Melatonin Decreases Alveolar Bone Loss in Rats with Experimental Periodontitis and Osteoporosis: A Morphometric and Histopathologic Study
Abstract
1. Introduction
2. Materials and Methods
- Non-ligated control (C, n = 6);
- Ligated periodontitis-induced (LP, n = 8);
- Osteoporosis-induced (O, n = 6);
- Osteoporosis- and periodontitis-induced (O+LP, n = 8);
- Osteoporosis- and periodontitis-induced through 30 mg/kg melatonin administration (ML30, n = 8);
- Osteoporosis- and periodontitis-induced through 50 mg/kg melatonin administration (ML50, n = 8).
2.1. Measurement of Alveolar Bone Loss
2.2. Histopathological Evaluation
2.3. RANKL Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. Morphometric Analyses
3.2. Histopathological Analyses
3.3. RANKL Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahingur, S.E.; Cohen, R.E. Analysis of host responses and risk for disease progression. Periodontol. 2000 2004, 34, 57–83. [Google Scholar] [CrossRef]
- Covani, U.; Giammarinaro, E.; Panetta, D.; Salvadori, P.A.; Cosola, S.; Marconcini, S. Alveolar Bone Remodeling with or without Collagen Filling of the Extraction Socket: A High-Resolution X-ray Tomography Animal Study. J. Clin. Med. 2022, 11, 2493. [Google Scholar] [CrossRef]
- Balci Yuce, H.; Karatas, O.; Aydemir Turkal, H.; Gorgun, E.P.; Ocakli, S.; Benli, I.; Cayli, S. The Effect of Melatonin on Bone Loss, Diabetic Control, and Apoptosis in Rats With Diabetes With Ligature-Induced Periodontitis. J. Periodontol. 2016, 87, e35–e43. [Google Scholar] [CrossRef]
- Cutando, A.; López-Valverde, A.; de Diego, R.G.; de Vicente, J.; Reiter, R.; Fernández, M.H.; Ferrera, M.J. Effect of topical application of melatonin to the gingiva on salivary osteoprotegerin, RANKL and melatonin levels in patients with diabetes and periodontal disease. Odontology 2013, 102, 290–296. [Google Scholar] [CrossRef]
- Kurita-Ochiai, T.; Jia, R.; Cai, Y.; Yamaguchi, Y.; Yamamoto, M. Periodontal Disease-Induced Atherosclerosis and Oxidative Stress. Antioxidants 2015, 4, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Varela-López, A.; Quiles, J.; Cordero, M.; Giampieri, F.; Bullón, P. Oxidative Stress and Dietary Fat Type in Relation to Periodontal Disease. Antioxidants 2015, 4, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lv, B.; Wang, Y. Protein Phosphatase 2A Mediates Oxidative Stress Induced Apoptosis in Osteoblasts. Mediat. Inflamm. 2015, 2015, 804260. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, D.V.; Ferreira, C.L.; Nunes, C.M.M.; Tricoly, T.d.S.; de Moura, N.B.; Santamaria, M.P.; De Marco, A.C.; Jardini, M.A.N. Effects of the Pulsed Electromagnetic Fields on Experimental Periodontitis and Estrogen Deficiency. Bioelectromagnetics 2022, 43, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Goyal, T.; Gupta, N. Osteoporosis and periodontitis in postmenopausal women: A systematic review. J. Midlife Health 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Penoni, D.C.; Fidalgo, T.K.S.; Torres, S.R.; Varela, V.; Masterson, D.; Leão, A.; Maia, L. Bone Density and Clinical Periodontal Attachment in Postmenopausal Women: A Systematic Review and Meta-Analysis. J. Dent. Res. 2017, 96, 261–269. [Google Scholar] [CrossRef]
- Bullon, P.; Newman, H.N.; Battino, M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: A shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol. 2000 2014, 64, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Notsu, M.; Yamaguchi, T. Secondary osteoporosis or secondary contributors to bone loss in fracture. Effects of oxidative stress on bone metabolism. Clin. Calcium 2013, 23, 1285–1292. [Google Scholar] [PubMed]
