Transporters, Ion Channels, and Junctional Proteins in Choroid Plexus Epithelial Cells
Abstract
1. Introduction
2. Expressed Proteins in CPEs and Stroma
2.1. Expressed Proteins in CPEs
2.2. Junctional Proteins Expressed between CPEs
2.3. Proteins Expressed in CP Stroma
3. Localization of Several Kinds of Transporters in CPEs
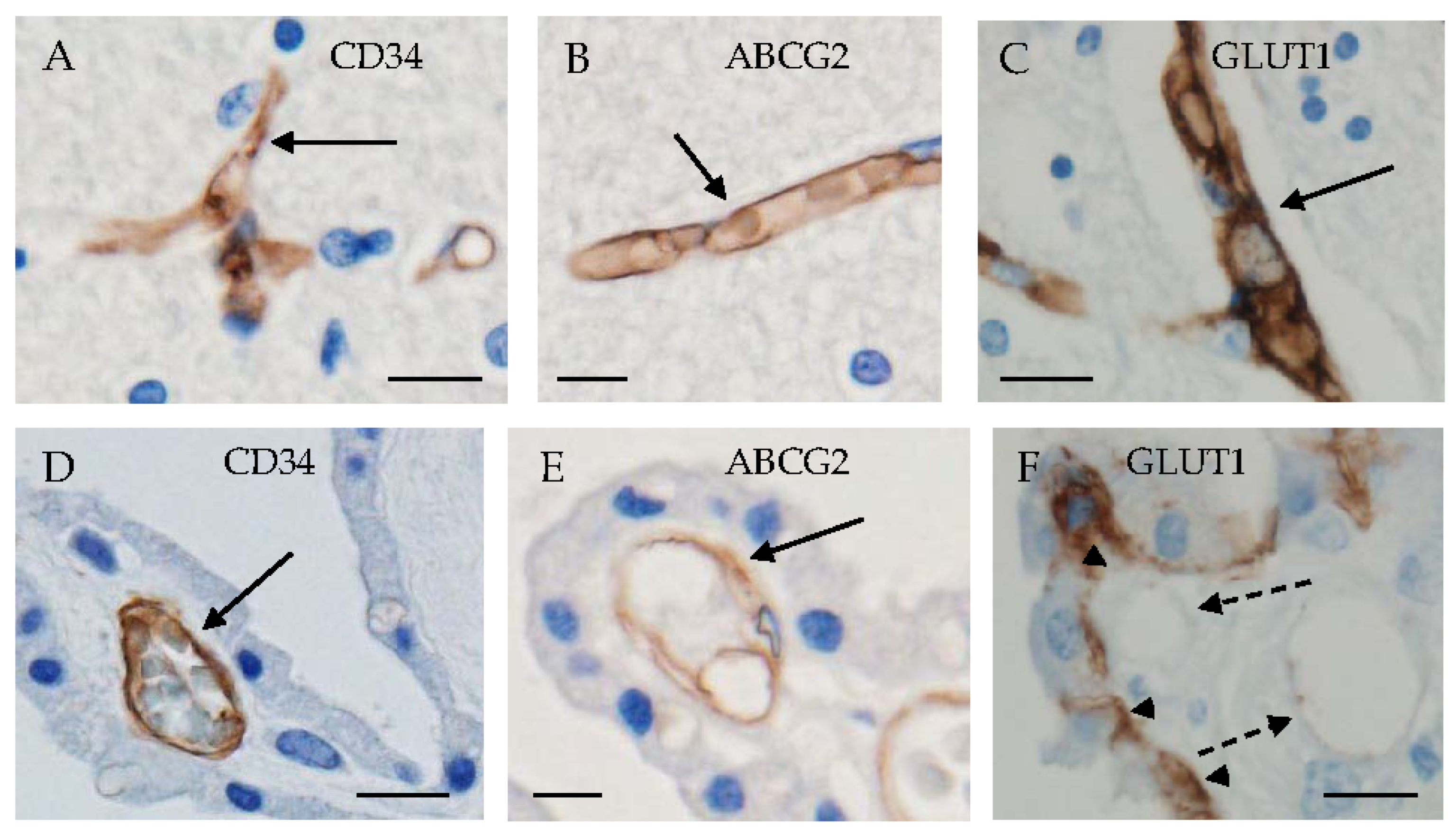
3.1. Glucose Transporters
3.2. Fructose Transporters
3.3. Urate Transporters
3.4. Lactate Transporters
3.5. Thyroid Hormone Transporters
3.6. Iron-Regulatory Proteins and Iron Transporters
3.7. Ions and Water Transporters
4. Localization of Transporters and Proteins in Junctions between Neighboring CPEs
4.1. Tight Junction
4.2. Adherens Junction
5. Alterations in CP Proteins with Aging and Brain Disorders
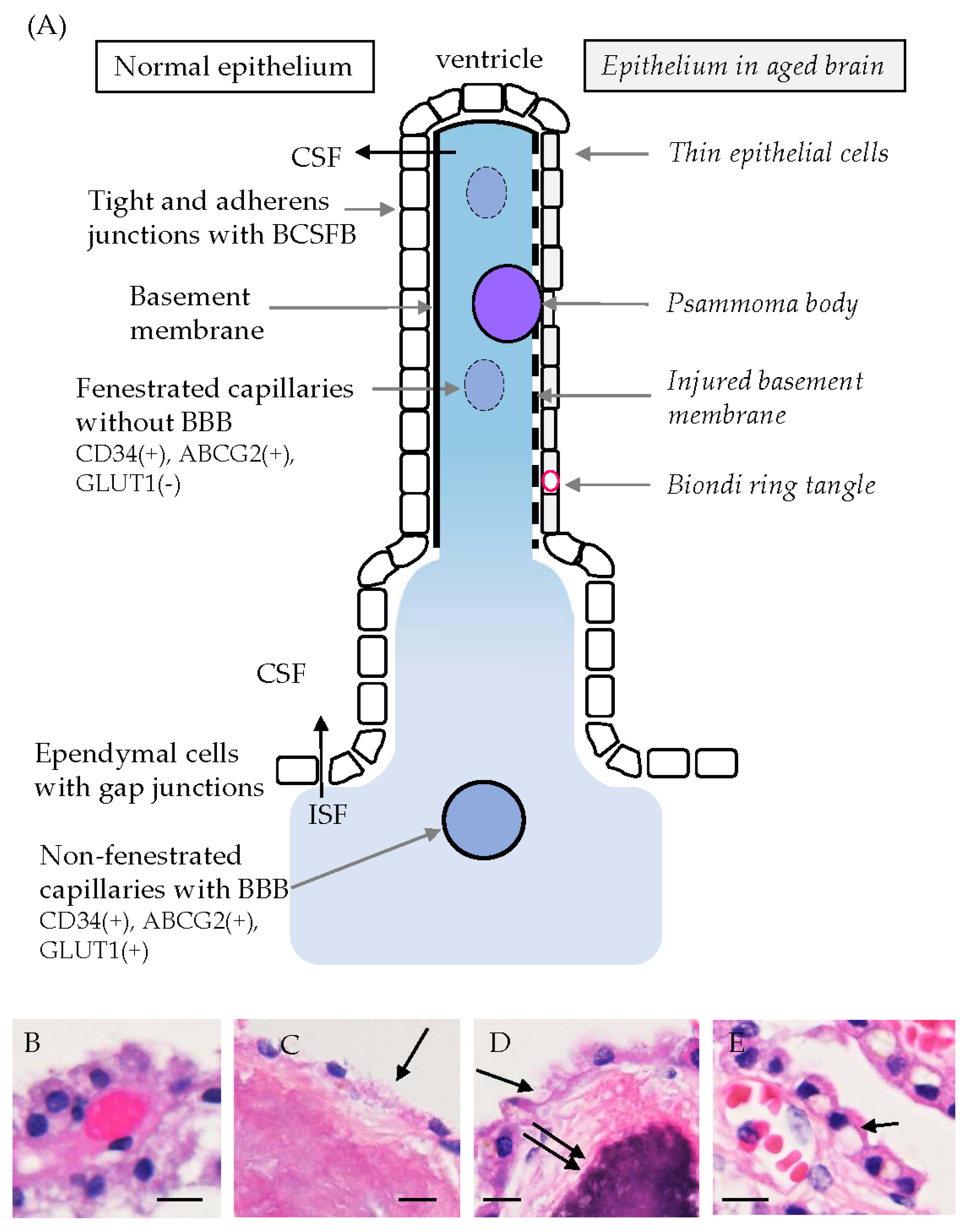
5.1. Age-Related Morphological Changes in CPEs
5.2. CP Changes in Brain Disorders
6. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Redzic, Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: Similarities and differences. Fluids Barriers CNS 2011, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Chiba, Y.; Murakami, R.; Matsumoto, K.; Fujihara, R.; Uemura, N.; Yanase, K.; Kamada, M. Disturbance of intracerebral fluid clearance and blood-brain barrier in vascular cognitive impairment. Int. J. Mol. Sci. 2019, 20, 2600. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Rodriguez-Cruz, V.; Felmlee, M.A. SLC and ABC transporters: Expression, localization, and species differences at the blood-brain and the blood-cerebrospinal fluid barriers. AAPS J. 2017, 19, 1317–1331. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, K.M.; Terasaki, T.; Urtti, A.; Montaser, A.B.; Uchida, Y. Pharmacoproteomics of brain barrier transporters and substrate design for the brain targeted drug delivery. Pharm. Res. 2022, 39, 1363–1392. [Google Scholar] [CrossRef]
- Damkier, H.; Praetorius, J. Structure of the mammalian choroid plexus. In Role of the Choroid Plexus in Health and Disease; Praetorius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 1–33. [Google Scholar]
- Gião, T.; Teixeira, T.; Almeida, M.R.; Cardoso, I. Choroid plexus in Alzheimer’s disease—The current state of knowledge. Biomedicines 2022, 10, 224. [Google Scholar] [CrossRef]
- Marques, F.; Sousa, J.C.; Brito, M.A.; Pahnke, J.; Santos, C.; Correia-Neves, M.; Palha, J.A. The choroid plexus in health and in disease: Dialogues into and out of the brain. Neurobiol. Dis. 2017, 107, 32–40. [Google Scholar] [CrossRef]
- Praetorius, J.; Damkier, H.H. Transport across the choroid plexus epithelium. Am. J. Physiol. Cell Physiol. 2017, 312, C673–C686. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef] [PubMed]
- van Cauwenberghe, C.; Gorlé, N.; Vandenbroucke, R.E. Role of the choroid plexus in aging. In Role of the Choroid Plexus in Health and Disease; Praetorius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 209–232. [Google Scholar]
- Orešković, D.; Radoš, M.; Klarica, M. Role of choroid plexus in cerebrospinal fluid hydrodynamics. Neuroscience 2017, 354, 69–87. [Google Scholar] [CrossRef]
- Xu, H.; Fame, R.M.; Sadegh, C.; Sutin, J.; Naranjo, C.; Syau, D.; Cui, J.; Shipley, F.B.; Vernon, A.; Gao, F.; et al. Choroid plexus NKCC1 mediates cerebrospinal fluid clearance during mouse early postnatal development. Nat. Commun. 2021, 12, 447. [Google Scholar] [CrossRef]
