels form through the growth of tiny vessels, and specialized cells called fibroblasts replace the initial blood clot with a tissue known as granulation tissue. Macrophages and regulatory T cells play crucial roles during this stage of the healing process. Eventually, the newly formed tissue undergoes further restructuring as fibroblasts reshape the deposited matrix, the blood vessels diminish in size, and specialized cells called myofibroblasts contribute to the overall contraction of the wound. Reproduced with permission from [23].
Stem cells are a class of cells with self-renewal and multidirectional differentiation capabilities, and they play an important role in tissue repair and regeneration [26]. MSCs are adult stem cells found in a wide range of tissues; Figure 2 provides an illustration of the sources of mesenchymal stem cells [27]. The low immunogenicity, ease of isolation and expansion, multiple differentiation potentials, and paracrine functions of MSCs make them ideal candidates for trauma therapy [28]. In recent years, more and more studies have shown that MSCs can promote wound healing through various mechanisms, such as through their differentiation potential, paracrine function, and promotion of angiogenesis [29,30]. Among them, the MSCs’ paracrine function refers to their involvement in the inflammatory, proliferative, and remodeling processes of wound healing by secreting a variety of bioactive molecules, such as growth factors, cytokines, chemokines, exosomes, etc., to influence the function and state of the surrounding cells and tissues [31]. Neovascularization is the division of new blood vessels from the surrounding normal blood vessels during the wound healing process due to the inflammatory response and angiogenic factors [32]. The proliferation and migration of endothelial cells in a wound form a new vascular network, thereby improving blood supply and oxygen delivery to the wound and promoting wound healing and tissue regeneration [33]. The newborn blood vessels can provide oxygen and nutrients to the wound tissue, promote cell proliferation and differentiation, and enhance the resistance and repair ability of the wound [32]. The newborn blood vessels also release some cytokines, such as vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), IL-6, etc., which regulate the inflammatory response and fibrotic process, inhibit the activation and secretion of inflammatory cells, and promote the proliferation of fibroblasts and collagen synthesis [31,34]. In addition, neovascularization improves the microcirculation in the wound tissue, reduces tissue hypoxia and edema, reduces the damage to and death of capillary endothelial cells, and maintains capillary patency [35]. The newborn blood vessels can influence the remodeling process of the wound tissue, promoting steps such as epithelial formation, collagen remodeling, fibrosis, and the formation of structures such as granulation tissue and scar tissue [36,37]. In conclusion, neovascularization plays an important role in wound healing as both a stimulating and regulatory factor. Neovascularization interacts with other factors that, together, determine the efficiency and quality of wound healing.
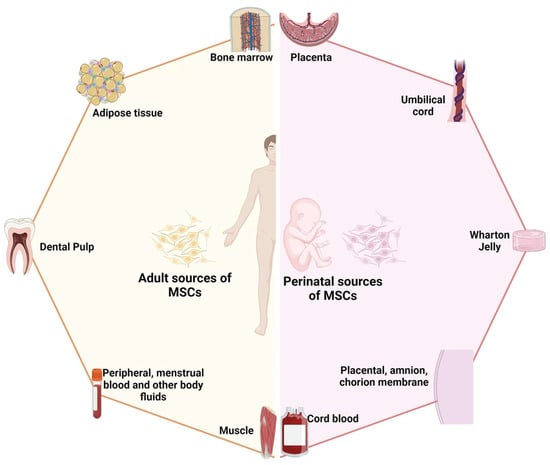
Figure 2.
Schematic diagram illustrating the two primary sources of mesenchymal stem cells: adult-derived and perinatal-derived sources. Reproduced with permission from [27].
MSCs can induce the proliferation, migration, and differentiation of peripheral vascular endothelial cells (VECs) by directly contacting or indirectly acting on VECs, thus promoting the formation and maturation of neovascularization of wounds, and improving the wound healing rate and functional recovery [38]. Endothelial cells are the main constituent cells of blood vessels and they play a key role in the process of neovascularization in wounds. Direct contact refers to the presence of physical or chemical interactions between MSCs and VECs, such as mechanical stretching, shear stress, and pharmacological inhibitors, which can enhance signaling and silencing of transcription factors between VECs and MSCs [39]. Indirect effects are defined as the effects of MSCs on the microenvironment around VECs through the release of exosomes or other factors, such as vascular endothelial growth factor A (VEGFA), epidermal growth factor (EGF), and fibroblast growth factor 2 (FGF2) [40,41]. The exosomes secreted by MSCs can bind to receptors on VECs, activating downstream signaling pathways such as the EGFR, ERK1/2, PI3K/Akt pathways, etc., and regulating the proliferation, migration, and differentiation of VECs, thereby promoting neovascularization [41].
MSCs are a class of adult stem cells with multiple differentiation potentials, which can differentiate into skin tissue cells, such as keratinocytes, fibroblasts, endothelial cells, etc., under the appropriate conditions, and thus participate in the re-epithelialization of wounds, formation of granulation tissue, and regeneration of blood vessels [37]. Keratinocytes are the main cell type of the skin’s surface layer and they play a key role in the re-epithelialization of wounds [42]. Re-epithelialization is the migration and proliferation of epithelial cells from the surface of the wound towards the center of the wound to form a new epithelial layer and restore the barrier function of the skin [43]. It has been found that exosomes isolated from human umbilical cord mesenchymal stem cells can accelerate re-epithelialization of burn wounds by enhancing the downstream effects of Wnt signaling through the increased nuclear translocation of β-catenin, thereby promoting the proliferation and migration of keratin-forming cells [44,45]. Exosomes are vesicles encapsulated by cell membranes, which can carry a wide range of biologically active molecules, such as proteins, nucleic acids, and lipids, thereby transmitting information between cells [46]. The Wnt/β-catenin signaling pathways are signal transduction pathways involved in cell proliferation, and play an important role in skin development and regeneration [47]. In recent years, it has been demonstrated that human umbilical cord mesenchymal stem cell exosomes could also accelerate the re-epithelialization of burn wounds by increasing the phosphorylation level of AKT and enhancing the downstream effects of AKT signaling, thereby promoting the proliferation and migration of keratin-forming cells [46]. The AKT signaling pathway is a signal transduction pathway involved in cell survival and proliferation, and plays an important role in skin regeneration [38]. Fibroblasts are the main cell type in the dermis of the skin and they play an important role in the proliferation and remodeling stages of wound healing [48]. Proliferation and remodeling refer to the synthesis and secretion of large amounts of extracellular matrix, such as collagen, elastic fibers, fibronectin, etc., by fibroblasts in the wound, thereby forming granulation tissue that fills in the wound defect and strengthens the wound [48]. It has been demonstrated that exosomes isolated from human adipose MSCs can reduce the proliferation of scar fibroblasts and collagen synthesis by inhibiting the TGF-β/Smad signaling pathway and improve scar formation in burn wounds [49]. Keloid fibroblasts are a specific type of fibroblasts that appear during the wound healing process; they are highly proliferative and secretory but lack the ability to degrade and remodel the extracellular matrix, leading to the excessive deposition of extracellular matrix and fibrosis of the tissue [50]. The TGF-β/Smad signaling pathway is a signal transduction pathway involved in cell proliferation and differentiation, and plays an important role in the regulation of scar formation [51]. Studies have shown that human adipose MSC exosomes can reduce scar formation in burn wounds by decreasing the expression and activity of TGF-β and inhibiting the phosphorylation and nuclear translocation of Smad2/3, thereby reducing scar fibroblast proliferation and collagen synthesis [52]. Additionally, MSCs also crosstalk with tissue cells such as macrophages to promote wound healing. Macrophages play a beneficial role in the wound repair process, and the anti-inflammatory M2 phenotype promotes wound healing in the later stages of wound healing [53]. Exosomes of MSCs are able to promote macrophage M2 polarization by targeting pknox1 so as to enhance wound healing [54].
3. The Potential of Mesenchymal Stem Cells in the Treatment of Clinical Diseases
The use of MSCs in clinical diseases is a cutting-edge research area that promises to provide new strategies and approaches for the treatment of a variety of skin injuries and diseases, such as chronic refractory wounds and burn injuries. The main aspects of acute and chronic wound healing are at the anatomical level.
MSC-derived extracellular vesicles (EVs) aid in tissue regeneration by facilitating the regrowth of the epidermis, dermis, hair follicles, nerves, and blood vessels, while also mitigating abnormal pigmentation (Figure 3) [55]. The roles of MSCs in wound healing are shown in Table 1. Chronic refractory wounds mean that the wounds cannot achieve structural and functional integrity in time and eventually cause a chronic inflammatory state [56]. They usually require long-term care and treatment to promote healing and prevent complications. The difficulty in the treatment of chronic refractory wounds lies in the lack of effective stimulating and growth factors, as well as the lack of precise control and regulation of the wound tissue. Burn injuries involve damage to the skin tissues due to friction, cold, heat, radiation, chemical, or electric sources [57]. They usually cause severe complications such as edema, necrosis, and infections [57]. The difficulties in the treatment of burn injuries lie in the lack of effective protective barriers and repair mechanisms, as well as the lack of individualized therapeutic treatments for different types and degrees of burns. In this section, we focus on the potential of MSCs in the treatment of clinical diseases from two perspectives: the treatment of chronic refractory wounds and burn injuries.