- Cervellati, C.; Bonaccorsi, G.; Cremonini, E.; Romani, A.; Fila, E.; Castaldini, M.C.; Ferrazzini, S.; Giganti, M.; Massari, L. Oxidative Stress and Bone Resorption Interplay as a Possible Trigger for Postmenopausal Osteoporosis. BioMed Res. Int. 2014, 2014, 569563. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-T. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Postmenopausal Osteoporosis. Med. Sci. Monit. 2015, 21, 2428–2432. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, S.; Iversen, H.K.; West, A.S. Melatonin profile in healthy, elderly subjects—A systematic literature review. Chronobiol. Int. 2022, 39, 476–492. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin as a chronobiotic/cytoprotective agent in bone. Doses involved. J. Pineal Res. 2024, 76, e12931. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Ramis, J.M.; Monjo, M. Anti-fibrotic and anti-inflammatory properties of melatonin on human gingival fibroblasts in vitro. Biochem. Pharmacol. 2013, 86, 1784–1790. [Google Scholar] [CrossRef]
- Pandıperumal, S.; Trakht, I.; Srınıvasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Histing, T.; Anton, C.; Scheuer, C.; Garcia, P.; Holstein, J.H.; Klein, M.; Matthys, R.; Pohlemann, T.; Menger, M.D. Melatonin Impairs Fracture Healing by Suppressing RANKL-Mediated Bone Remodeling. J. Surg. Res. 2012, 173, 83–90. [Google Scholar] [CrossRef]
- Koyama, H.; Nakade, O.; Takada, Y.; Kaku, T.; Lau, K.-H.W. Melatonin at Pharmacologic Doses Increases Bone Mass by Suppressing Resorption Through Down-Regulation of the RANKL-Mediated Osteoclast Formation and Activation. J. Bone Miner. Res. 2002, 17, 1219–1229. [Google Scholar] [CrossRef]
- Arabacı, T.; Kermen, E.; Özkanlar, S.; Köse, O.; Kara, A.; Kızıldağ, A.; Duman, B.; Ibişoğlu, E. Therapeutic Effects of Melatonin on Alveolar Bone Resorption After Experimental Periodontitis in Rats: A Biochemical and Immunohistochemical Study. J. Periodontol. 2015, 86, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Kose, O.; Arabaci, T.; Kara, A.; Yemenoglu, H.; Kermen, E.; Kizildag, A.; Gedikli, S.; Ozkanlar, S. Effects of Melatonin on Oxidative Stress Index and Alveolar Bone Loss in Diabetic Rats With Periodontitis. J. Periodontol. 2016, 87, e82–e90. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.K.; Sikjaer, T.; Heickendorff, L.; Mosekilde, L.; Rejnmark, L. Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: A randomized controlled trial. J. Pineal Res. 2015, 59, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kotlarczyk, M.P.; Lassila, H.C.; O’Neil, C.K.; D’amico, F.; Enderby, L.T.; Witt-Enderby, P.A.; Balk, J.L. Melatonin osteoporosis prevention study (MOPS): A randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J. Pineal Res. 2012, 52, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-L.; Meng, H.-Z.; Yang, R.-F.; Yang, M.-W.; Sun, G.-H.; Liu, J.-H.; Shi, P.-X.; Liu, F.; Yang, B. Melatonin suppresses autophagy in type 2 diabetic osteoporosis. Oncotarget 2016, 7, 52179–52194. [Google Scholar] [CrossRef] [PubMed]
- Ladizesky, M.G.; Cutrera, R.A.; Boggio, V.; Somoza, J.; Centrella, J.M.; Mautalen, C.; Cardinali, D.P. Effect of melatonin on bone metabolism in ovariectomized rats. Life Sci. 2001, 70, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, Z.; Kos-Kudla, B.; Marek, B.; Kajdaniuk, D.; Staszewicz, P.; Szapska, B.; Strzelczyk, J. The influence of pinealectomy and melatonin administration on the dynamic pattern of biochemical markers of bone metabolism in experimental osteoporosis in the rat. Neuro Endocrinol. Lett. 2002, 23 (Suppl. 1), 104–109. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12019362 (accessed on 10 March 2024).
