The Influence of Cross-Reactive T Cells in COVID-19
Abstract
1. Introduction: SARS-CoV-2 and COVID-19
2. Adaptive Immune Response to SARS-CoV-2
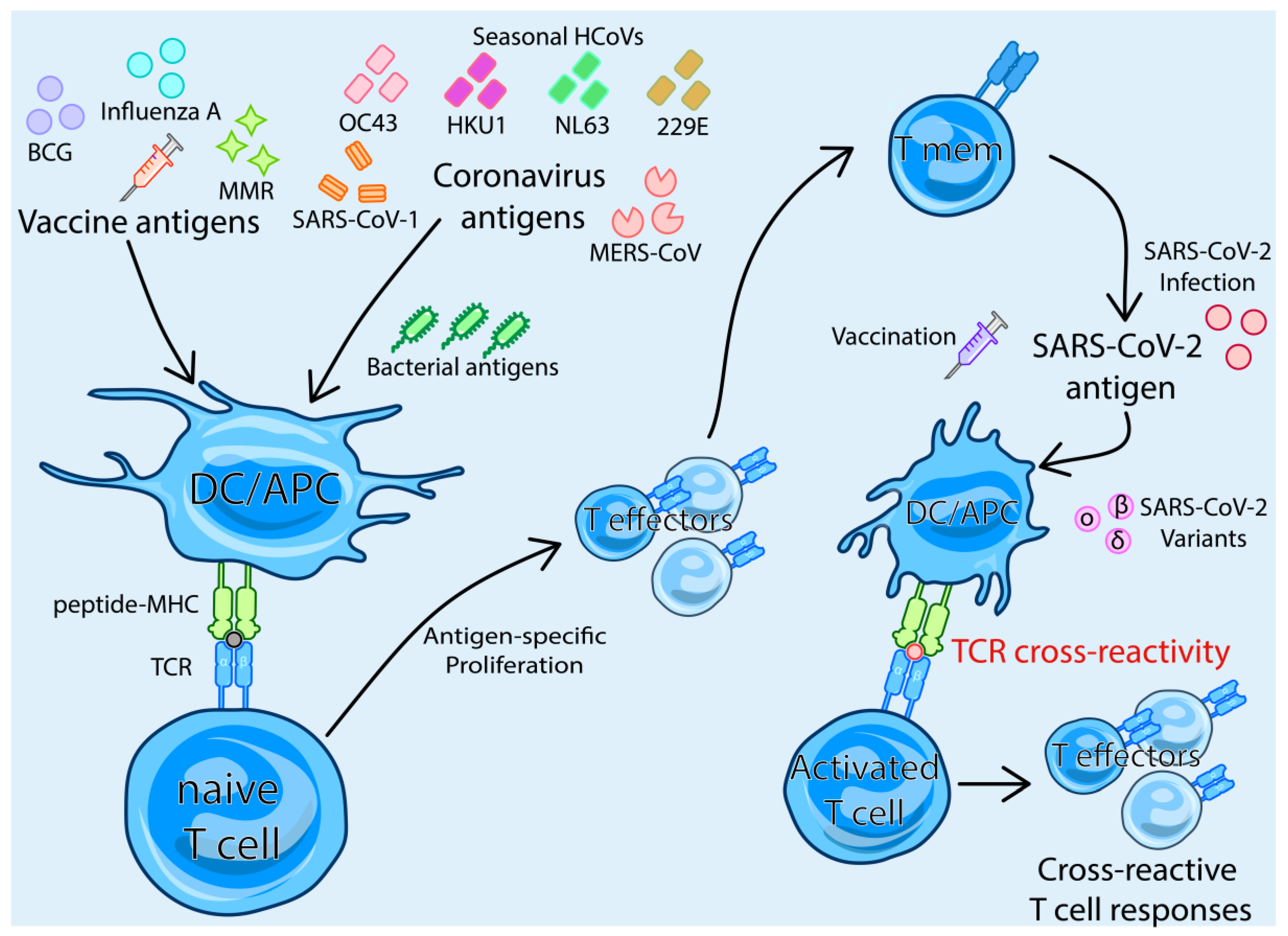
3. TCR-Dependent Cross-Reactivity
4. T cell Cross-Reactivity between SARS-CoV-2 and Other Human Coronaviruses
| HLA Association | SARS-CoV-2 Epitope | SARS-CoV-2 Sequence | Reference |
|---|---|---|---|
| Cross-reactive Spike Epitopes | |||
| HLA-DP | S355–364 | RKRISNCVAD | [63] |
| HLA-DR | S506–525 | QPYRVVVLSFELLHAPATVC | [63] |
| NA | S556–564 | NKKFLPFQQ | [80] |
| NA | S770–777 | IAVEQDKN | [80] |
| NA | S810–816 | KPSKRS | [57] |
| NA | S817–824 | FIEDLLFN | [80] |
| HLA-DP | S816–830 | SFIEDLLFNKVTLAD | [42,44,56,63] |
| NA | S851–856 | CAQKFN | [57] |
| NA | S901–906 | QMAYRF | [57] |
| HLA-B*15:01 | S919–927 | NQKLIANQF | [73] |
| HLA-A*02:01 | S976–984 | VLNDILSRL | [81] |
| NA | S997–1002 | ITGRLQ | [57] |
| HLA-DP | S981–1000 | LSRLDKVEAEVQIDRLITGR | [63] |
| NA | S1040–1044 | VDFCG | [57] |
| NA | S1148–1157 | FKEELDKYFK | [80,82] |
| NA | S1150–1156 | EELDKYF | [80,82] |
| NA | S1205–1212 | KYEQYIKW | [57] |
| NA | S1206–1220 | YEQYIKWPWYIWLGF | [42] |
| HLA-A*24 | S1207–1215 | QYIKWPWYI | [43] |
| Cross-reactive NSP Epitopes | |||
| HLA-A*02:01 | NSP1 (ORF184–92) | VMVELVAEL | [81] |
| HLA-A*01:01 | NSP3 (ORF11637–1646) | TTDPSFLGRY | [43] |
| HLA-A*02:01 | NSP5 (ORF13467–3475) | VLAWLYAAV | [81] |
| HLA-B*08 | NSP5 (ORF13361–3369) | TPKYKFVRI | [43] |
| HLA-A*02:01 | NSP6 (ORF13690–3698) | KLKDCVMYA | [83] |
| HLA-B*35 | NSP736–50 | HNDILLAKDTTEAFE | [33] |
| NA | NSP726–40 | SKLWAQCVQLHNDIL | [33] |
| HLA-A*02:01 | NSP8 (ORF14032–4040) | MLFTMLRKL | [81] |
| NA | NSP8 (ORF13976–3990) | VLKKLKKSLNVAKSE | [42] |
| HLA-B*08 | NSP10 (ORF14344–4352) | DLKGKYVQI | [43] |
| NA | NSP12 (ORF15246–5260) | LMIERFVSLAIDAYP | [42] |
| HLA-A*24 | NSP12 (ORF15137–5145) | FYAYLRKHF | [83] |
| NA | NSP12 (ORF15136–5150) | EFYAYLRKHFSMMIL | [42] |
| NA | NSP12 (ORF14966–4980) | KLLKSIAATRGATVV | [42] |
| HLA-A*02:01 | NSP12 (ORF14515–4523) | TMADLVYAL | [81] |
| NA | NSP13 (ORF15881–5895) | NVNRFNVAITRAKVG | [42] |
| HLA-A*03 | NSP13 (ORF15455–5463) | KLFAAETLK | [43] |
| NA | NSP13 (ORF15361–5375) | TSHKLVLSVNPYVCN | [42] |
| HLA-B*40 | NSP14 (ORF16219–6228) | IEYPIIGDEL | [43] |
| NA | NSP15 (ORF16751–6765) | LDDFVEIIKSQDLSV | [43] |
| NA | ORF843–57 | SKWYIRVGARKSAPL | [43] |
| NA | ORF7a90–104 | QEEVQELYSPIFLIV | [43] |
| HLA-B*40 | ORF7a40–49 | YEGNSPFHPL | [43] |
| HLA-DR | ORF626–40 | IWNLDYIINLIIKNL | [43] |
| HLA-A*01 | ORF620–31 | RTFKVSIWNLDY | [43] |
| Cross-reactive Nucleocapsid Epitopes | |||
| HLA-DR | N50–64 | ASWFTALTQHGKEDL | [43] |
| HLA-B*07 | N101–120 | MKDLSPRWYFYYLGTGPEAG | [33,43] |