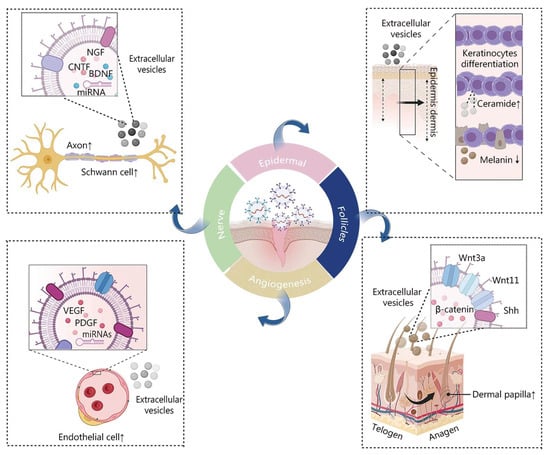
Figure 3.
Illustration depicting the impact of MSC-derived EVs on the process of wound healing. Reproduced with permission from [55].

Table 1.
MSC therapy in animal models to promote wound healing.
To date, the treatments for chronic refractory wounds have included debridement, topical antibiotics, compression bandages, skin grafts, and cytokines [67,68,69,70]. However, all of these methods have certain limitations and side effects, for example, debridement can cause infection and bleeding [71], etc. Therefore, there is an urgent need to explore some new approaches. MSC therapy is a novel therapy with great potential to restart the normal healing response in old wounds by increasing the accumulation of MSCs in the wound. The mechanisms of stem cell effects in chronic refractory wound healing include cell recruitment, cell differentiation, immunomodulation, antimicrobial effects, pro-angiogenic effects, and epidermal replantation [72,73]. Stem cells are used in chronic refractory wounds by directly injecting MSCs into the wound [74,75]. Direct injection can rapidly increase the number and distribution of MSCs, promoting wound healing and tissue regeneration [75]. Several clinical trials and studies have been conducted to investigate the efficacy and safety of MSCs in the treatment of chronic refractory wounds [55]. In short, MSCs have great potential and advantages in the treatment of chronic refractory wounds, and can be used in a variety of ways to achieve repair of and tissue regeneration in wounds. However, there are still some challenges and problems, such as the need for the further optimization and validation of stem cell sources, quality, quantity, preservation, transport, and safety. More high-quality clinical trials are needed in the future to assess the efficacy and safety of MSCs in different types and degrees of chronic refractory wounds and to explore the optimal use and dosage.
Besides their superiority in treatments for chronic refractory wounds, MSCs also have great potential in the treatment of burn injuries and can promote the healing of burn wounds in several ways. Firstly, MSCs can inhibit the excessive inflammatory response after a burn injury by reducing the infiltration of inflammatory cells and releasing inflammatory factors, thus reducing the severity of the burn injury and the risk of complications [76]. They can also trigger the polarization of macrophages from the pro-inflammatory M1 type to pro-healing M2 type, thereby promoting wound cleaning and repair [77]. In addition, MSCs can stimulate the formation of neovascularization by secreting growth factors such as VEGF and FGF, which increase blood perfusion and provide nutrients to burn wounds, thereby accelerating wound healing [78]. They can improve the structure and function of wounds by secreting matrix proteins such as collagen, elastin, and fibronectin, which promote the reconstruction of the dermis and enhance the strength and elasticity of the wound [79]. MSCs can also stimulate the proliferation and differentiation of epidermal cells and promote the regeneration of the epidermal layer through the secretion of growth factors such as transforming growth factor-β (TGF-β), epidermal growth factor (EGF), and keratinocyte growth factor (KGF), thereby restoring the barrier function of the skin [23]. Furthermore, MSCs can regulate the remodeling of the extracellular matrix (ECM) by secreting enzymes such as matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), which balances matrix synthesis and degradation, thereby reducing scar formation after a burn [80]. They can also inhibit keloid formation by restraining the proliferation and secretion of fibroblasts, thus improving the restoration effects [81].
In summary, MSCs have multifaceted advantages in the treatment of burns and can influence the healing process of burn wounds at multiple levels, including inflammation, vascularity, and scarring, in the dermis and epidermis to improve the quality of life and survival rate of burn patients. Currently, several clinical studies and case reports have confirmed the efficacy and safety of MSCs in the treatment of burn injuries [82], but further exploration of the optimal source, dosage, delivery, timing, and mechanism of MSCs is still needed to provide a more optimal treatment plan for burn patients.
4. Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation
The use of the regeneration ability of MSCs is not limited to disease treatments and is also used in the aesthetic field. MSCs have received attention due to their multilineage differentiation abilities. Some studies have reported that mesenchymal stem cell exosomes can be utilized in the treatment of skin aging. Except for chronological aging, human skin also undergoes photoaging, a kind of sun-induced skin aging. Actually, in the past 30 years, the research on the molecular mechanisms of skin photoaging such as the production of intracellular ROS has made substantial progress. The generation of reactive oxygen species (ROS) damages the connective tissues in human skin [83]. The exposure to ultraviolet rays for a long time has been proven to decrease collagen protein production.
In contrast, MSCs, with their antioxidation and anti-apoptosis effects, can reduce the production of metalloproteinases (MMPs) and activate the proliferation of dermal fibroblasts [84]. A study compared adipose-derived stem cells (ADSCs) with fibroblasts in the improvement of skin wrinkles caused by photoaging and found that ADSCs are as effective as fibroblasts in promoting the production of collagen protein [85]. There are plenty of studies that have demonstrated that therapies and medicines based on stem cells can inhibit the signaling cascades which participate in telomere shortening, estrogen depletion, and excessive ROS production [86]. It has been reported that ADSCs have anti-aging effects on aging cells and in animal models of premature aging. ADSCs can accelerate mitophagy, eliminate intracellular ROS, and eventually improve the number of mitochondria [87]. Exosomes from the conditioned medium of human induced pluripotent stem cells (iPSCs) have a therapeutic potential in the treatment of skin aging caused by photoaging and natural senescence. Pretreated induced pluripotent stem cells exosomes (iPSCs-Exo’s) can inhibit the overexpression of MMP-1/3 and attenuate the human dermal fibroblast (HDF) injury caused by UVB and finally restore the expression of type I collagen [88]. By injecting autologous adipose-derived mesenchymal stem cells expanded in vitro, completely de novo oxytalan and elaunin fiber production in the subepidermal area and the rebuilding of the dermis–epidermal junction structure were observed, indicating the complete rescue of solar elastic deformation [89].
Moreover, adipose stem cell-conditioned culture medium (ADSC-CM) can reduce UVB-induced apoptosis and stimulate collagen synthesis by HDFs to reduce the appearance of wrinkles, which was confirmed by a shortening of the subG1 phase of HDFs. ADSC-CM can also increase the expression of type I collagen in HDFs and increase the level of metalloproteinase 1 [90]. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells (USC-CM) contains epithelial growth factor (EGF), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), collagen type 1, and growth differentiation factor-11 (GDF-11), one of the youth growth factors [91]. In an experiment, GDF-11 significantly accelerated the growth and migration of HDFs and also improved the production of extracellular matrix (ECM). USC-CM exosomes (USC-CM Exo’s) contain the growth factor related to skin rejuvenation which acts on HDFs to promote cell migration and collagen synthesis [92].
In recent years, the extracts secreted by mesenchymal stem cells have been used as a prospective biological therapy. However, the extracts are unstable and non-specific which could be resolved using artificial intelligence. Due to the ability of machine learning and artificial intelligence to predict and simulate protein folding and peptide/protein structure interactions, they have been used for screening promising biomimetic peptide components in mesenchymal stem cell secretions. One peptide virtual screening model which identified EQ-9 as a peptide with anti-aging and skin repair functions. EQ-9 potentially inhibits inflammation through increasing the fibroblast survival rate and decreasing intracellular ROS levels [93]. It has been reported that microfragmented adipose tissue containing adipose stem cells combined with a crosslinked hyaluronic acid scaffold can improve soft tissue defects such as deep wrinkles [94].
5. Methods for Enhancing the Effects of MSCs
5.1. Stem Cell Delivery into Skin Wounds
A dermal substitute is able to promote wound healing by shortening the healing time and improving the impaired function of the injured tissues. As a prospective carrier of stem cells, SECM-MC hydrogel, composed of soluble ECM (sECM) and methyl cellulose (MC), was inserted into a full-layer skin wound, which led to wound healing through re-epithelialization and neovascularization [95].
Through the combination of biomaterials and living cells, tissue engineering technology can promote the development of regenerative medicine. Tissue engineering scaffolds can transport stem cells to the sites in need of repair, eventually increasing the retention and implantation rates of stem cell transplants [96,97]. The rise of 3D printing technology further enriches the structural design and composition of tissue engineering scaffolds, and also provides convenience for the loading and delivery of live cells. Transplanting human gingival tissue pluripotent mesenchymal stem cells/stromal cells in 3Dprinted medical grade polycaprolactone (mPCL) dressings resulted in wound contracture and significantly improved skin regeneration through granulation and re-epithelialization [98]. One study showed that dermal vascular endothelial cells in wounds treated with chitosan exhibited better immunoreactivity [99]. A new type of chitosan/decellularized dermal matrix (CHS/ADM) stem cell delivery system can overcome the limitation of the traditional collagen delivery system, which lacks a response to high ROS environments. In a high ROS microenvironment, the new system acts as a protective screen and effectively clears a certain amount of the ROS, protecting mesenchymal stem cells (MSCs) from oxidative stress [100]. Mesenchymal stem cells derived from rat adipose tissue seeded on collagen–chitosan scaffolds and implanted into wounds can completely heal the wound, restoring the epidermis and dermis to a normal state [101]. Decellularized human amniotic membranes (dAMs) and matrix (sAM) were used as wound dressing scaffolds. AdMSCs were seeded onto dAMs or sAM, and the results showed that they can promote wound healing by enhancing angiogenesis and collagen remodeling [102]. A scaffold made of polyethylene terephthalate (PET) can be used to load mesenchymal stromal cells (MSCs) and promote the rate of wound re-epithelialization [103]. BM-MSCs delivered by EGF microspheres into an engineered skin model improved skin wound healing and repaired sweat glands [104]. Stem cells and nano-specific simvastatin, which both have the effect of enhancing wound healing, can be locally applied in the same tissue scaffold (TS) to provide a more effective choice for diabetic wound healing [105]. A DADM/MSC scaffold containing bone marrow mesenchymal stem cells (BM MSCs) and a degenerated decellularized dermal matrix (DADM) promoted wound healing in deep, extensive burn wounds. It was reported that, combined with cell therapy, a pre-synthesized novel nano scaffold made of nanocellulose, type I collagen, and carboxymethyl diethylaminoethyl cellulose has a synergistic effect on wound healing in rats [106]. The researchers developed an in situ cell electrospinning system which overcomes the shortcomings of some stem cell delivery methods, such as a lack of targeting and easy cell loss. The system increased collagen deposition to enhance extracellular matrix remodeling without negatively impacting surface marker expression and the differentiation ability of MSCs.