- Ladizesky, M.G.; Boggio, V.; Albornoz, L.E.; Castrillón, P.O.; Mautalen, C.; Cardinali, D.P. Melatonin increases oestradiol-induced bone formation in ovariectomized rats. J. Pineal Res. 2003, 34, 143–151. [Google Scholar] [CrossRef]
- Ladizesky, M.G.; Boggio, V.; Cutrera, R.A.; Mondelo, N.; Mastaglia, S.; Somoza, J.; Cardinali, D.P. Melatonin effect on bone metabolism in rats treated with methylprednisolone. J. Pineal Res. 2006, 40, 297–304. [Google Scholar] [CrossRef]
- Oktem, G.; Uslu, S.; Vatansever, S.H.; Aktug, H.; Yurtseven, M.E.; Uysal, A. Evaluation of the relationship between inducible nitric oxide synthase (iNOS) activity and effects of melatonin in experimental osteoporosis in the rat. Surg. Radiol. Anat. 2006, 28, 157–162. [Google Scholar] [CrossRef]
- Munmun, F.; Mohiuddin, O.A.; Hoang, V.T.; Burow, M.E.; Bunnell, B.A.; Sola, V.M.; Carpentieri, A.R.; Witt-Enderby, P.A. The role of MEK1/2 and MEK5 in melatonin-mediated actions on osteoblastogenesis, osteoclastogenesis, bone microarchitecture, biomechanics, and bone formation. J. Pineal Res. 2022, 73, e12814. [Google Scholar] [CrossRef]
- Kara, A.; Akman, S.; Ozkanlar, S.; Tozoglu, U.; Kalkan, Y.; Canakci, C.F.; Tozoglu, S. Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic. Biol. Med. 2013, 55, 21–26. [Google Scholar] [CrossRef]
- Fabris, G.B.M.; Nocini, P.F.; Gelpi, F.; Lotti, J.; Favero, V.; Zanotti, G.; Jurlaro, A.; Rosskopf, I.; Lotti, T.; Barone, A.; et al. Treatment of skin defects with growth factors and biodegradable collagen carrier: Histological evaluation in animal model. J. Biol. Regul. Homeost. Agents 2017, 31 (Suppl. 2), 1–13. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28702959 (accessed on 10 March 2024).
- Blair, H.C.; Robinson, L.J.; Huang, C.L.-H.; Sun, L.; Friedman, P.A.; Schlesinger, P.H.; Zaidi, M. Calcium and bone disease. BioFactors 2011, 37, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Balci Yuce, H.; Toker, H.; Yildirim, A.; Tekin, M.B.; Gevrek, F.; Altunbas, N. The effect of luteolin in prevention of periodontal disease in Wistar rats. J. Periodontol. 2019, 90, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Karatas, O.; Balci Yuce, H.; Taskan, M.M.; Gevrek, F.; Yarkac, F.U.; Keskin, A.; Karatas, S.F.O.; Toker, H. The effect of vanillic acid on ligature-induced periodontal disease in Wistar rats. Arch. Oral Biol. 2019, 103, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, M.A.; Bral, M. Laboratory animal models in periodontology. J. Clin. Periodontol. 1999, 26, 335–340. [Google Scholar] [CrossRef]
- Karakan, N.C.; Akpınar, A.; Göze, F.; Poyraz, Ö. Investigating the Effects of Systemically Administered Strontium Ranelate on Alveolar Bone Loss Histomorphometrically and Histopathologically on Experimental Periodontitis in Rats. J. Periodontol. 2017, 88, e24–e31. [Google Scholar] [CrossRef]
- Toker, H.; Ozdemir, H.; Balci Yuce, H.; Goze, F. The effect of boron on alveolar bone loss in osteoporotic rats. J. Dent. Sci. 2016, 11, 331–337. [Google Scholar] [CrossRef]
- Karatas, O.; Balci Yuce, H.; Taskan, M.M.; Gevrek, F.; Alkan, C.; Kara, G.I.; Temiz, C. Cinnamic acid decreases periodontal inflammation and alveolar bone loss in experimental periodontitis. J. Periodontal Res. 2020, 55, 676–685. [Google Scholar] [CrossRef]
- Çalışır, M.; Akpınar, A.; Poyraz, Ö.; Göze, F.; Çınar, Z. Humic Acid, a Polyphenolic Substance, Decreases Alveolar Bone Loss in Experimental Periodontitis in Rats. J. Vet. Dent. 2019, 36, 257–265. [Google Scholar] [CrossRef]
- Toker, H.; Ozdemir, H.; Balcı, H.; Ozer, H. N-acetylcysteine decreases alveolar bone loss on experimental periodontitis in streptozotocin-induced diabetic rats. J. Periodontal Res. 2012, 47, 793–799. [Google Scholar] [CrossRef]
- Xu, P.; Guo, X.; Zhang, Y.; Li, Y.; Cao, J.; Xiong, Y. The effect of retinoic acid on induction of osteoporotic model rats and the possible mechanism. Sichuan Da Xue Xue Bao Yi Xue Ban 2005, 36, 229–232. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15807274 (accessed on 10 March 2024).