| HLA-B*07:01 | N105–113 | SPRWYFYYL | [25,26,65,69,83] |
| HLA-DR | N127–141 | KDGIIWVATEGALNT | [43] |
| NA | N221–235 | LLLLDRLNQLESKMS | [43] |
| HLA-A*02:01 | N221–230 | LLLLDRLNQL | [83] |
| HLA-DR | N311–325 | ASAFFGMSRIGMEVT | [43] |
| NA | N326–340 | PSGTWLTYTGAIKLD | [42] |
| NA | N328–342 | GTWLTYTGAIKLDDK | [43] |
5. T Cell Cross-Reactivity between SARS-CoV-2 and Novel SARS-CoV-2 Variants
6. T Cell Cross-Reactivity between SARS-CoV-2 and Different Vaccines or Pathogens
7. T Cell Cross-Reactivity from the Bacillus Calmette–Guérin Vaccine
8. T Cell Cross-Reactivity from the Influenza Vaccine
9. T Cell Cross-Reactivity from the Measles, Mumps and Rubella Vaccine
10. T Cell Cross-Reactivity from Microbial Antigens
11. Conclusions and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Le, T.T.; Cramer, J.P.; Chen, R.; Mayhew, S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 667–668. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Pere, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Moller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Madden, E.A.; Diamond, M.S. Host cell-intrinsic innate immune recognition of SARS-CoV-2. Curr. Opin. Virol. 2022, 52, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
- Notarbartolo, S.; Ranzani, V.; Bandera, A.; Gruarin, P.; Bevilacqua, V.; Putignano, A.R.; Gobbini, A.; Galeota, E.; Manara, C.; Bombaci, M.; et al. Integrated longitudinal immunophenotypic, transcriptional and repertoire analyses delineate immune responses in COVID-19 patients. Sci. Immunol. 2021, 6, eabg5021. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, L.; Mescia, F.; Turner, L.; Hanson, A.L.; Kotagiri, P.; Dunmore, B.J.; Ruffieux, H.; De Sa, A.; Huhn, O.; Morgan, M.D.; et al. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity 2021, 54, 1257–1275.e8. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Klein, J.; Sundaram, M.E.; Liu, F.; Wong, P.; Silva, J.; Mao, T.; Oh, J.E.; Mohanty, S.; Huang, J.; et al. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat. Med. 2021, 27, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Laing, A.G.; Lorenc, A.; Del Molino Del Barrio, I.; Das, A.; Fish, M.; Monin, L.; Munoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.B.; Braciale, V.L.; Braciale, T.J. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J. Exp. Med. 1994, 180, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Altmann, D.M.; Boyton, R.J. SARS-CoV-2 T cell immunity: Specificity, function, durability, and role in protection. Sci. Immunol. 2020, 5, eabd6160. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Sidney, J.; Kidd, C.K.; Dan, J.M.; Ramirez, S.I.; Yu, E.D.; Mateus, J.; da Silva Antunes, R.; Moore, E.; Rubiro, P.; et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med. 2021, 2, 100204. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Vita, R.; Peters, B.; Crotty, S.; Weiskopf, D.; Sette, A. SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19. Cell Host Microbe 2021, 29, 1076–1092. [Google Scholar] [CrossRef]