- Sadegh, C.; Xu, H.; Sutin, J.; Fatou, B.; Gupta, S.; Pragana, A.; Taylor, M.; Kalugin, P.N.; Zawadzki, M.E.; Alturkistani, O.; et al. Choroid plexus-targeted NKCC1 overexpression to treat post-hemorrhagic hydrocephalus. Neuron 2023, 111, 1591–1608. [Google Scholar] [CrossRef]
- Yamada, S.; Mase, M. Cerebrospinal fluid production and absorption and ventricular enlargement mechanisms in hydrocephalus. Neurol. Med.-Chir. 2023, 63, 141–151. [Google Scholar] [CrossRef]
- Xiang, J.; Hua, Y.; Xi, G.; Keep, R.F. Mechanisms of cerebrospinal fluid and brain interstitial fluid production. Neurobiol. Dis. 2023, 183, 106159. [Google Scholar] [CrossRef]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev. 2013, 93, 1847–1892. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Carare, R.O.; Hawkes, C.A.; Weller, R.O. Afferent and efferent immunological pathways of the brain. Anatomy, function and failure. Brain Behav. Immun. 2014, 36, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain—Implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, L.M.; Plá, V.; Giannetto, M.; Vinitsky, H.S.; Stæger, F.F.; Metcalfe, T.M.; Nguyen, R.; Benrais, A.; Nedergaard, M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 2020, 11, 4411. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Chiba, Y.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Kamada, M.; Nonaka, W.; Uemura, N.; Yanase, K.; Ueno, M. Metabolites and biomarker compounds of neurodegenerative diseases in cerebrospinal fluid. Metabolites 2022, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Chiba, Y.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Wakamatsu, K.; Takebayashi, G.; Uemura, N.; Yanase, K. Distribution of monocarboxylate transporters in brain and choroid plexus epithelium. Pharmaceutics 2023, 15, 2062. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Murakami, R.; Matsumoto, K.; Wakamatsu, K.; Nonaka, W.; Uemura, N.; Yanase, K.; Kamada, M.; Ueno, M. Glucose, fructose, and urate transporters in the choroid plexus epithelium. Int. J. Mol. Sci. 2020, 21, 7230. [Google Scholar] [CrossRef] [PubMed]
- Steinemann, A.; Galm, I.; Chip, S.; Nitsch, C.; Maly, I.P. Claudin-1, -2 and -3 are selectively expressed in the epithelia of the choroid plexus of the mouse from early development and into adulthood while claudin-5 is restricted to endothelial cells. Front. Neuroanat. 2016, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Christensen, I.B.; Mogensen, E.N.; Damkier, H.H.; Praetorius, J. Choroid plexus epithelial cells express the adhesion protein P-cadherin at cell-cell contacts and syntaxin-4 in the luminal membrane domain. Am. J. Physiol. Cell Physiol. 2018, 314, C519–C533. [Google Scholar] [CrossRef] [PubMed]
- Christensen, I.B.; Gyldenholm, T.; Damkier, H.H.; Praetorius, J. Polarization of membrane associated proteins in the choroid plexus epithelium from normal and slc4a10 knockout mice. Front. Physiol. 2013, 4, 344. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, G.; Chiba, Y.; Wakamatsu, K.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Uemura, N.; Yanase, K.; Shirakami, G.; Ogino, Y.; et al. E-cadherin is expressed in epithelial cells of the choroid plexus in human and mouse brains. Curr. Issues Mol. Biol. 2023, 45, 7813–7826. [Google Scholar] [CrossRef]
- Bihlmaier, R.; Deffner, F.; Mattheus, U.; Neckel, P.H.; Hirt, B.; Mack, A.F. Aquaporin-1 and aquaporin-4 expression in ependyma, choroid plexus and surrounding transition zones in the human brain. Biomolecules 2023, 13, 212. [Google Scholar] [CrossRef]