Furthermore, it also increased the expression of vascular endothelial growth factor (VEGF) and the formation of small blood vessels to promote angiogenesis but significantly reduced the expression of interleukin-6 (IL-6), and ultimately promoted skin wound healing [107]. When comparing poly (ε-caprolactone) (PCL) and poly (ε-caprolactone)/type I collagen (PCol) in the ability to promote biological signaling, wound coverage, and tissue repair processes, it was found that PCol/human Wharton’s jelly mesenchymal stromal cells hWJ-MSCs had a better effect on skin tissue repair [108]. The rheological properties of hydrogels are similar to those of the natural extracellular matrix of skin and they can simulate various functions. Therefore, hydrogels have good development prospects as stem cell delivery vehicles [109]. Hydrogels derived from porcine myocardial matrix have entered clinical trials (NCT02305602) for the prevention and treatment of heart failure after myocardial infarction.
Additionally, natural polymer hydrogels have great advantages in accelerating chronic wound healing. Adipose-derived stem cell (ADSC)- and platelet-rich plasma (PRP)-supported hydrogel systems based on methacrylate gelatin (GelMA) and methacrylate fibroin protein (SFMA) have been developed as cell and growth factor delivery carriers for the treatment of pressure ulcers, and have shown good efficacy [110]. In addition, studies have indicated a great potential for using Poloxam hydrogel as a cell carrier to support human mesenchymal stromal cells (hMSCs) [111]. hUCMSCs, human umbilical cord mesenchymal stem cells encapsulated in a functional injectable thermosensitive hydrogel (chitosan/sodium glycerophosphite/cellulose nanocrystalline, CS/GP/CNC), can be used to repair full-layer skin wounds and significantly accelerate wound closure, microcirculation, tissue remodeling, re-epithelialization, and hair follicle regeneration [112]. Through promoting the transformation of M1-type macrophages into M2-type macrophages and accelerating wound angiogenesis, carboxyethyl chitosan (CEC)-dialdehyde carboxymethyl cellulose hydrogel (MSC-Exos@CEC-DCMC HG) loaded on bone marrow mesenchymal stem cell-derived exosomes (MSC-Exo) resulted in a reduction in inflammation [113]. Microgels consisting of aligned silk nanofibers were used to load MSCs and regulate paracrine signaling; dispersing the MSCs into these injectable silk nanofiber hydrogels can protect and stabilize these cells in wounds.
At the same time, the system is adjustable which enhanced the effect of the MSCs [114]. Recently, a novel polysaccharide-based hydrogel scaffold was made using alginate to create a suitable microenvironment for the delivery of adipose-derived mesenchymal stem cells (ASCs) and was demonstrated to improve wound healing processes and accelerate wound closure [115]. In situ hydrogel systems composed of hyperbranched polyethylene glycol diacrylate (HB-PEGDA) polymers, sulfhydryl functionalized hyaluronic acid (HA-SH), and short RGD peptides bound to adipose-derived stem cells (ASCs) significantly enhanced neovascularization and accelerated wound healing [116]. With the aim of reducing scar formation, bone marrow mesenchymal stem cell (BMSC)-derived nanovesicles (NVs) released by hydrogels were enhanced by genipin and BSA, resulting in efficient ROS clearance and good immunomodulatory activity, and the promoted the proliferation and migration of fibroblasts and vascular endothelial cells, which effectively treated diabetic wounds [117]. Injectable hydrogels from adipose acellular matrix hydrogels (hDAT-gel) combined with human adipose stem cells (hASCs) can accelerate the formation of blood vessels at the wound site to a certain extent and accelerate wound healing, which has great potential in the field of wound healing [118].
It has been found that the stimulation of rat adipose stem cells (rASC) with a 5 μA electrical stimulation (ES), namely 5 μA PFS, can enhance their paracrine function, and delivering the 5 μA PFS with a heparinized PGA–host–guest hydrogel (PGA–Hp hydrogel) effectively accelerated the repair process in a rat full-layer wound model. Amino-functionalized mesoporous silica nanoparticles (MSNs) can enhance the stability of hydrogel beads and significantly improve the proliferative properties of human adipose-derived mesenchymal stem cells (hASCs). In one study, treating circulating monocytes with mesenchymal stem cell (MSC) superserum to produce activated macrophages in a double-layer scaffold composed of hydrogels and nanofibers resulted in faster wound healing rates [119]. In another similar study, a double-layer scaffold composed of hydrogels and nanofibers was also prepared, and ADSCs were inoculated onto it; the optimal performance of re-epithelialization, collagen tissue production, neovascularization, and reduction in inflammation in the wound area were observed [120].
Currently, synthetic cell vectors can be produced by polymerizing acrylic plasma onto medical-grade silica gel for the purpose of delivering hBM-MSCs into the skin [121]. Silk fibroin (SF) was shown to significantly improve the adhesion of bone mesenchymal stem cells (BMSCs), while Col/TSF hybrid scaffolds have excellent skin affinity, good air and water permeability, and good wound healing potential [122].
5.2. The Effect of Stem Cell Sheets
Scaffolds have disadvantages such as high requirements for a sufficient supply of cells and correct cell injection positions. In contrast, cell sheets, cells that are either self-supporting or that are delivered from a supporting material but where the material plays no long-term role in the therapy [123], do not need a scaffold and have a higher cell density, which is one of the crucial factors for enhancing the therapeutic function of cell transplantation. Due to their high cell density, cell sheets show a longer retention time at the transplant site as well as more local delivery of growth factors and cytokines.
Cell sheet engineering (CSE) has attracted increasing attention as a competitive alternative to traditional cell-based or stent-based approaches due to its inherent advantages of higher cell survival and biocompatibility [124]. In addition to heart tissue, the benefits of ASC tablets on tissue regeneration are mainly reflected in the skin [125]. Early studies have found that the combination of ASC tablets and artificial skin grafts accelerates wound healing and blood vessel formation [126]. A ROS-induced cell sheet stacking method was designed, and the newly prepared hematoporphyrin was incorporated into a polyketone membrane (Hp-PK membrane), which could improve the delivery efficiency of cell sheets and be effectively applied for wound healing [127]. Human umbilical cord mesenchymal stem cells (hUC-MSCs) were cultivated on Col-T scaffolds to prepare stem cell tablets, which could restore the structure and function of damaged tissues [128]. The injection of disintegrated human amniotic fluid stem cells hAFSC tablets can exert anti-fibrotic properties without delaying wound closure, thereby accelerating skin wound healing and reducing fibrotic scarring, similar to fetal wound healing.
Compared to dissociated cells, treating wound tissues with ASC tablets has the advantages of faster wound healing and minimal risk of long-term side effects [129]. Compared with normal people, diabetic patients with foot ulcers usually show prolonged wound healing due to diabetic neuropathy and blood flow disorders. However, it had been indicated that the direct injection of human fat stem cells (hASCs) can effectively accelerate wound healing in diabetic patients, and this study also pointed out that hASCs have the disadvantage of relative instability [130]. Studies of skin pressure sore healing induced by the injection of MSC-based cell sheets (CSs) in C57Bl/6 mice found that, despite a brief retention of the CSs on the ulcer surface (3–7 days), there was an increase in granulation tissue (GT) thickness and increased vascular maturity, while at the same time, compared to the mesenchymal stromal cell (MSC) exosome group, the CSs had a unique function of skin repair using skin appendages [131]. Additionally, the use of the peritoneum as the support for the precise transplantation of ASC tablets to the back of SD rats had better effects on gross and histopathological repair than that of simply injecting the ASC tablets [132]. Compared with fibroblast tablets, complete tablets composed of amniotic mesenchymal stem cells have a higher tendency to disintegrate, and have the potential to treat burn wounds [133].
Moreover, adipose-derived stem cell (ADSC) tablets can also promote peripheral nerve regeneration [134] and repair ulcerative oral mucosa [135]. In order to harvest cells from a culture medium with intact extracellular matrix (ECM) and preserved intercellular connections, many new materials and methods have been developed, among which nanomaterials are a hot topic. For example, TiO2 nanodots are the most commonly used nanomaterials in photoinduced cell sheet technology, and two-dimensional (2D) nanomaterials such as graphene have also been applied in photoinduced cell sheet technology [136].
5.3. Effects of Conditioned Media
There are some limitations to the clinical use of ASCs. For autologous ASCs, ASCs need to be cultured for several weeks to obtain a sufficient number of cells, and the process comes with significant costs and requires staff to maintain the cell culture and cell processing facilities.