- Quadeer, A.A.; Ahmed, S.F.; McKay, M.R. Landscape of epitopes targeted by T cells in 852 individuals recovered from COVID-19: Meta-analysis, immunoprevalence, and web platform. Cell Rep. Med. 2021, 2, 100312. [Google Scholar] [CrossRef]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef]
- Verhagen, J.; van der Meijden, E.D.; Lang, V.; Kremer, A.E.; Volkl, S.; Mackensen, A.; Aigner, M.; Kremer, A.N. Human CD4+ T cells specific for dominant epitopes of SARS-CoV-2 Spike and Nucleocapsid proteins with therapeutic potential. Clin. Exp. Immunol. 2021, 205, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; Tan, H.X.; Lee, W.S.; Reynaldi, A.; Kelly, H.G.; Wragg, K.; Esterbauer, R.; Kent, H.E.; Batten, C.J.; Mordant, F.L.; et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020, 26, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.; Qin, K.; Files, J.K.; Russell, R.M.; Stoltz, R.; Bibollet-Ruche, F.; Bansal, A.; Erdmann, N.; Hahn, B.H.; Goepfert, P.A. SARS-CoV-2-specific circulating T follicular helper cells correlate with neutralizing antibodies and increase during early convalescence. PLoS Pathog. 2021, 17, e1009761. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, E.; Reynolds, G.; Botting, R.A.; Calero-Nieto, F.J.; Morgan, M.D.; Tuong, Z.K.; Bach, K.; Sungnak, W.; Worlock, K.B.; Yoshida, M.; et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat. Med. 2021, 27, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Felce, S.L.; Dong, D.; Penkava, F.; Mentzer, A.J.; Yao, X.; Liu, G.; Yin, Z.; Chen, J.L.; Lu, Y.; et al. An immunodominant NP(105-113)-B*07:02 cytotoxic T cell response controls viral replication and is associated with less severe COVID-19 disease. Nat. Immunol. 2022, 23, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Lineburg, K.E.; Grant, E.J.; Swaminathan, S.; Chatzileontiadou, D.S.M.; Szeto, C.; Sloane, H.; Panikkar, A.; Raju, J.; Crooks, P.; Rehan, S.; et al. CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. Immunity 2021, 54, 1055–1065.e5. [Google Scholar] [CrossRef]
- Ferretti, A.P.; Kula, T.; Wang, Y.; Nguyen, D.M.V.; Weinheimer, A.; Dunlap, G.S.; Xu, Q.; Nabilsi, N.; Perullo, C.R.; Cristofaro, A.W.; et al. Unbiased Screens Show CD8+ T Cells of COVID-19 Patients Recognize Shared Epitopes in SARS-CoV-2 that Largely Reside outside the Spike Protein. Immunity 2020, 53, 1095–1107.e3. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med. 2021, 2, 100354. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Adamo, S.; Michler, J.; Zurbuchen, Y.; Cervia, C.; Taeschler, P.; Raeber, M.E.; Baghai Sain, S.; Nilsson, J.; Moor, A.E.; Boyman, O. Signature of long-lived memory CD8+ T cells in acute SARS-CoV-2 infection. Nature 2022, 602, 148–155. [Google Scholar] [CrossRef]