- French, D.L.; Muir, J.M.; Webber, C.E. The ovariectomized, mature rat model of postmenopausal osteoporosis: An assessment of the bone sparing effects of curcumin. Phytomedicine 2008, 15, 1069–1078. [Google Scholar] [CrossRef]
- Frost, H.M. The regional acceleratory phenomenon: A review. Henry Ford Hosp. Med. J. 1983, 31, 3–9. Available online: http://www.ncbi.nlm.nih.gov/pubmed/6345475 (accessed on 10 March 2024). [PubMed]
- Watts, N.B.; Bilezikian, J.P.; Camacho, P.M.; Greenspan, S.L.; Harris, S.T.; Hodgson, S.F.; Kleerekoper, M.; Luckey, M.M.; McClung, M.R.; Pollack, R.P.; et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the Prevention and Treatment of Postmenopausal Osteoporosis: 2001 Edition, with Selected Updates for 2003. Endocr Pract. 2003, 9, 544–564. [Google Scholar] [CrossRef]
- Simon Turner, A. Animal models of osteoporosis—necessity and limitations. Eur. Cells Mater. 2001, 1, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Allam, E.; Draz, A.; Hassan, A.; Neamat, A.; Galal, M.; Windsor, L.J. Expression of receptor activator of nuclear factor κB ligand in ligature-induced periodontitis in osteoporotic and non-osteoporotic rats. J. Periodontal Res. 2010, 45, 136–142. [Google Scholar] [CrossRef]
- Luo, K.; Ma, S.; Guo, J.; Huang, Y.; Yan, F.; Xiao, Y. Association between Postmenopausal Osteoporosis and Experimental Periodontitis. BioMed Res. Int. 2014, 2014, 316134. [Google Scholar] [CrossRef]
- Amadei, S.U.; de Souza, D.M.; Brandão, A.A.H.; da Rocha, R.F. Influence of different durations of estrogen deficiency on alveolar bone loss in rats. Braz. Oral Res. 2011, 25, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.B.; Reinhardt, R.A.; Nummikoski, P.V.; Patil, K.D. Longitudinal Alveolar Bone Loss in Postmenopausal Osteoporotic/Osteopenic Women. Osteoporos. Int. 1999, 10, 34–40. [Google Scholar] [CrossRef]