At present, there is increasing evidence that mesenchymal stem cells can promote skin repair and regeneration through paracrine actions [137,138,139]. Mesenchymal stem cell-conditioned medium, as a cell-free therapy, can accelerate the wound healing process and avoid the risks of live-cell therapy. Not only that, CM can be easily manufactured, stored, and transported. A study found that Wharton’s jelly mesenchymal stem cell (WJ-MSC)-derived conditioned medium (MSC-CM) secreted some factors that promoted HUVEC multiplication, increased the regeneration of sebaceous glands, and enhanced the angiogenesis induced by human umbilical vein endothelial cells. By promoting the expression of α-SMA, MSC-CM significantly increased the number of skin blood vessels in healed wounds. At the same time, MSCCM was used to treat radiation dermatitis in rats for the first time in [140]. Another similar study showed that WJ-MSCs had a greater ability in sweat gland repair and skin regeneration after skin injury, and the MSC-CM group had the smallest wound area and the highest Col1A2 expression [141]. The conditioned medium of human cord blood mesenchymal stem cells (USC-CM) has an anti-inflammatory effect through the growth factors and cytokines in it, such as EGF [142]. ASC-CM treatment was shown to promote the anti-inflammatory phenotype of macrophages, partially protecting the skin barrier damaged by PMA exposure, and has broad application prospects in wound healing and skin inflammation [143].
Moreover, USC-CM also includes growth factors associated with skin rejuvenation, such as growth differentiation factor-11 (GDF-11) [144]. It has also been found that after injecting a medium conditioned by mesenchymal stromal cells into a wound, a lower inflammation level or enhanced epithelialization were observed [145]. By preparing a diabetic foot ulcer (DFU) model and pretreating the ulcer with rat bone marrow MSC-conditioned medium, it was finally demonstrated that MSC-CM injected into DFU rats promotes the wound healing process by accelerating wound closure, cell proliferation, and angiogenesis, without increasing ulcer cell death [146].
Excessive wound repair can lead to hyperplastic scars or keloids, and it is generally believed that skin fibroblasts play an important role in the scarring process. Hypoxic-conditioned media of placenta-derived mesenchymal stem cells (PMSCs) reduced scarring in vivo and inhibited the proliferation and migration of skin fibroblasts in vitro [147], suggesting that PMSCS may be a promising wound treatment therapy.
5.4. Other Practicable Methods to Deliver Stem Cells
Before the transplantation of human adipose-derived stromal cell (hASC) globules (PBM-globules), photobiological regulation can stimulate angiogenesis and tissue regeneration in mouse flaps to improve skin tissue functional recovery. When the wound tissue is treated with cell sheets composed of adipose-derived stem cells (ASCs), more transplanted ASCs can be observed in the wound tissue, and the new skin formed from it has a thickness similar to normal skin and a highly organized collagen structure, which can ultimately improve skin wound healing and reduce scarring [129]. The injection of cell sheets made from disintegrated human amniotic fluid stem cells (hAFSCs) can exert anti-fibrotic properties without delaying wound closure [148].
Studies have found that three-dimensional graphene foam (GF) scaffolds have good biocompatibility, and the combination with bone marrow mesenchymal stem cells (MSCs) promotes the growth and proliferation of MSCs, which can improve skin wound healing [149]. Platelet-rich plasma (PRP) products are believed to have a pro-angiogenic effect and are currently recommended for the treatment of chronic wounds. In one study, the researchers added PRP to irradiated HDMEC and hASC cultures to prevent a large radiation-induced drop in cell numbers, rescuing the proliferation defects caused by external radiation. This method may be beneficial for treating chronic wounds with defective healing processes [150]. Modified acellular dermal matrix (DADM), as a skin substitute combined with bone marrow mesenchymal stem cells (BM-MSCs) implanted on mouse skin wounds, has good survival characteristics and represents a promising alternative therapy for deep, extensive burn wound healing [151]. To verify that preconditioning ASCs with hypoxia leads to enhanced functions of the adipose-derived stem cells (ASCs), porcine adipose stem cells (pASCs) cultured under hypoxia (PASCS-HYP) were transplanted into a mouse model of full-layer skin wound resection and were shown to promote the expression of the angiogenesis marker VegfA and decreased the level of proliferation-promoting Tgfβ1 [152]. Low-level laser therapy (LLLT) can increase the survival rate of ASCs, thereby stimulating the secretion of growth factors [153]. Gene-activated scaffolds (GASs) transfected with genes were loaded with bone marrow-derived mesenchymal stem cells (BM-MSCs) to form GAS/BM-MSCs constructs; these constructs were shown to accelerate the wound healing process and induce in situ regeneration of full-layer skin with sweat glands [154]. The use of micronized amniotic membrane (mAM) as a microcarrier can improve the in vitro expansion efficiency of mesenchymal stem cells [155]. Studies have shown that the pre-treatment of MSCs with bioactive compounds can improve their survival rate and regenerative potential. Human umbilical cord mesenchymal stem cells (hUC-MSCs) can significantly improve healing after treatment with quercetin [156]. Rosuvastatin calcium-loaded scaffolds were prepared and combined with MSCs and implanted into mouse wounds, and it was found that the mesenchymal stem cell–drug scaffolds showed complete skin healing after 30 days [157].
In one study, a 3D radial and vertically aligned nanofiber scaffold was developed that perfectly matched the size, depth, and shape of diabetic wounds to transplant bone marrow mesenchymal stem cells (BMSCs) to promote granulation tissue formation, angiogenesis, and collagen deposition, and tilt the immune response in the pro-regeneration direction [158]. The cord platelet gel (CBPG) developed by the Cord Blood Unit (CBU) has been successfully applied to induce wound closure and tissue regeneration [159].
6. Summary and Outlook
In recent years, due to the development of tissue engineering technology, the use of mesenchymal stem cells as a new method for wound healing has garnered extensive research, for uses such as nanotherapy, stem cell therapy, and 3D bioprinting, and due to the low amount of ethical controversy associated with it, there are many tissue sources for mesenchymal stem cells. One of the benefits of mesenchymal stem cells is that bone marrow, adipose tissue, umbilical cord blood, Wharton’s jelly stem cells and amniotic fluid can be easily collected.
In general, the mechanisms through which mesenchymal stem cells promote wound healing have been fully discussed at the molecular level and cellular level. Preclinical and clinical studies on the application of mesenchymal stem cells in the treatment of burns and chronic wounds have fully demonstrated their potential in the field of wound healing. Moreover, mesenchymal stem cells, combined with other tissue engineering techniques, can play a greater role in skin tissue repair. For example, through 3D-printing technology, medical-grade materials such as polycaprolactone (mPCL) and polyethylene terephthalate (PET) can be used to effectively create scaffolds, transporting stem cells to the desired repair site. The scaffold made by combining chitosan and collagen can overcome the limitations of traditional collagen scaffolds and even achieve complete wound healing. In addition, hydrogels can be used as the main raw material for delivery carriers because their rheological properties are similar to those of skin. When combined with emerging materials, mesenchymal stem cells have also shown good performance in promoting wound healing in many studies.
However, many relevant studies are still in the pre-clinical stage, and further exploration is still needed before they are truly transformed into clinical wound healing treatments. However, mesenchymal stem cells have demonstrated their ability, and we have sufficient confidence that mesenchymal stem cells will become the backbone of wound healing in the future.