- Jung, J.H.; Rha, M.S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.H.; Jeong, H.W.; Choi, W.S.; et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat. Commun. 2021, 12, 4043. [Google Scholar] [CrossRef]
- Rha, M.S.; Jeong, H.W.; Ko, J.H.; Choi, S.J.; Seo, I.H.; Lee, J.S.; Sa, M.; Kim, A.R.; Joo, E.J.; Ahn, J.Y.; et al. PD-1-Expressing SARS-CoV-2-Specific CD8+ T Cells Are Not Exhausted, but Functional in Patients with COVID-19. Immunity 2021, 54, 44–52.e3. [Google Scholar] [CrossRef]
- Bilich, T.; Nelde, A.; Heitmann, J.S.; Maringer, Y.; Roerden, M.; Bauer, J.; Rieth, J.; Wacker, M.; Peter, A.; Horber, S.; et al. T cell and antibody kinetics delineate SARS-CoV-2 peptides mediating long-term immune responses in COVID-19 convalescent individuals. Sci. Transl. Med. 2021, 13, eabf7517. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Chaisawangwong, W.; Wang, H.; Kouo, T.; Salathe, S.F.; Isser, A.; Bieler, J.G.; Zhang, M.L.; Livingston, N.K.; Li, S.; Horowitz, J.J.; et al. Cross-reactivity of SARS-CoV-2- and influenza A-specific T cells in individuals exposed to SARS-CoV-2. JCI Insight 2022, 7, e158308. [Google Scholar] [CrossRef] [PubMed]
- Mysore, V.; Cullere, X.; Settles, M.L.; Ji, X.; Kattan, M.W.; Desjardins, M.; Durbin-Johnson, B.; Gilboa, T.; Baden, L.R.; Walt, D.R.; et al. Protective heterologous T cell immunity in COVID-19 induced by the trivalent MMR and Tdap vaccine antigens. Med. 2021, 2, 1050–1071.e7. [Google Scholar] [CrossRef]
- Maurice, N.J.; Taber, A.K.; Prlic, M. The Ugly Duckling Turned to Swan: A Change in Perception of Bystander-Activated Memory CD8 T Cells. J. Immunol. 2021, 206, 455–462. [Google Scholar] [CrossRef]
- Raue, H.P.; Brien, J.D.; Hammarlund, E.; Slifka, M.K. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J. Immunol. 2004, 173, 6873–6881. [Google Scholar] [CrossRef]
- Gilbertson, B.; Germano, S.; Steele, P.; Turner, S.; Fazekas de St Groth, B.; Cheers, C. Bystander activation of CD8+ T lymphocytes during experimental mycobacterial infection. Infect. Immun. 2004, 72, 6884–6891. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cornberg, M.; Wang, X.Z.; Chen, H.D.; Selin, L.K.; Welsh, R.M. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J. Exp. Med. 2005, 201, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Salkowska, A.; Karwaciak, I.; Karas, K.; Dastych, J.; Ratajewski, M. SARS-CoV-2 Proteins Induce IFNG in Th1 Lymphocytes Generated from CD4+ Cells from Healthy, Unexposed Polish Donors. Vaccines 2020, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, E.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370, 89–94. [Google Scholar] [CrossRef]