- Svedha, P.; Mahendra, J.; Theayarajar, R.; Namachivayam, A. Comparison of bone mineral density among pre- and post-menopausal women with and without chronic generalized periodontitis. J. Indian. Soc. Periodontol. 2017, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Ichimaru, R.; Tominari, T.; Yoshinouchi, S.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Numabe, Y.; Murphy, G.; Nagase, H.; Miyaura, C.; et al. Raloxifene reduces the risk of local alveolar bone destruction in a mouse model of periodontitis combined with systemic postmenopausal osteoporosis. Arch. Oral Biol. 2018, 85, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Weyant, R.J.; Pearlstein, M.E.; Churak, A.P.; Forrest, K.; Famili, P.; Cauley, J.A. The Association between Osteopenia and Periodontal Attachment Loss in Older Women. J. Periodontol. 1999, 70, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Anbinder, A.L.; Prado, M.d.A.; Spalding, M.; Balducci, I.; Carvalho, Y.R.; da Rocha, R.F. Estrogen deficiency and periodontal condition in rats: A radiographic and macroscopic study. Braz. Dent. J. 2006, 17, 201–207. [Google Scholar] [CrossRef]
- Santoro, N.; Epperson, C.N.; Mathews, S.B. Menopausal Symptoms and Their Management. Endocrinol. Metab. Clin. N. Am. 2015, 44, 497–515. [Google Scholar] [CrossRef]
- Toffol, E.; Kalleinen, N.; Haukka, J.; Vakkuri, O.; Partonen, T.; Polo-Kantola, P. Melatonin in perimenopausal and postmenopausal women. Menopause 2014, 21, 493–500. [Google Scholar] [CrossRef]
- Tresguerres, I.F.; Tamimi, F.; Eimar, H.; Barralet, J.E.; Prieto, S.; Torres, J.; Calvo-Guirado, J.L.; Tresguerres, J.A. Melatonin Dietary Supplement as an Anti-Aging Therapy for Age-Related Bone Loss. Rejuvenation Res. 2014, 17, 341–346. [Google Scholar] [CrossRef]