Author Contributions
All authors made substantial contributions to this review. Z.Z., H.Y. and Z.Y. conceived and designed the review., S.W., S.S. and W.F. retrieved and reviewed the papers and wrote the manuscript. Z.Z., H.Y. and Z.Y. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82202884), Training Fund for Open Projects at the Clinical Institutes and Departments of Capital Medical University (No. CCMU2022ZKYXY008), National Key Technologies R&D Program (No. 2015BAI13B09), National Key Technologies R&D Program of China (No. 2017YFC0110904), and Clinical Center for Colorectal Cancer, Capital Medical University (No. 1192070313).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sheng, G. The developmental basis of mesenchymal stem/stromal cells (MSCs). BMC Dev. Biol. 2015, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V.J.; Melguizo-Rodriguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shi, Y. Mesenchymal stem/stromal cells (MSCs): Origin, immune regulation, and clinical applications. Cell. Mol. Immunol. 2023, 20, 555–557. [Google Scholar] [CrossRef]
- Liang, L.; Kaufmann, A.M. The Significance of Cancer Stem Cells and Epithelial-Mesenchymal Transition in Metastasis and Anti-Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 2555. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Lee, A.J.; Barabino, G.A. Coculture-driven mesenchymal stem cell-differentiated articular chondrocyte-like cells support neocartilage development. Stem Cells Transl. Med. 2012, 1, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef]
- Li, B.; Ouchi, T.; Cao, Y.; Zhao, Z.; Men, Y. Dental-Derived Mesenchymal Stem Cells: State of the Art. Front. Cell Dev. Biol. 2021, 9, 654559. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Li, G.; Yu, J.; Fang, F.; Qiu, W. Dental-derived mesenchymal stem cell sheets: A prospective tissue engineering for regenerative medicine. Stem Cell Res. Ther. 2022, 13, 38. [Google Scholar] [CrossRef]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell. Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef]
- Tang, H.; Luo, H.; Zhang, Z.; Yang, D. Mesenchymal Stem Cell-Derived Apoptotic Bodies: Biological Functions and Therapeutic Potential. Cells 2022, 11, 3879. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Osteogenesis imperfecta, rehabilitation medicine, fundamental research and mesenchymal stem cells. Connect. Tissue Res. 1995, 31, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, J.; Li, J. Isolation, culture, and delivery considerations for the use of mesenchymal stem cells in potential therapies for acute liver failure. Front. Immunol. 2023, 14, 1243220. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Chen, H.; Wen, X.; Liu, S.; Sun, T.; Song, H.; Wang, F.; Xu, J.; Zhang, Y.; Zhao, Y.; Yu, J.; et al. Dissecting Heterogeneity Reveals a Unique BAMBI(high) MFGE8(high) Subpopulation of Human UC-MSCs. Adv. Sci. 2022, 10, e2202510. [Google Scholar] [CrossRef]
- Wei, P.; Jia, M.; Kong, X.; Lyu, W.; Feng, H.; Sun, X.; Li, J.; Yang, J.J. Human umbilical cord-derived mesenchymal stem cells ameliorate perioperative neurocognitive disorder by inhibiting inflammatory responses and activating BDNF/TrkB/CREB signaling pathway in aged mice. Stem Cell Res. Ther. 2023, 14, 263. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, B.; Zhai, J.; Wang, A.; Cao, N.; Liao, T.; Su, R.; He, L.; Li, Y.; Pei, X.; et al. Clinical-grade human umbilical cord-derived mesenchymal stem cells improved skeletal muscle dysfunction in age-associated sarcopenia mice. Cell Death Dis. 2023, 14, 321. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, D.; Jing, L.; Wen, X.; Xia, N.; Ma, W.; Zhang, X.; Jin, Z.; Shen, W.; Yao, G.; et al. Allogenic Umbilical Cord-Derived Mesenchymal Stromal Cells Sustain Long-Term Therapeutic Efficacy Compared With Low-Dose Interleukin-2 in Systemic Lupus Erythematosus. Stem Cells Transl. Med. 2023, 12, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Wu, Y.; Song, G.; Azizi, R.; Zamani, A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: A comprehensive review. Stem Cell Res. Ther. 2022, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Wood, F.M.; Middelkoop, E.; Bayat, A.; Teot, L.; Ogawa, R.; Gauglitz, G.G. Scars. Nat. Rev. Dis. Primers 2023, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Baptista-Hon, D.T.; Wang, Z.; Li, G.; Herrler, T.; Dai, C.; Liu, K.; Yu, B.; Chen, X.; Yang, M.; et al. Bioengineered human tissue regeneration and repair using endogenous stem cells. Cell Rep. Med. 2023, 4, 101156. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Gou, M.; Da, L.C.; Zhang, W.Q.; Xie, H.Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part. B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- Deng, D.; Li, X.; Zhang, J.J.; Yin, Y.; Tian, Y.; Gan, D.; Wu, R.; Wang, J.; Tian, B.M.; Chen, F.M.; et al. Biotin-Avidin System-Based Delivery Enhances the Therapeutic Performance of MSC-Derived Exosomes. ACS Nano 2023, 17, 8530–8550. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, L.; Zou, H.; He, Y.; Pan, Y.; Ye, L.; Huang, Y.; Fan, W.; Zhang, J.; Ma, Y.; et al. Optogenetic engineered umbilical cord MSC-derived exosomes for remodeling of the immune microenvironment in diabetic wounds and the promotion of tissue repair. J. Nanobiotechnology 2023, 21, 176. [Google Scholar] [CrossRef]
- Stenqvist, A.C.; Nagaeva, O.; Baranov, V.; Mincheva-Nilsson, L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J. Immunol. 2013, 191, 5515–5523. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Walimbe, T.; Chen, H.; Gao, K.; Kumar, P.; Wei, Y.; Hao, D.; Liu, R.; Farmer, D.L.; Lam, K.S.; et al. Bioactive extracellular matrix scaffolds engineered with proangiogenic proteoglycan mimetics and loaded with endothelial progenitor cells promote neovascularization and diabetic wound healing. Bioact. Mater. 2022, 10, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Mokarizadeh, A.; Delirezh, N.; Morshedi, A.; Mosayebi, G.; Farshid, A.A.; Mardani, K. Microvesicles derived from mesenchymal stem cells: Potent organelles for induction of tolerogenic signaling. Immunol. Lett. 2012, 147, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Rebling, J.; Ben-Yehuda Greenwald, M.; Wietecha, M.; Werner, S.; Razansky, D. Long-Term Imaging of Wound Angiogenesis with Large Scale Optoacoustic Microscopy. Adv. Sci. 2021, 8, 2004226. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Azari, A.A.; Safapour, S. Therapeutic approaches for corneal neovascularization. Eye Vis. 2017, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Solarte David, V.A.; Güiza-Argüello, V.R.; Arango-Rodríguez, M.L.; Sossa, C.L.; Becerra-Bayona, S.M. Decellularized Tissues for Wound Healing: Towards Closing the Gap Between Scaffold Design and Effective Extracellular Matrix Remodeling. Front. Bioeng. Biotechnol. 2022, 10, 821852. [Google Scholar] [CrossRef]
- Jere, S.W.; Abrahamse, H.; Houreld, N.N. Interaction of the AKT and β-catenin signalling pathways and the influence of photobiomodulation on cellular signalling proteins in diabetic wound healing. J. Biomed. Sci. 2023, 30, 81. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Zhang, W.; Ling, Y.; Sun, Y.; Xiao, F.; Wang, L. Extracellular Vesicles Derived from Mesenchymal Stem Cells Promote Wound Healing and Skin Regeneration by Modulating Multiple Cellular Changes: A Brief Review. Genes 2023, 14, 1516. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Li, T.; Shen, K.; Wang, K.J.; Tian, C.; Hu, D. Adipose Mesenchymal Stem Cell Derived Exosomes Promote Keratinocytes and Fibroblasts Embedded in Collagen/Platelet-Rich Plasma Scaffold and Accelerate Wound Healing. Adv. Mater. 2023, 35, e2303642. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kuri, P.; Aubert, Y.; Brewster, M.; Li, N.; Farrelly, O.; Rice, G.; Bae, H.; Prouty, S.; Dentchev, T.; et al. Lgr6 marks epidermal stem cells with a nerve-dependent role in wound re-epithelialization. Cell Stem Cell 2021, 28, 1582–1596.e6. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, J.; Fan, Y.; Yang, L. Mechanisms of human umbilical cord mesenchymal stem cells-derived exosomal lncRNA GAS5 in alleviating EMT of HPMCs via Wnt/beta-catenin signaling pathway. Aging 2023, 15, 4144–4158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, M.; Liao, Y.; Chen, H.; Su, D.; Tao, Y.; Li, J.; Luo, K.; Wu, L.; Zhang, X.; et al. Human umbilical cord mesenchymal stem cell-derived exosomes promote murine skin wound healing by neutrophil and macrophage modulations revealed by single-cell RNA sequencing. Front. Immunol. 2023, 14, 1142088. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Pang, Y.; Zhang, C.; Liu, L.; Bi, Y. Exosomes derived from human umbilical cord mesenchymal stem cells inhibit vein graft intimal hyperplasia and accelerate reendothelialization by enhancing endothelial function. Stem Cell Res. Ther. 2020, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Yuan, R.; Dai, X.; Li, Y.; Li, C.; Liu, L. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-beta2/Smad3 signaling. Mol. Med. Rep. 2021, 24, 758. [Google Scholar] [CrossRef]