- Mack, A.F.; Bihlmaier, R.; Deffner, F. Shifting from ependyma to choroid plexus epithelium and the changing expressions of aquaporin-1 and aquaporin-4. J. Physiol. 2023; ahead of print. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Chiba, Y.; Murakami, R.; Matsumoto, K.; Miyai, Y.; Kawauchi, M.; Yanase, K.; Uemura, N.; Ueno, M. Immunohistochemical expression of osteopontin and collagens in choroid plexus of human brains. Neuropathology 2022, 42, 117–125. [Google Scholar] [CrossRef]
- Tomioka, N.H.; Tamura, Y.; Takada, T.; Shibata, S.; Suzuki, H.; Uchida, S.; Hosoyamada, M. Immunohistochemical and in situ hybridization study of urate transporters GLUT9/URATv1, ABCG2, and URAT1 in the murine brain. Fluids Barriers CNS 2016, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Elimination of substances from the brain parenchyma: Efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS 2018, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Sugiyama, Y.; Nishi, N.; Nonaka, W.; Murakami, R.; Ueno, M. Sodium/glucose cotransporter 2 is expressed in choroid plexus epithelial cells and ependymal cells in human and mouse brains. Neuropathology 2020, 40, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.A.; Carruthers, A.; Vannucci, S.J. Supply and demand in cerebral energy metabolism: The role of nutrient transporters. J. Cereb. Blood Flow Metab. 2007, 27, 1766–1791. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Hou, T.H.; Yu, H.P.; Li, A.; Liu, F.C. Cerebrospinal fluid electrolytes and acid-base in diabetic patients. Transl. Neurosci. 2021, 12, 448–455. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Dziegielewska, K.M.; Ek, C.J.; Habgood, M.D.; Bauer, H.; Bauer, H.C.; Lindsay, H.; Wakefield, M.J.; Strazielle, N.; Kratzer, I.; et al. Mechanisms that determine the internal environment of the developing brain: A transcriptomic, functional and ultrastructural approach. PLoS ONE 2013, 8, e65629. [Google Scholar] [CrossRef]
- Ueno, M.; Nishi, N.; Nakagawa, T.; Chiba, Y.; Tsukamoto, I.; Kusaka, T.; Miki, T.; Sakamoto, H.; Yamaguchi, F.; Tokuda, M. Immunoreactivity of glucose transporter 5 is located in epithelial cells of the choroid plexus and ependymal cells. Neuroscience 2014, 260, 149–157. [Google Scholar] [CrossRef]
- Uchida, Y.; Zhang, Z.; Tachikawa, M.; Terasaki, T. Quantitative targeted absolute proteomics of rat blood-cerebrospinal fluid barrier transporters: Comparison with a human specimen. J. Neurochem. 2015, 134, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Chiba, Y.; Tsuboi, K.; Matsumoto, K.; Kawauchi, M.; Fujihara, R.; Mashima, M.; Kanenishi, K.; Yamamoto, T.; Ueno, M. Immunoreactivity of glucose transporter 8 is localized in epithelial cells of the choroid plexus and in ependymal cells. Histochem. Cell Biol. 2016, 146, 231–236. [Google Scholar] [CrossRef]
- Uemura, N.; Murakami, R.; Chiba, Y.; Yanase, K.; Fujihara, R.; Mashima, M.; Matsumoto, K.; Kawauchi, M.; Shirakami, G.; Ueno, M. Immunoreactivity of urate transporters, GLUT9 and URAT1, is located in epithelial cells of the choroid plexus of human brains. Neurosci. Lett. 2017, 659, 99–103. [Google Scholar] [CrossRef]
- Toyoda, Y.; Takada, T.; Miyata, H.; Matsuo, H.; Kassai, H.; Nakao, K.; Nakatochi, M.; Kawamura, Y.; Shimizu, S.; Shinomiya, N.; et al. Identification of GLUT12/SLC2A12 as a urate transporter that regulates the blood urate level in hyperuricemia model mice. Proc. Natl. Acad. Sci. USA 2020, 117, 18175–18177. [Google Scholar] [CrossRef] [PubMed]