- Nelde, A.; Bilich, T.; Heitmann, J.S.; Maringer, Y.; Salih, H.R.; Roerden, M.; Lubke, M.; Bauer, J.; Rieth, J.; Wacker, M.; et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2021, 22, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Loyal, L.; Braun, J.; Henze, L.; Kruse, B.; Dingeldey, M.; Reimer, U.; Kern, F.; Schwarz, T.; Mangold, M.; Unger, C.; et al. Cross-reactive CD4+ T cells enhance SARS-CoV-2 immune responses upon infection and vaccination. Science 2021, 374, eabh1823. [Google Scholar] [CrossRef]
- Gorse, G.J.; Patel, G.B.; Vitale, J.N.; O’Connor, T.Z. Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin. Vaccine Immunol. 2010, 17, 1875–1880. [Google Scholar] [CrossRef]
- Guthmiller, J.J.; Wilson, P.C. Remembering seasonal coronaviruses. Science 2020, 370, 1272–1273. [Google Scholar] [CrossRef] [PubMed]
- Edridge, A.W.D.; Kaczorowska, J.; Hoste, A.C.R.; Bakker, M.; Klein, M.; Loens, K.; Jebbink, M.F.; Matser, A.; Kinsella, C.M.; Rueda, P.; et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020, 26, 1691–1693. [Google Scholar] [CrossRef]
- Saletti, G.; Gerlach, T.; Jansen, J.M.; Molle, A.; Elbahesh, H.; Ludlow, M.; Li, W.; Bosch, B.J.; Osterhaus, A.; Rimmelzwaan, G.F. Older adults lack SARS-CoV-2 cross-reactive T lymphocytes directed to human coronaviruses OC43 and NL63. Sci. Rep. 2020, 10, 21447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Alshukairi, A.N.; Baharoon, S.A.; Ahmed, W.A.; Bokhari, A.A.; Nehdi, A.M.; Layqah, L.A.; Alghamdi, M.G.; Al Gethamy, M.M.; Dada, A.M.; et al. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci. Immunol. 2017, 2, eaan5393. [Google Scholar] [CrossRef]
- Mok, C.K.P.; Zhu, A.; Zhao, J.; Lau, E.H.Y.; Wang, J.; Chen, Z.; Zhuang, Z.; Wang, Y.; Alshukairi, A.N.; Baharoon, S.A.; et al. T-cell responses to MERS coronavirus infection in people with occupational exposure to dromedary camels in Nigeria: An observational cohort study. Lancet Infect. Dis. 2021, 21, 385–395. [Google Scholar] [CrossRef]
- Gussow, A.B.; Auslander, N.; Faure, G.; Wolf, Y.I.; Zhang, F.; Koonin, E.V. Genomic determinants of pathogenicity in SARS-CoV-2 and other human coronaviruses. Proc. Natl. Acad. Sci. USA 2020, 117, 15193–15199. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; van den Akker, J.P.C.; Molenkamp, R.; Koopmans, M.P.G.; van Gorp, E.C.M.; et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5, eabd2071. [Google Scholar] [CrossRef] [PubMed]