- Lee, C.C.; Tsai, C.H.; Chen, C.H.; Yeh, Y.C.; Chung, W.H.; Chen, C.B. An updated review of the immunological mechanisms of keloid scars. Front. Immunol. 2023, 14, 1117630. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.F.; Wang, Z.C.; Lou, D.; Fang, Q.Q.; Hu, Y.Y.; Zhao, W.Y.; Zhang, L.Y.; Wu, L.H.; Tan, W.Q. Current potential therapeutic strategies targeting the TGF-beta/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020, 129, 110287. [Google Scholar] [CrossRef] [PubMed]
- Li, S.N.; Wu, J.F. TGF-beta/SMAD signaling regulation of mesenchymal stem cells in adipocyte commitment. Stem Cell Res. Ther. 2020, 11, 41. [Google Scholar] [CrossRef]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.Y.; Chen, M.J.; Wu, L.F.; Shu, G.F.; Fang, S.J.; Li, Z.Y.; Chu, X.R.; Li, X.K.; Wang, Z.G.; Ji, J.S. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: Roles, opportunities and challenges. Mil. Med. Res. 2023, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, F.; Feng, J.; Wu, B.; Li, H.; Xiao, S.; Lu, F.; Wei, Z.; Deng, C. Long-term follow-up and exploration of the mechanism of stromal vascular fraction gel in chronic wounds. Stem Cell Res. Ther. 2023, 14, 163. [Google Scholar] [CrossRef]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Hou, Y.; Chai, J.; Duan, H.; Chu, W.; Zhang, H.; Hu, Q.; Du, J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS ONE 2014, 9, e88348. [Google Scholar] [CrossRef]
- Arno, A.I.; Amini-Nik, S.; Blit, P.H.; Al-Shehab, M.; Belo, C.; Herer, E.; Tien, C.H.; Jeschke, M.G. Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res. Ther. 2014, 5, 28. [Google Scholar] [CrossRef]
- Bai, H.; Kyu-Cheol, N.; Wang, Z.; Cui, Y.; Liu, H.; Liu, H.; Feng, Y.; Zhao, Y.; Lin, Q.; Li, Z. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J. Tissue Eng. 2020, 11, 2041731420947242. [Google Scholar] [CrossRef]
- Luo, G.; Cheng, W.; He, W.; Wang, X.; Tan, J.; Fitzgerald, M.; Li, X.; Wu, J. Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair Regen. 2010, 18, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Vander Beken, S.; Wlaschek, M.; et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J. Investig. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yong, X.; Li, C.; Lü, M.; Liu, D.; Chen, L.; Hu, J.; Teng, M.; Zhang, D.; Fan, Y.; et al. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J. Surg. Res. 2013, 183, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Gao, H.; Li, H.; Ge, S.; Zhang, F.; Wang, L.; Shi, H.; Han, A. Self-Assembly of a Multifunction DNA Tetrahedron for Effective Delivery of Aptamer PL1 and Pcsk9 siRNA Potentiate Immune Checkpoint Therapy for Colorectal Cancer. ACS Appl. Mater. Interfaces 2022, 14, 31634–31644. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lam, P.K.; Siu, W.S.; Tong, C.S.W.; Lo, K.K.Y.; Koon, C.M.; Wu, X.X.; Li, X.; Cheng, W.; Shum, W.T.; et al. Adipose Tissue-Derived Mesenchymal Stem Cells (ADMSCs) and ADMSC-Derived Secretome Expedited Wound Healing in a Rodent Model—A Preliminary Study. Clin. Cosmet. Investig. Dermatol. 2021, 14, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Martinello, T.; Gomiero, C.; Perazzi, A.; Iacopetti, I.; Gemignani, F.; DeBenedictis, G.M.; Ferro, S.; Zuin, M.; Martines, E.; Brun, P.; et al. Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin. BMC Vet. Res. 2018, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, K.; Liu, C.; Qu, X. Harnessing strategies for enhancing diabetic wound healing from the perspective of spatial inflammation patterns. Bioact. Mater. 2023, 28, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Xie, X.; Hu, L.; Zhou, W.; Mi, B.; Xiong, Y.; Xue, H.; Zhang, K.; Zhang, Y.; Hu, Y.; et al. Hypoxia endothelial cells-derived exosomes facilitate diabetic wound healing through improving endothelial cell function and promoting M2 macrophages polarization. Bioact. Mater. 2024, 33, 157–173. [Google Scholar] [CrossRef]
- Luo, Z.; Bian, Y.; Zheng, R.; Song, Y.; Shi, L.; Xu, H.; Wang, H.; Li, X.; Tao, Z.; Wang, A.; et al. Combination of chemically modified SDF-1alpha mRNA and small skin improves wound healing in diabetic rats with full-thickness skin defects. Cell Prolif. 2022, 55, e13318. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.Q.; Li, Z.B.; Wu, Y.L.; Li, D.W. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef]
- Tang, J.; Guan, H.; Dong, W.; Liu, Y.; Dong, J.; Huang, L.; Zhou, J.; Lu, S. Application of Compound Polymyxin B Ointment in the Treatment of Chronic Refractory Wounds. Int. J. Low. Extrem. Wounds 2022, 21, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, M.; Liang, F.; Li, J. Recent strategies for enhancing the therapeutic efficacy of stem cells in wound healing. Stem Cell Res. Ther. 2021, 12, 588. [Google Scholar] [CrossRef] [PubMed]
- Watt, S.M.; Pleat, J.M. Stem cells, niches and scaffolds: Applications to burns and wound care. Adv. Drug Deliv. Rev. 2018, 123, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Tanios, E.; Ahmed, T.M.; Shafik, E.A.; Sherif, M.F.; Sayed, D.; Gaber, N.; Hassan, Y. Efficacy of adipose-derived stromal vascular fraction cells in the management of chronic ulcers: A randomized clinical trial. Regen. Med. 2021, 16, 975–988. [Google Scholar] [CrossRef]
- Moon, K.C.; Suh, H.S.; Kim, K.B.; Han, S.K.; Young, K.W.; Lee, J.W.; Kim, M.H. Potential of Allogeneic Adipose-Derived Stem Cell-Hydrogel Complex for Treating Diabetic Foot Ulcers. Diabetes 2019, 68, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.H.; Haitsma, J.J.; Dos Santos, C.C.; Deng, Y.; Lai, P.F.; Slutsky, A.S.; Liles, W.C.; Stewart, D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010, 182, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, X.; Lv, Y.; Zheng, C.; Dong, Y.; Dou, G.; Zhu, B.; Liu, A.; Wang, W.; Zhou, J.; et al. Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res. Ther. 2020, 11, 507. [Google Scholar] [CrossRef]
- Xia, X.; Yin, T.; Yan, J.; Yan, L.; Jin, C.; Lu, C.; Wang, T.; Zhu, X.; Zhi, X.; Wang, J.; et al. Mesenchymal Stem Cells Enhance Angiogenesis and Follicle Survival in Human Cryopreserved Ovarian Cortex Transplantation. Cell Transplant. 2015, 24, 1999–2010. [Google Scholar] [CrossRef]
- Liu, J.; Gao, J.; Liang, Z.; Gao, C.; Niu, Q.; Wu, F.; Zhang, L. Mesenchymal stem cells and their microenvironment. Stem Cell Res. Ther. 2022, 13, 429. [Google Scholar] [CrossRef]
- Burk, J.; Sassmann, A.; Kasper, C.; Nimptsch, A.; Schubert, S. Extracellular Matrix Synthesis and Remodeling by Mesenchymal Stromal Cells Is Context-Sensitive. Int. J. Mol. Sci. 2022, 23, 1758. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, B.; Yang, Y.; Jiang, Q.; Li, T.; Gong, J.; Tang, H.; Zhang, Q. Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res. Ther. 2023, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, X.; Lei, X.; Tan, J.; Xie, H. Mesenchymal stem cell-based therapy for burn wound healing. Burns Trauma 2021, 9, tkab002. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yang, S.; Lv, M.; Lv, J.; Sui, Y.; Guo, S. Protective roles of mesenchymal stem cells on skin photoaging: A narrative review. Tissue Cell 2022, 76, 101746. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Fan, Y.; You, G.Y.; Choi, T.H.; Kim, S. Improvement of photoaged skin wrinkles with cultured human fibroblasts and adipose-derived stem cells: A comparative study. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xuan, M.; Leung, V.Y.; Cheng, B. Stem cells and aberrant signaling of molecular systems in skin aging. Ageing Res. Rev. 2015, 19, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhang, S.; Jiang, B.; Cao, S.; Dong, Y.; Cao, L.; Guo, S. Adipose-derived stem cells regulate metabolic homeostasis and delay aging by promoting mitophagy. FASEB J. 2021, 35, e21709. [Google Scholar] [CrossRef]
- Oh, M.; Lee, J.; Kim, Y.J.; Rhee, W.J.; Park, J.H. Exosomes Derived from Human Induced Pluripotent Stem Cells Ameliorate the Aging of Skin Fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715. [Google Scholar] [CrossRef]
- Charles-de-Sa, L.; Gontijo-de-Amorim, N.F.; Rigotti, G.; Sbarbati, A.; Bernardi, P.; Benati, D.; Bizon Vieira Carias, R.; Maeda Takiya, C.; Borojevic, R. Photoaged Skin Therapy with Adipose-Derived Stem Cells. Plast. Reconstr. Surg. 2020, 145, 1037e–1049e. [Google Scholar] [CrossRef]
- Kim, W.S.; Park, B.S.; Park, S.H.; Kim, H.K.; Sung, J.H. Antiwrinkle effect of adipose-derived stem cell: Activation of dermal fibroblast by secretory factors. J. Dermatol. Sci. 2009, 53, 96–102. [Google Scholar] [CrossRef]