- Miyata, H.; Toyoda, Y.; Takada, T.; Hiragi, T.; Kubota, Y.; Shigesawa, R.; Koyoma, R.; Ikegaya, Y.; Suzuki, H. Identification of an exporter that regulates vitamin C supply from blood to the brain. iScience 2022, 25, 103642. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. The SLC16 gene family—Structure, role and regulation in health and disease. Mol. Asp. Med. 2013, 34, 337–349. [Google Scholar] [CrossRef]
- Iwanaga, T.; Kishimoto, A. Cellular distributions of monocarboxylate transporters: A review. Biomed. Res. 2015, 36, 279–301. [Google Scholar] [CrossRef]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate transporters (SLC16): Function, regulation, and role in health and disease. Pharmacol. Rev. 2020, 72, 466–485. [Google Scholar] [CrossRef]
- Murakami, R.; Chiba, Y.; Nishi, N.; Matsumoto, K.; Wakamatsu, K.; Yanase, K.; Uemura, N.; Nonaka, W.; Ueno, M. Immunoreactivity of receptor and transporters for lactate located in astrocytes and epithelial cells of choroid plexus of human brain. Neurosci. Lett. 2021, 741, 135479. [Google Scholar] [CrossRef]
- Philp, N.J.; Yoon, H.; Lombardi, L. Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. Am. J. Physiol. Cell Physiol. 2001, 280, C1319–C1326. [Google Scholar] [CrossRef]
- Friesema, E.C.H.; Ganguly, S.; Abdalla, A.; Manning Fox, J.E.; Halestrap, A.P.; Visser, T.J. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J. Biol. Chem. 2003, 278, 40128–40135. [Google Scholar] [CrossRef]
- Visser, W.E.; Friesema, E.C.; Jansen, J.; Visser, T.J. Thyroid hormone transport in and out of cells. Trends Endocrinol. Metab. 2008, 19, 50–56. [Google Scholar] [CrossRef]
- Roberts, L.M.; Woodford, K.; Zhou, M.; Black, D.S.; Haggerty, J.E.; Tate, E.H.; Grindstaff, K.K.; Mengesha, W.; Raman, C.; Zerangue, N. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 2008, 149, 6251–6261. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, H. Aceruloplasminemia. Neuropathology 2015, 35, 83–90. [Google Scholar] [CrossRef]
- Li, B.; Yu, W.; Verkhratsky, A. Trace metals and astrocytes physiology and pathophysiology. Cell Calcium 2024, 118, 102843. [Google Scholar] [CrossRef]
- Rishi, G.; Wallace, D.F.; Subramaniam, V.N. Hepcidin: Regulation of the master iron regulator. Biosci. Rep. 2015, 35, e00192. [Google Scholar] [CrossRef] [PubMed]
- Vela, D. Hepcidin, an emerging and important player in brain iron homeostasis. J. Transl. Med. 2018, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, C.N.; Xin, V.; Lu, Y.; Savage, T.; Anderson, G.J.; Jormakka, M. Large scale expression and purification of secreted mouse hephaestin. PLoS ONE 2017, 12, e0184366. [Google Scholar] [CrossRef] [PubMed]
- Hametner, S.; Wimmer, I.; Haider, L.; Pfeifenbring, S.; Brück, W.; Lassmann, H. Iron and neurodegeneration in the multiple sclerosis brain. Ann. Neurol. 2013, 74, 848–861. [Google Scholar] [CrossRef]
- Raha, A.A.; Vaishnav, R.A.; Friedland, R.P.; Bomford, A.; Raha-Chowdhury, R. The systemic iron-regulatory proteins hepcidin and ferroportin are reduced in the brain in Alzheimer’s disease. Acta Neuropathol. Commun. 2013, 1, 55. [Google Scholar] [CrossRef]
- Yanase, K.; Uemura, N.; Chiba, Y.; Murakami, R.; Fujihara, R.; Matsumoto, K.; Shirakami, G.; Araki, N.; Ueno, M. Immunoreactivities for hepcidin, ferroportin, and hephaestin in astrocytes and choroid plexus epithelium of human brains. Neuropathology 2020, 40, 75–83. [Google Scholar] [CrossRef]