- Cecon, E.; Oishi, A.; Jockers, R. Melatonin receptors: Molecular pharmacology and signalling in the context of system bias. Br. J. Pharmacol. 2018, 175, 3263–3280. [Google Scholar] [CrossRef]
- Li, J.; Somers, V.K.; Xu, H.; Lopez-Jimenez, F.; Covassin, N. Trends in Use of Melatonin Supplements Among US Adults, 1999-2018. JAMA 2022, 327, 483–485. [Google Scholar] [CrossRef]
- Galley, H.F.; Lowes, D.A.; Allen, L.; Cameron, G.; Aucott, L.S.; Webster, N.R. Melatonin as a potential therapy for sepsis: A phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J. Pineal Res. 2014, 56, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.H.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- Sarıtekin, E.; Üreyen Kaya, B.; Aşcı, H.; Özmen, Ö. Anti-inflammatory and antiresorptive functions of melatonin on experimentally induced periapical lesions. Int. Endod. J. 2019, 52, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Halıcı, M.; Öner, M.; Güney, A.; Canöz, Ö.; Narin, F.; Halıcı, C. Melatonin promotes fracture healing in the rat model. Eklem Hastalik. Cerrahisi 2010, 21, 172–177. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21067500 (accessed on 10 March 2024). [PubMed]
- Liu, R.-Y.; Li, L.; Zhang, Z.-T.; Wu, T.; Lin, S.; Zhang, X.-T. Clinical efficacy of melatonin as adjunctive therapy to non-surgical treatment of periodontitis: A systematic review and meta-analysis. Inflammopharmacology 2022, 30, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Bashır, A.; Mak, Y.; Sankaralıngam, S.; Cheung, J.; Mc Gowan, N.; Grigoriadis, A.; Fogelman, I.; Hampson, G. Changes in RANKL/OPG/RANK gene expression in peripheral mononuclear cells following treatment with estrogen or raloxifene. Steroids 2005, 70, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Cappellen, D.; Luong-Nguyen, N.