- Kim, Y.J.; Seo, D.H.; Lee, S.H.; Lee, S.H.; An, G.H.; Ahn, H.J.; Kwon, D.; Seo, K.W.; Kang, K.S. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem. Biophys. Rep. 2018, 16, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yoo, S.M.; Park, H.H.; Lim, H.J.; Kim, Y.L.; Lee, S.; Seo, K.W.; Kang, K.S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Feifei, W.; Wenrou, S.; Sining, K.; Siyu, Z.; Xiaolei, F.; Junxiang, L.; Congfen, H.; Xuhui, L. A novel functional peptide, named EQ-9 (ESETRILLQ), identified by virtual screening from regenerative cell secretome and its potential anti-aging and restoration effects in topical applications. Peptides 2023, 169, 171078. [Google Scholar] [CrossRef] [PubMed]
- Svolacchia, L.; Prisco, C.; Giuzio, F.; Svolacchia, F. Adipose Autologous Micrograft and Its Derived Mesenchymal Stem Cells in a Bio Cross-Linked Hyaluronic Acid Scaffold for Correction Deep Wrinkles, Facial Depressions, Scars, Face Dermis and Its Regenerations: A Pilot Study and Cases Report. Medicina 2022, 58, 1692. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Choi, J.S.; Kim, J.S.; Choi, Y.C.; Cho, Y.W. Injectable and Thermosensitive Soluble Extracellular Matrix and Methylcellulose Hydrogels for Stem Cell Delivery in Skin Wounds. Biomacromolecules 2016, 17, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, M.; Zhao, J.; Chai, R.; Kang, J. Looking into the Future: Toward Advanced 3D Biomaterials for Stem-Cell-Based Regenerative Medicine. Adv. Mater. 2018, 30, e1705388. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Cavalcanti, A.S.; Saidy, N.T.; Schneidereit, D.; Friedrich, O.; Ravichandran, A.; De-Juan-Pardo, E.M.; Hutmacher, D.W. Convergence of 3D printed biomimetic wound dressings and adult stem cell therapy. Biomaterials 2021, 268, 120558. [Google Scholar] [CrossRef]
- El Sadik, A.O.; El Ghamrawy, T.A.; Abd El-Galil, T.I. The Effect of Mesenchymal Stem Cells and Chitosan Gel on Full Thickness Skin Wound Healing in Albino Rats: Histological, Immunohistochemical and Fluorescent Study. PLoS ONE 2015, 10, e0137544. [Google Scholar] [CrossRef]
- Lin, W.; Qi, X.; Guo, W.; Liang, D.; Chen, H.; Lin, B.; Deng, X. A barrier against reactive oxygen species: Chitosan/acellular dermal matrix scaffold enhances stem cell retention and improves cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 383. [Google Scholar] [CrossRef]
- Shokrgozar, M.A.; Fattahi, M.; Bonakdar, S.; Ragerdi Kashani, I.; Majidi, M.; Haghighipour, N.; Bayati, V.; Sanati, H.; Saeedi, S.N. Healing potential of mesenchymal stem cells cultured on a collagen-based scaffold for skin regeneration. Iran. Biomed. J. 2012, 16, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, V.; Rahvarian, J.; Esmaeilzadeh, Z.; Mohammad-Pour, N.; Babaki, D.; Sadeghifar, F.; Esfehani, R.J.; Bidkhori, H.R.; Roshan, N.M.; Momeni-Moghaddam, M.; et al. Adipose-derived human mesenchymal stem cells seeded on denuded or stromal sides of the amniotic membrane improve angiogenesis and collagen remodeling and accelerate healing of the full-thickness wound. Acta Histochem. 2023, 125, 152027. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.; Duque, K.; Ramos-Gonzalez, G.; Diaz-Solano, D.; Wittig, O.; Zamora, M.; Gledhill, T.; Cardier, J.E. Wound healing by transplantation of mesenchymal stromal cells loaded on polyethylene terephthalate scaffold: Implications for skin injury treatment. Injury 2023, 54, 1071–1081. [Google Scholar] [CrossRef]
- Huang, S.; Lu, G.; Wu, Y.; Jirigala, E.; Xu, Y.; Ma, K.; Fu, X. Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J. Dermatol. Sci. 2012, 66, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Orgul, D.; Eroglu, H.; Tiryaki, M.; Pinarli, F.A.; Hekimoglu, S. In-vivo evaluation of tissue scaffolds containing simvastatin loaded nanostructured lipid carriers and mesenchymal stem cells in diabetic wound healing. J. Drug Deliv. Sci. Technol. 2021, 61, 102140. [Google Scholar] [CrossRef]
- Khadivar, P.; Alipour, M. Synergic effect of bone marrow derived mesenchymal stem cells and differentiated keratinocytes-like cells with a novel cellulose and collagen nanoscaffold on wound healing in rats. Biomed. Pharmacother. 2023, 160, 114404. [Google Scholar] [CrossRef]
- Wen, Z.; Chen, Y.; Liao, P.; Wang, F.; Zeng, W.; Liu, S.; Wu, H.; Wang, N.; Moroni, L.; Zhang, M.; et al. In Situ Precision Cell Electrospinning as an Efficient Stem Cell Delivery Approach for Cutaneous Wound Healing. Adv. Healthc. Mater. 2023, 12, e2300970. [Google Scholar] [CrossRef]
- Lizarazo-Fonseca, L.; Correa-Araujo, L.; Prieto-Abello, L.; Camacho-Rodriguez, B.; Silva-Cote, I. In vitro and in vivo evaluation of electrospun poly (epsilon-caprolactone)/collagen scaffolds and Wharton’s jelly mesenchymal stromal cells (hWJ-MSCs) constructs as potential alternative for skin tissue engineering. Regen. Ther. 2023, 24, 11–24. [Google Scholar] [CrossRef]
- Safari, B.; Aghazadeh, M.; Davaran, S.; Roshangar, L. Exosome-loaded hydrogels: A new cell-free therapeutic approach for skin regeneration. Eur. J. Pharm. Biopharm. 2022, 171, 50–59. [Google Scholar] [CrossRef]
- Lu, K.; Li, K.; Zhang, M.; Fang, Z.; Wu, P.; Feng, L.; Deng, K.; Yu, C.; Deng, Y.; Xiao, Y.; et al. Adipose-derived stem cells (ADSCs) and platelet-rich plasma (PRP) loaded gelatin/silk fibroin hydrogels for improving healing in a murine pressure ulcer model. Chem. Eng. J. 2021, 424, 130429. [Google Scholar] [CrossRef]
- Galocha-Leon, C.; Antich, C.; Voltes-Martinez, A.; Marchal, J.A.; Mallandrich, M.; Halbaut, L.; Rodriguez-Lagunas, M.J.; Souto, E.B.; Clares-Naveros, B.; Galvez-Martin, P. Development and characterization of a poloxamer hydrogel composed of human mesenchymal stromal cells (hMSCs) for reepithelization of skin injuries. Int. J. Pharm. 2023, 647, 123535. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, S.; Wang, J.; Lan, Y.; Feng, L.; Zhu, M.; Xiao, Y.; Cheng, B.; Xue, W.; Guo, R. Enhanced cutaneous wound healing by functional injectable thermo-sensitive chitosan-based hydrogel encapsulated human umbilical cord-mesenchymal stem cells. Int. J. Biol. Macromol. 2019, 137, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Qi, Y.; Liu, X.; Shi, Y.; Li, H.; Zhao, L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater. Adv. 2022, 133, 112613. [Google Scholar] [CrossRef]
- Zheng, X.; Ding, Z.; Cheng, W.; Lu, Q.; Kong, X.; Zhou, X.; Lu, G.; Kaplan, D.L. Microskin-Inspired Injectable MSC-Laden Hydrogels for Scarless Wound Healing with Hair Follicles. Adv. Healthc. Mater. 2020, 9, e2000041. [Google Scholar] [CrossRef] [PubMed]
- Khandan-Nasab, N.; Mahdipour, E.; Askarian, S.; Kalantari, M.R.; Ramezanian, N.; Kazemi Oskuee, R. Design and characterization of adipose-derived mesenchymal stem cell loaded alginate/pullulan/hyaluronic acid hydrogel scaffold for wound healing applications. Int. J. Biol. Macromol. 2023, 241, 124556. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Su, M.; Zhang, S.; Xiao, L.; Xiao, Y.; Zhang, M.; Bei, Y.; Li, M.; Zhang, F.; Yuan, Q.; et al. A mesenchymal stem cell-derived nanovesicle-biopotentiated bovine serum albumin-bridged gelatin hydrogel for enhanced diabetic wound therapy. Mater. Des. 2023, 230, 111960. [Google Scholar] [CrossRef]
- Pu, W.; Ren, J.; Chen, Y.; Shu, J.; Cui, L.; Han, Y.; Xi, J.; Pei, X.; Yue, W.; Han, Y. Injectable human decellularized adipose tissue hydrogel containing stem cells enhances wound healing in mouse. Colloids Surf. A-Physicochem. Eng. Asp. 2020, 604, 125268. [Google Scholar] [CrossRef]
- Ebrahimi, L.; Samadikuchaksaraei, A.; Joghataei, M.T.; Safa, M.; Abtahi Froushani, S.M.; Ghasemian, M.; Zolfaghari, S.; Mozafari, M.; Brouki Milan, P. Transplantation of decellularised human amniotic membranes seeded with mesenchymal stem cell-educated macrophages into animal models. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1637–1650. [Google Scholar] [CrossRef]
- Lashkari, M.; Rahmani, M.; Yousefpoor, Y.; Ahmadi-Zeidabadi, M.; Faridi-Majidi, R.; Ameri, Z.; Salary, M.; Azizi, S.; Shahabi, A.; Rahi, A.; et al. Cell-based wound dressing: Bilayered PCL/gelatin nanofibers-alginate/collagen hydrogel scaffold loaded with mesenchymal stem cells. Int. J. Biol. Macromol. 2023, 239, 124099. [Google Scholar] [CrossRef]
- Walker, N.G.; Mistry, A.R.; Smith, L.E.; Eves, P.C.; Tsaknakis, G.; Forster, S.; Watt, S.M.; Macneil, S. A chemically defined carrier for the delivery of human mesenchymal stem/stromal cells to skin wounds. Tissue Eng. Part C Methods 2012, 18, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Zhang, C.; Gan, B.; Liu, W.; Liang, J.; Fan, Z.; Wen, Y.; Yang, Y.; Peng, X.; Zhou, Y. Collagen-tussah silk fibroin hybrid scaffolds loaded with bone mesenchymal stem cells promote skin wound repair in rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110611. [Google Scholar] [CrossRef] [PubMed]
- Kirby, G.T.S.; Michelmore, A.; Smith, L.E.; Whittle, J.D.; Short, R.D. Cell sheets in cell therapies. Cytotherapy 2018, 20, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Lin, R.; Liang, S.; Qiu, X.; Hou, H. Smart surface-based cell sheet engineering for regenerative medicine. Trends Chem. 2023, 5, 88–101. [Google Scholar] [CrossRef]