- Steffensen, A.B.; Oernbo, E.K.; Stoica, A.; Gerkau, N.J.; Barbuskaite, D.; Tritsaris, K.; Rose, C.R.; MacAulay, N. Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat. Commun. 2018, 9, 2167. [Google Scholar] [CrossRef] [PubMed]
- Johanson, C.E.; Keep, R.F. Blending established and new perspectives on choroid plexus-CSF dynamics. In Role of the Choroid Plexus in Health and Disease; Praetorius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 35–81. [Google Scholar]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Lapajne, L.; Rudzitis, C.N.; Cullimore, B.; Ryskamp, D.; Lakk, M.; Redmon, S.N.; Yarishkin, O.; Krizaj, D. TRPV4: Cell type-specific activation, regulation and function in the vertebrate eye. Curr. Top Membr. 2022, 89, 189–219. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.; Simpson, S.; Halm, D.; Hochstetler, A.; Schwerk, C.; Schroten, H.; Blazer-Yost, B.L. Activation of TRPV4 stimulates transepithelial ion flux in a porcine choroid plexus cell line. Am. J. Physiol. Cell Physiol. 2018, 315, C357–C366. [Google Scholar] [CrossRef] [PubMed]
- Balda, M.S.; Flores-Maldonado, C.; Cereijido, M.; Matter, K. Multiple domains of occludin are involved in the regulation of paracellular permeability. J. Cell Biochem. 2000, 78, 85–96. [Google Scholar] [CrossRef]
- Saitou, M.; Furuse, M.; Sasaki, H.; Schulzke, J.D.; Fromm, M.; Takano, H.; Noda, T.; Tsukita, S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 2000, 11, 4131–4142. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Wolburg-Buchholz, K.; Liebner, S.; Engelhardt, B. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci. Lett. 2001, 307, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Günzel, D.; Krug, S.M.; Schulzke, J.-D.; Fromm, M.; Yu, A.S.L. Claudin-2-mediated cation and water transport share a common pore. Acta Physiol. 2017, 219, 521–536. [Google Scholar] [CrossRef]
- Wei, J.; Wu, L.; Yang, S.; Zhang, C.; Feng, L.; Wang, M.; Li, H.; Wang, F. E-cadherin to N-cadherin switching in the TGF-β1 mediated retinal pigment epithelial to mesenchymal transition. Exp. Eye Res. 2022, 220, 109085. [Google Scholar] [CrossRef]
- Rubtsova, S.N.; Zhitnyak, I.Y.; Gloushankova, N.A. Dual role of E-cadherin in cancer cells. Tissue Barriers 2022, 10, 2005420. [Google Scholar] [CrossRef]
- Matsuo, M.; Seo, K.; Taruno, A.; Mizoro, Y.; Yamaguchi, Y.; Doi, M.; Nakao, R.; Kori, H.; Abe, T.; Ohmori, H.; et al. A light-induced small G-protein gem limits the circadian clock phase-shift magnitude by inhibiting voltage-dependent calcium channels. Cell Rep. 2022, 39, 110844. [Google Scholar] [CrossRef]
- Scarpetta, V.; Bodaleo, F.; Salio, C.; Agarwal, A.; Sassoè-Pognetto, M.; Patrizi, A. Morphological and mitochondrial changes in murine choroid plexus epithelial cells during healthy aging. Fluids Barriers CNS 2023, 20, 19. [Google Scholar] [CrossRef]
- Wen, G.; Wisniewski, H.M.; Kascsak, R.J. Biondi ring tangles in the choroid plexus of Alzheimer’s disease and normal aging brains: A quantitative study. Brain Res. 1999, 832, 40–46. [Google Scholar] [CrossRef]
- Ben-Shoshan, S.D.; Lolansen, S.D.; Mathiesen, T.I.; MacAulay, N. CSF hypersecretion versus impaired CSF absorption in posthemorrhagic hydrocephalus: A systematic review. Acta Neurochir. 2023, 165, 3271–3287. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Z.; Chen, Y.; Liao, J.; Wang, Y.; Liu, J.; Lin, Z.; Xiao, G. Choroid plexus epithelium and its role in neurological diseases. Front. Mol. Neurosci. 2022, 15, 949231. [Google Scholar] [CrossRef]