-H.; Bongiovanni, S.; Grenet, O.; Wanke, C.; Šuša, M. Transcriptional Program of Mouse Osteoclast Differentiation Governed by the Macrophage Colony-stimulating Factor and the Ligand for the Receptor Activator of NFκB. J. Biol. Chem. 2002, 277, 21971–21982. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Goultschın, J.; Dean, D.D.; Boyan, B.D. Mechanisms of alveolar bone destruction in periodontitis. Periodontol. 2000 1997, 14, 158–172. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Jilka, R.L. Bone Marrow, Cytokines, and Bone Remodeling—Emerging Insights into the Pathophysiology of Osteoporosis. N. Engl. J. Med. 1995, 332, 305–311. [Google Scholar] [CrossRef] [PubMed]
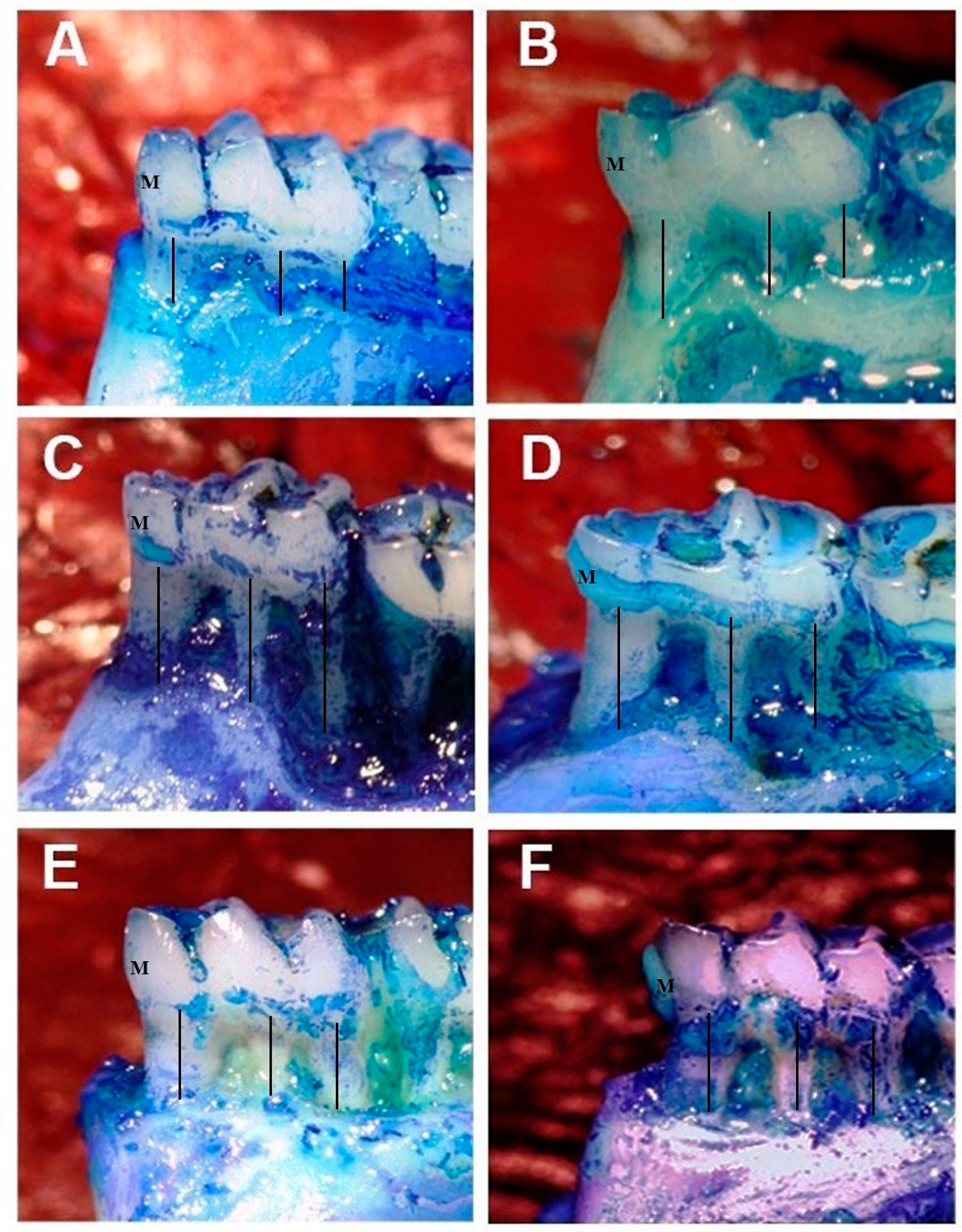
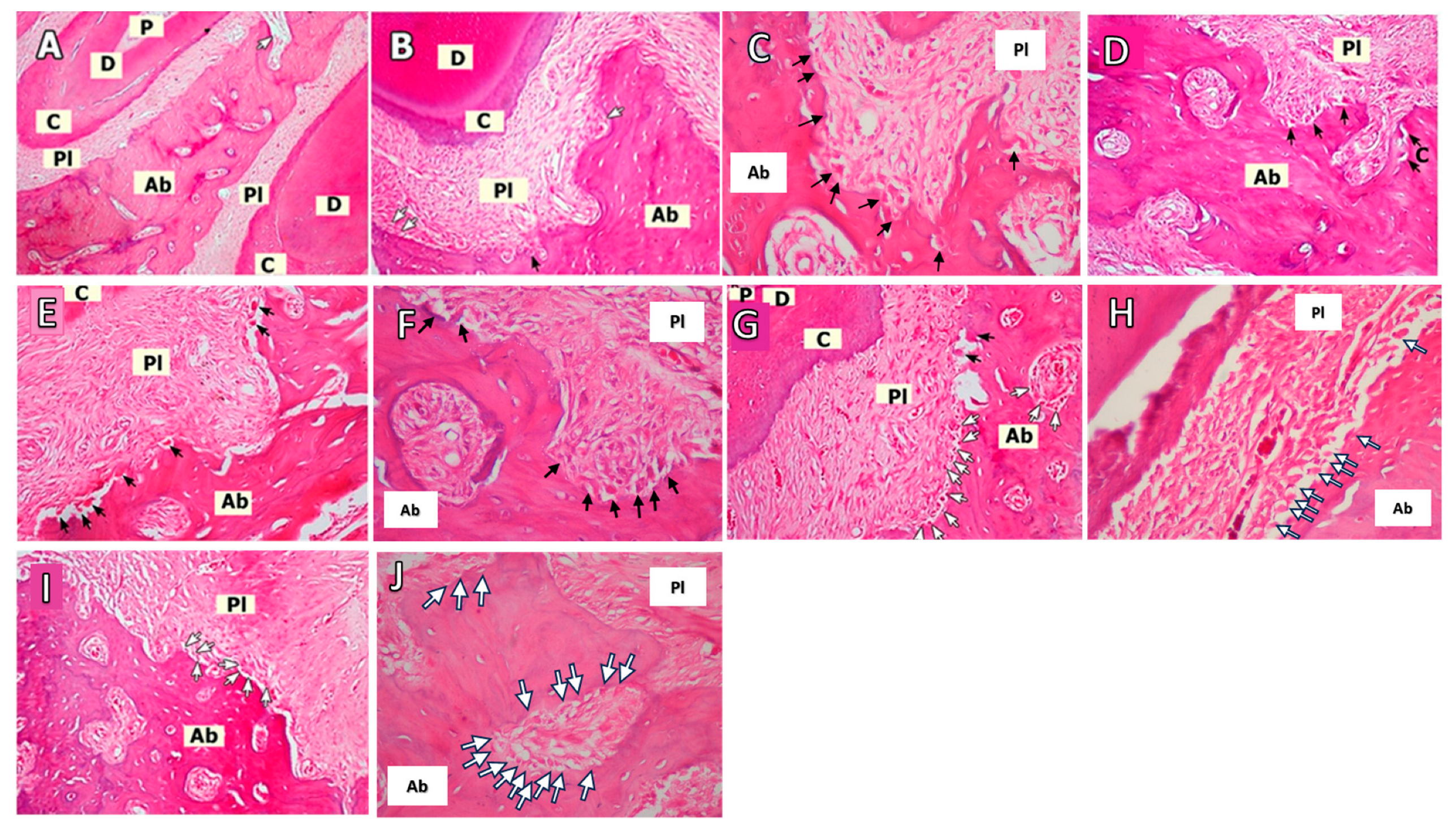
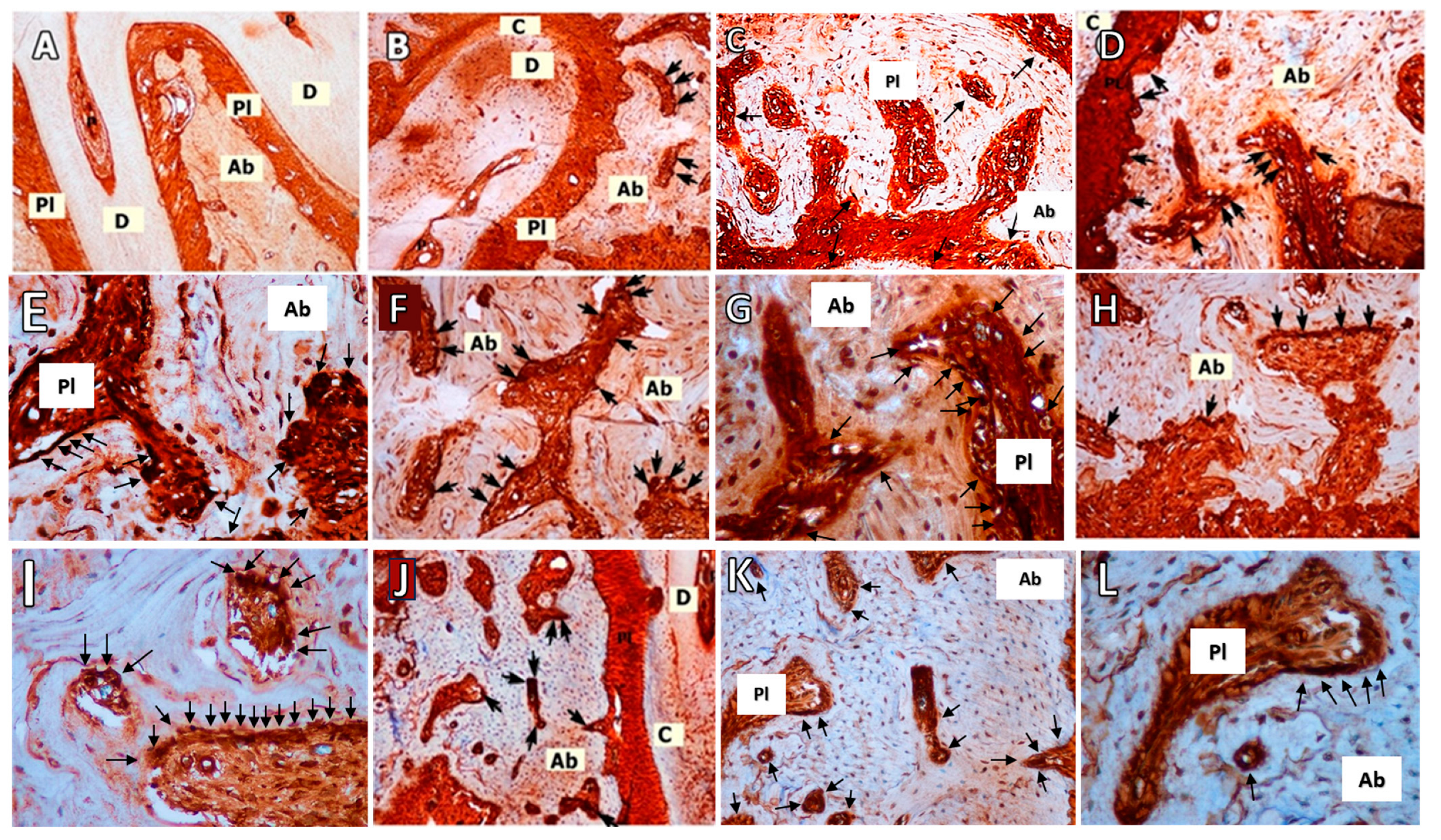
| Variables | C Group | O Group | LP Group | O+LP Group | ML30 Group | ML50 Group | p Value | |
|---|---|---|---|---|---|---|---|---|
| Mean alveolar bone loss | Mean ± SD | 0.58 ± 0.16 | 0.65 ± 0.19 | 1.21 ± 0.23 | 1.16 ± 0.17 | 0.86 ± 0.18 | 0.96 ± 0.19 | <0.001 a |
| Median (Min-Max) | 0.59 (0.33–0.75) | 0.57 (0.49–1.02) | 1.12 (0.92–1.54) | 1.19 (0.83–1.32) | 0.91 (0.53–1.04) | 0.98 (0.57–1.14) | ||
| Osteoclast numbers | Mean ± SD | 1.17 ± 1.17 | 4.83 ± 1.94 | 20.00 ± 12.40 | 28.00 ± 14.98 | 10.13 ± 3.83 | 12.38 ± 3.74 | <0.001 a |
| Median (Min-Max) | 1.0 (0.0–3.0) | 4.5 (3.0–8.0) | 16.5 (5.0–40.0) | 26.5 (9.0–50.0) | 9.0 (6.0–16.0) | 11.50 (8.0–19.0) | ||
| Osteoblast numbers | Mean ± SD | 20.67 ± 9.07 | 34.50 ± 10.31 | 69.38 ± 10.46 | 64.13 ± 9.14 | 93.75 ± 16.68 | 82.88 ± 14.18 | <0.001 a |