- Sukho, P.; Cohen, A.; Hesselink, J.W.; Kirpensteijn, J.; Verseijden, F.; Bastiaansen-Jenniskens, Y.M. Adipose Tissue-Derived Stem Cell Sheet Application for Tissue Healing In Vivo: A Systematic Review. Tissue Eng. Part B Rev. 2018, 24, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Iwata, T.; Morikawa, S.; Yamato, M.; Okano, T.; Uchigata, Y. Allogeneic Transplantation of an Adipose-Derived Stem Cell Sheet Combined With Artificial Skin Accelerates Wound Healing in a Rat Wound Model of Type 2 Diabetes and Obesity. Diabetes 2015, 64, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.A.; Hee Hong, S.; Hee Lee, M.; Kwon, B.J.; Mi Seon, G.; Sung Kim, M.; Kim, D.; Chang Nam, K.; Park, J.C. Effective stacking and transplantation of stem cell sheets using exogenous ROS-producing film for accelerated wound healing. Acta Biomater. 2019, 95, 418–426. [Google Scholar] [CrossRef]
- Le-Buu Pham, T.; Nguyen, T.M.; Phu-Hai Nguyen, D.; Tran, H.N.; Thi-Thanh Nguyen, T.; Binh, N.T.; Nguyen, Q.D.; Bui, H.T. Stem cell sheet fabrication from human umbilical cord mesenchymal stem cell and Col-T scaffold. Stem Cell Res. 2022, 65, 102960. [Google Scholar] [CrossRef]
- Yu, J.; Wang, M.Y.; Tai, H.C.; Cheng, N.C. Cell sheet composed of adipose-derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomater. 2018, 77, 191–200. [Google Scholar] [CrossRef]
- Hamada, M.; Iwata, T.; Kato, Y.; Washio, K.; Morikawa, S.; Sakurai, H.; Yamato, M.; Okano, T.; Uchigata, Y. Xenogeneic transplantation of human adipose-derived stem cell sheets accelerate angiogenesis and the healing of skin wounds in a Zucker Diabetic Fatty rat model of obese diabetes. Regen. Ther. 2017, 6, 65–73. [Google Scholar] [CrossRef]
- Lin, Y.C.; Grahovac, T.; Oh, S.J.; Ieraci, M.; Rubin, J.P.; Marra, K.G. Evaluation of a multi-layer adipose-derived stem cell sheet in a full-thickness wound healing model. Acta Biomater. 2013, 9, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N.; Ohki, T.; Aoyama, S.; Yamaguchi, S.; Itabashi, M.; Egawa, H.; Yamamoto, M. Transplantation of hybrid adipose-derived stem cell sheet with autologous peritoneum: An in vivo feasibility study. Heliyon 2023, 9, e12992. [Google Scholar] [CrossRef]
- Kitala, D.; Klama-Baryła, A.; Kraut, M.; Łabuś, W.; Glik, J.; Kawecki, M.; Trzebicka, B.; Dworak, A.; Adamus-Włodarczyk, A.; Komasa, J.; et al. Amniotic Stem Cells Cultured on Thermoresponsive Polymers Allow Obtaining a Full Cell Sheet. Transplant. Proc. 2020, 52, 2198–2203. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Tada, K.; Nakada, M.; Matsuta, M.; Tsuchiya, H. Facilitatory effects of artificial nerve filled with adipose-derived stem cell sheets on peripheral nerve regeneration: An experimental study. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2021, 26, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.-j.; Kim, S.-j.; Choi, J.S.; Eom, M.R.; Shin, H.; Kwon, S.K. Adipose-derived mesenchymal stem cell spheroid sheet accelerates regeneration of ulcerated oral mucosa by enhancing inherent therapeutic properties. J. Ind. Eng. Chem. 2020, 91, 296–310. [Google Scholar] [CrossRef]
- Jiang, Z.; He, J.; Wang, X.; Zhu, D.; Li, N.; Ren, L.; Yang, G. Nanomaterial-based cell sheet technology for regenerative medicine and tissue engineering. Colloids Surf. B-Biointerfaces 2022, 217, 112661. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, J.; Leyh, B. Paracrine effects of stem cells in wound healing and cancer progression (Review). Int. J. Oncol. 2014, 44, 1789–1798. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012, 26, 1166–1173. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Song, X.; Zhu, J.; Zhu, Q. The Healing Effects of Conditioned Medium Derived from Mesenchymal Stem Cells on Radiation-Induced Skin Wounds in Rats. Cell Transplant. 2019, 28, 105–115. [Google Scholar] [CrossRef]
- Kusindarta, D.L.; Wihadmadyatami, H.; Fibrianto, Y.H.; Nugroho, W.S.; Susetya, H.; Musana, D.K.; Wijayanto, H.; Prihatna, S.A.; Wahyuni, A.E. Human umbilical mesenchymal stem cells conditioned medium promote primary wound healing regeneration. Vet. World 2016, 9, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ahn, H.J.; Lee, S.H.; Lee, M.H.; Kang, K.S. Effects of conditioned media from human umbilical cord blood-derived mesenchymal stem cells in the skin immune response. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 131, 110789. [Google Scholar] [CrossRef] [PubMed]
- Yano, F.; Takeda, T.; Kurokawa, T.; Tsubaki, T.; Chijimatsu, R.; Inoue, K.; Tsuji, S.; Tanaka, S.; Saito, T. Effects of conditioned medium obtained from human adipose-derived stem cells on skin inflammation. Regen. Ther. 2022, 20, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Chung, P.S.; Ahn, J.C.; Leproux, A. Human adipose-derived stem cell spheroid treated with photobiomodulation irradiation accelerates tissue regeneration in mouse model of skin flap ischemia. Lasers Med. Sci. 2017, 32, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Payushina, O.V.; Butorina, N.N.; Sheveleva, O.N.; Domaratskaya, E.I. Effect of Mesenchymal Stromal Cells and Conditioned Media on Healing of Skin Wound. Bull. Exp. Biol. Med. 2018, 165, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Wu, Y.X.; Wang, H.M.; Gao, C.H.; Xu, Y.Y.; Yan, Y. Bone marrow-derived mesenchymal stem cell-conditioned medium ameliorates diabetic foot ulcers in rats. Clinics 2023, 78, 100181. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Lv, R.; Yang, X.; Cheng, S.; Ma, T.; Xu, J. Hypoxic conditioned medium of placenta-derived mesenchymal stem cells protects against scar formation. Life Sci. 2016, 149, 51–57. [Google Scholar] [CrossRef]
- Ochiai, D.; Abe, Y.; Fukutake, M.; Sato, Y.; Ikenoue, S.; Kasuga, Y.; Masuda, H.; Tanaka, M. Cell sheets using human amniotic fluid stem cells reduce tissue fibrosis in murine full-thickness skin wounds. Tissue Cell 2021, 68, 101472. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yang, B.; Sun, Y.; Huo, R. Three-dimensional graphene foams loaded with bone marrow derived mesenchymal stem cells promote skin wound healing with reduced scarring. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 181–188. [Google Scholar] [CrossRef]
- Haubner, F.; Muschter, D.; Schuster, N.; Pohl, F.; Ahrens, N.; Prantl, L.; Gassner, H.G. Platelet-rich plasma stimulates dermal microvascular endothelial cells and adipose derived stem cells after external radiation. Clin. Hemorheol. Microcirc. 2015, 61, 279–290. [Google Scholar] [CrossRef]
- Qi, Y.; Dong, Z.; Chu, H.; Zhao, Q.; Wang, X.; Jiao, Y.; Gong, H.; Pan, Y.; Jiang, D. Denatured acellular dermal matrix seeded with bone marrow mesenchymal stem cells for wound healing in mice. Burn. J. Int. Soc. Burn. Inj. 2019, 45, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, J.; Słyszewska, M.; Kopcewicz, M.; Walendzik, K.; Machcińska, S.; Stałanowska, K.; Gawrońska-Kozak, B. Comparative studies on the effect of pig adipose-derived stem cells (pASCs) preconditioned with hypoxia or normoxia on skin wound healing in mice. Exp. Cell Res. 2022, 418, 113263. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, K.; Kweon, O.K.; Kim, W.H. Enhanced wound healing effect of canine adipose-derived mesenchymal stem cells with low-level laser therapy in athymic mice. J. Dermatol. Sci. 2012, 68, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kolakshyapati, P.; Li, X.; Chen, C.; Zhang, M.; Tan, W.; Ma, L.; Gao, C. Gene-activated matrix/bone marrow-derived mesenchymal stem cells constructs regenerate sweat glands-like structure in vivo. Sci. Rep. 2017, 7, 17630. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xun, J.; Wu, C.; Ji, C.; Ji, S.; Shu, F.; Wang, Y.; Chen, H.; Zheng, Y.; Xiao, S. Acceleration of burn wound healing by micronized amniotic membrane seeded with umbilical cord-derived mesenchymal stem cells. Mater. Today Bio 2023, 20, 100686. [Google Scholar] [CrossRef] [PubMed]
- Irfan, F.; Jameel, F.; Khan, I.; Aslam, R.; Faizi, S.; Salim, A. Role of quercetin and rutin in enhancing the therapeutic potential of mesenchymal stem cells for cold induced burn wound. Regen. Ther. 2022, 21, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.; Abdelkhalek, A.A.; Mahmoud, A.A.; Salah, S.; Ammar, M.M.; Ghorab, M.M. Mesenchymal stem cells associated with chitosan scaffolds loaded with rosuvastatin to improve wound healing. Eur. J. Pharm. Sci. 2019, 127, 185–198. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Su, Y.; John, J.V.; McCarthy, A.; Wong, S.L.; Xie, J. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater. 2020, 108, 153–167. [Google Scholar] [CrossRef]
- Mallis, P.; Alevrogianni, V.; Sarri, P.; Velentzas, A.D.; Stavropoulos-Giokas, C.; Michalopoulos, E. Effect of Cord Blood Platelet Gel on wound healing capacity of human Mesenchymal Stromal Cells. Transfus. Apher. Sci. 2020, 59, 102734. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).