- Peng, K.; Koduri, S.; Xia, F.; Gao, F.; Hua, Y.; Keep, R.F.; Xi, G. Impact of sex differences on thrombin-induced hydrocephalus and white matter injury: The role of neutrophils. Fluids Barriers CNS 2021, 18, 38. [Google Scholar] [CrossRef]
- Senay, O.; Seethaler, M.; Makris, N.; Yeterian, E.; Rushmore, J.; Cho, K.l.K.; Rizzoni, E.; Pasternak, O.; Szczepankiewicz, F.; Westin, C.F.; et al. A preliminary choroid plexus volumetric study in individuals with psychosis. Hum. Brain Mapp. 2023, 44, 2465–2478. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Huang, J.-C.; Zhang, P.; Fan, F.-M.; Chen, S.; Fan, H.-Z.; Cui, Y.-M.; Luo, X.-G.; Tan, S.-P.; Wang, Z.-R.; et al. Choroid plexus enlargement and allostatic load in schizophrenia. Schizophr. Bull. 2020, 46, 722–731. [Google Scholar] [CrossRef]
- Williams, M.R.; Macdonald, C.M.; Turkheimer, F.E. Histological examination of choroid plexus epithelia changes in schizophrenia. Brain Behav. Immun. 2023, 111, 292–297. [Google Scholar] [CrossRef]
- Ota, M.; Sato, N.; Nakaya, M.; Shigemoto, Y.; Kimura, Y.; Chiba, E.; Yokoi, Y.; Tsukamoto, T.; Matsuda, H. Relationship between the tau protein and choroid plexus volume in Alzheimer’s disease. Neuroreport 2023, 34, 546–550. [Google Scholar] [CrossRef]
- Choi, J.D.; Moon, Y.; Kim, H.J.; Yim, Y.; Lee, S.; Moon, W.J. Choroid plexus volume and permeability at brain MRI within the Alzheimer disease clinical spectrum. Radiology 2022, 304, 635–645. [Google Scholar] [CrossRef]
- Čarna, M.; Onyango, I.G.; Katina, S.; Holub, D.; Novotny, J.S.; Nezvedova, M.; Jha, D.; Nedelska, Z.; Lacovich, V.; Vyvere, T.V.; et al. Pathogenesis of Alzheimer’s disease: Involvement of the choroid plexus. Alzheimers Dement. 2023, 19, 3537–3554. [Google Scholar] [CrossRef]
- Leitner, D.F.; Kanshin, E.; Faustin, A.; Thierry, M.; Friedman, D.; Devore, S.; Ueberheide, B.; Devinsky, O.; Wisneiewski, T. Localization proteomic differences in the choroid plexus of Alzheimer’s disease and epilepsy patients. Front. Neurol. 2023, 14, 1221775. [Google Scholar] [CrossRef]
- Quintela, T.; Furtado, A.; Duarte, A.C.; Gonçalves, I.; Myung, J.; Santos, C.R.A. The role of circadian rhythm in choroid plexus functions. Prog. Neurobiol. 2021, 205, 102129. [Google Scholar] [CrossRef]
- Furtado, A.; Astaburuaga, R.; Costa, A.; Duarte, A.C.; Gonçalves, I.; Cipolla-Neto, J.; Lemos, M.C.; Carro, E.; Relógio, A.; Santos, C.R.A.; et al. The rhythmicity of clock genes is disrupted in the choroid plexus of the APP/PS1 mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2020, 77, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Jeong, H.-J.; Sunwoo, M.K.; Ahn, S.S.; Lee, S.-K.; Lee, P.H.; Kim, Y.J.; Sohn, Y.H.; Park, C.J.; Chung, S.J. Association between choroid plexus volume and cognition in Parkinson disease. Eur. J. Neurol. 2023, 30, 3114–3123. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Park, C.J.; Jeong, H.-J.; Sunwoo, M.K.; Ahn, S.S.; Lee, S.-K.; Lee, P.H.; Kim, Y.J.; Sohn, Y.H.; Chung, S.J. Association of choroid plexus volume with motor symptoms and dopaminergic degeneration in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2023, 94, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Assogna, M.; Premi, E.; Gazzina, S.; Benussi, A.; Ashton, N.J.; Zetterberg, H.; Blennow, K.; Gasparotti, R.; Padovani, A.; Tadayon, E.; et al. Association of choroid plexus volume with serum biomarkers, clinical features, and disease severity in patients with frontotemporal lobar degeneration spectrum. Neurology 2023, 101, e1218–e1230. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.; Wang, X.H.; Chiang, G.C.; Li, Y.; Zhou, L.; Xi, K.; Wickramasuriya, N.; Tanzi, E.; Spector, E.; Ozsahin, I.; et al. Choroid plexus calcification correlates with cortical microglial activation in humans: A multimodal PET, CT, MRI study. Am. J. Neuroradiol. 2023, 44, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Ricigliano, V.A.G.; Morena, E.; Columbi, A.; Tonietto, M.; Hamzaoui, M.; Poirion, E.; Bottlaender, M.; Gervais, P.; Louapre, C.; Bodini, B.; et al. Choroid plexus enlargement in inflammatory multiple sclerosis: 3.0-T MRI and translocator protein PET evaluation. Radiology 2021, 301, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, V.; Gonzalez-Escamilla, G.; Ciolac, D.; Albrecht, P.; Küry, P.; Gruchot, J.; Dietrich, M.; Hecker, C.; Müntefering, T.; Bock, S.; et al. Translational value of choroid plexus imaging for tracking neuroinflammation in mice and humans. Proc. Natl. Acad. Sci. USA 2021, 118, e2025000118. [Google Scholar] [CrossRef]
- Steffen, B.J.; Breier, G.; Butcher, E.C.; Schulz, M.; Engelhardt, B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am. J. Pathol. 1996, 148, 1819–1838. [Google Scholar] [PubMed]
- Kuhbandner, K.; Hammer, A.; Haase, S.; Terbrack, E.; Hoffmann, A.; Schippers, A.; Wagner, N.; Hussain, R.Z.; Miller-Little, W.A.; Koh, A.Y.; et al. MAdCAM-1-mediated intestinal lymphocyte homing is critical for the development of active experimental autoimmune encephalomyelitis. Front. Immunol. 2019, 10, 903. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.C.; Kern, F.; Losada, P.M.; Agam, M.R.; Maat, C.A.; Schmartz, G.P.; Fehlmann, T.; Stein, J.A.; Schaum, N.; Lee, D.P.; et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 2021, 595, 565–571. [Google Scholar] [CrossRef]
- Gorlé, N.; Blaecher, C.; Bauwens, E.; Vandendriessche, C.; Balusu, S.; Vandewalle, J.; Van Cauwenberghe, C.; Van Wonterghem, E.; Van Imschoot, G.; Liu, C.; et al. The choroid plexus epithelium as a novel player in the stomach-brain axis during Helicobacter infection. Brain Behav. Immun. 2018, 69, 35–47. [Google Scholar] [CrossRef]


| [1] Morphometry of human brains by imaging techniques | ||
| Psychosis | An increase in CP volume in early psychosis and a positive correlation between higher CP and higher lateral ventricle volumes in chronic psychosis. | [80] |
| Schizophrenia | CP enlargement and allostatic load. | [81] |
| Alzheimer’s disease | The CP volume is a good marker for the evaluation of tau deposition and neuroinflammation. | [83] |
| A larger CP volume is associated with the severity of cognitive impairment in the AD spectrum. | [84] | |
| Increased CP volumes in AD correlate with age and cognitive performance. | [85] | |
| Parkinson’s disease | The CP volume is associated with frontal or executive function, followed by the dementia conversion risk. | [89] |
| The CP volume has the potential to serve as a biomarker of motor disabilities. | [90] | |
| FTLD | The CP volume can assist in differentiating patients with FTLD from healthy controls and characterizing disease severity. | [91] |
| Multiple sclerosis | CP enlargement and inflammation | [93] |
| CP enlargement is closely linked to emerging functional impairment. | [94] | |
| [2] Pathological findings of CPEs and interstitium by histological or molecular biological techniques | ||