| Median (Min-Max) | 17.5 (12.0–35.0) | 33.5 (20.0–48.0) | 69.0 (54.0–85.0) | 65.5 (48.0–76.0) | 91.0 (74.0–120.0) | 83.0 (64.0–108.0) | ||
| RANKL scores | Mean ± SD | 0.67 ± 0.52 | 0.83 ± 0.75 | 2.13 ± 0.99 | 2.88 ± 0.35 | 0.88 ± 0.64 | 1.25 ± 0.46 | <0.001 a |
| Median (Min-Max) | 1.0 (0.0–1.0) | 1.0 (0.0–2.0) | 2.5 (1.0–3.0) | 3.0 (2.0–3.0) | 1.0 (0.0–2.0) | 1.0 (1.0–2.0) | ||
| Inflammatory cell infiltration scores | Mean ± SD | 0.67 ± 0.52 | 1.0 ± 0.0 | 2.0 ± 0.76 | 2.13 ± 0.64 | 1.13 ± 0.35 | 1.50 ± 0.53 | <0.001 a |
| Median (Min-Max) | 1.0 (0.0–1.0) | 1.0 (1.0–1.0) | 2.00 (1.0–3.0) | 2.0 (1.0–3.0) | 1.0 (1.0–2.0) | 1.5 (1.0–2.0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altıntepe Doğan, S.S.; Toker, H.; Göze, Ö.F. Melatonin Decreases Alveolar Bone Loss in Rats with Experimental Periodontitis and Osteoporosis: A Morphometric and Histopathologic Study. Biomedicines 2024, 12, 684. https://doi.org/10.3390/biomedicines12030684
Altıntepe Doğan SS, Toker H, Göze ÖF. Melatonin Decreases Alveolar Bone Loss in Rats with Experimental Periodontitis and Osteoporosis: A Morphometric and Histopathologic Study. Biomedicines. 2024; 12(3):684. https://doi.org/10.3390/biomedicines12030684
Chicago/Turabian StyleAltıntepe Doğan, Suat Serhan, Hülya Toker, and Ömer Fahrettin Göze. 2024. "Melatonin Decreases Alveolar Bone Loss in Rats with Experimental Periodontitis and Osteoporosis: A Morphometric and Histopathologic Study" Biomedicines 12, no. 3: 684. https://doi.org/10.3390/biomedicines12030684
APA StyleAltıntepe Doğan, S. S., Toker, H., & Göze, Ö. F. (2024). Melatonin Decreases Alveolar Bone Loss in Rats with Experimental Periodontitis and Osteoporosis: A Morphometric and Histopathologic Study. Biomedicines, 12(3), 684. https://doi.org/10.3390/biomedicines12030684