| Hydrocephalus | Overexpression of NKCC1 mitigates posthemorrhagic hydrocephalus. | [15] |
| Increased CSF secretion and impaired CSF absorption in the posthemorrhagic state. | [77] | |
| The relationship between abnormal CPEs and hydrocephalus or stroke. | [78] | |
| Periventricular white matter injury with neutrophil infiltration into CP and white matter in thrombin-induced hydrocephalus. | [79] | |
| Schizophrenia | Increased somal width of CPEs. | [82] |
| Alzheimer’s disease | Biondi ring tangles. | [76] |
| Increased amyloid-β deposition, reduced TJ formation, and decreased expression of LRP-1. | [78] | |
| Changes in signaling pathways associated with cell metabolism including activated fatty acid beta-oxidation and inhibited glycolysis. | [86] | |
| Multiple sclerosis | A large number of HLA-DR immunostained T lymphocytes in CPEs. | [78] |
| Aging, inflammation, or others | Decreases in total volume, height, and length of microvilli of CPEs in the elderly. | [9,12] |
| The basement membrane immunopositive for type IV collagen is destroyed and covering CPEs are thin or have disappeared. | [33] | |
| Age-related changes in flattening of CPEs, reduction in microvilli length, an increase in interrupted tight junctions, and a decrease in mitochondrial density with elongation of mitochondria of mice. | [75] | |
| Biondi ring tangles are present in aged brains. | [76] | |
| Roles of Na+/K+-ATPase, GLUT1, and transporters related to Aβ clearance in circadian regulation in CPEs. | [87] | |
| CP calcification may be a specific and relatively easily acquired biomarker of neuroinflammation and CP pathology in humans. | [92] | |
| MAdCAM-1 is upregulated in CPEs during experimental autoimmune encephalitis and may facilitate the entry of leukocyte subsets into CP. | [95] | |
| In human brains with COVID-19, barrier cells of the CP sense and relay inflammation into the brain with infiltration of increased number of CD68-positive macrophages into the stroma of CP. | [97] | |
| Helicobacter suis infection induces brain inflammation associated with cognitive decline, including CP inflammation, and the CP is a novel player in the stomach–brain axis. | [98] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, M.; Chiba, Y.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Wakamatsu, K.; Nakagawa, T.; Takebayashi, G.; Uemura, N.; Yanase, K.; et al. Transporters, Ion Channels, and Junctional Proteins in Choroid Plexus Epithelial Cells. Biomedicines 2024, 12, 708. https://doi.org/10.3390/biomedicines12040708
Ueno M, Chiba Y, Murakami R, Miyai Y, Matsumoto K, Wakamatsu K, Nakagawa T, Takebayashi G, Uemura N, Yanase K, et al. Transporters, Ion Channels, and Junctional Proteins in Choroid Plexus Epithelial Cells. Biomedicines. 2024; 12(4):708. https://doi.org/10.3390/biomedicines12040708
Chicago/Turabian StyleUeno, Masaki, Yoichi Chiba, Ryuta Murakami, Yumi Miyai, Koichi Matsumoto, Keiji Wakamatsu, Toshitaka Nakagawa, Genta Takebayashi, Naoya Uemura, Ken Yanase, and et al. 2024. "Transporters, Ion Channels, and Junctional Proteins in Choroid Plexus Epithelial Cells" Biomedicines 12, no. 4: 708. https://doi.org/10.3390/biomedicines12040708
APA StyleUeno, M., Chiba, Y., Murakami, R., Miyai, Y., Matsumoto, K., Wakamatsu, K., Nakagawa, T., Takebayashi, G., Uemura, N., Yanase, K., & Ogino, Y. (2024). Transporters, Ion Channels, and Junctional Proteins in Choroid Plexus Epithelial Cells. Biomedicines, 12(4), 708. https://doi.org/10.3390/biomedicines12040708