- Meckiff, B.J.; Ramirez-Suastegui, C.; Fajardo, V.; Chee, S.J.; Kusnadi, A.; Simon, H.; Eschweiler, S.; Grifoni, A.; Pelosi, E.; Weiskopf, D.; et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4+ T Cells in COVID-19. Cell 2020, 183, 1340–1353.e16. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Pre-existing immunity to SARS-CoV-2: The knowns and unknowns. Nat. Rev. Immunol. 2020, 20, 457–458. [Google Scholar] [CrossRef]
- Bacher, P.; Rosati, E.; Esser, D.; Martini, G.R.; Saggau, C.; Schiminsky, E.; Dargvainiene, J.; Schroder, I.; Wieters, I.; Khodamoradi, Y.; et al. Low-Avidity CD4+ T Cell Responses to SARS-CoV-2 in Unexposed Individuals and Humans with Severe COVID-19. Immunity 2020, 53, 1258–1271.e5. [Google Scholar] [CrossRef] [PubMed]
- Dykema, A.G.; Zhang, B.; Woldemeskel, B.A.; Garliss, C.C.; Cheung, L.S.; Choudhury, D.; Zhang, J.; Aparicio, L.; Bom, S.; Rashid, R.; et al. Functional characterization of CD4+ T cell receptors crossreactive for SARS-CoV-2 and endemic coronaviruses. J. Clin. Investig. 2021, 131, e146922. [Google Scholar] [CrossRef]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 40–49. [Google Scholar] [CrossRef]
- Sagar, M.; Reifler, K.; Rossi, M.; Miller, N.S.; Sinha, P.; White, L.F.; Mizgerd, J.P. Recent endemic coronavirus infection is associated with less-severe COVID-19. J. Clin. Investig. 2021, 131, e143380. [Google Scholar] [CrossRef]
- Kundu, R.; Narean, J.S.; Wang, L.; Fenn, J.; Pillay, T.; Fernandez, N.D.; Conibear, E.; Koycheva, A.; Davies, M.; Tolosa-Wright, M.; et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat. Commun. 2022, 13, 80. [Google Scholar] [CrossRef]
- Sette, A.; Saphire, E.O. Inducing broad-based immunity against viruses with pandemic potential. Immunity 2022, 55, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Shen, M.; Han, X.; Chen, Q.; Li, L.; Chen, S.; Zhang, J.; Gao, F.; Wang, W.; Wang, Y.; et al. Identification of cross-reactive CD8+ T cell receptors with high functional avidity to a SARS-CoV-2 immunodominant epitope and its natural mutant variants. Genes. Dis. 2022, 9, 216–229. [Google Scholar] [CrossRef]
- Low, J.S.; Vaqueirinho, D.; Mele, F.; Foglierini, M.; Jerak, J.; Perotti, M.; Jarrossay, D.; Jovic, S.; Perez, L.; Cacciatore, R.; et al. Clonal analysis of immunodominance and cross-reactivity of the CD4 T cell response to SARS-CoV-2. Science 2021, 372, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.M.; Malhotra, U.; Kim, Y.G.; Gomez, R.; Krist, M.P.; Wald, A.; Koelle, D.M.; Kwok, W.W. Cross-reactive and mono-reactive SARS-CoV-2 CD4+ T cells in prepandemic and COVID-19 convalescent individuals. PLoS Pathog. 2021, 17, e1010203. [Google Scholar] [CrossRef]
- Schulien, I.; Kemming, J.; Oberhardt, V.; Wild, K.; Seidel, L.M.; Killmer, S.; Sagar; Daul, F.; Salvat Lago, M.; Decker, A.; et al. Characterization of pre-existing and induced SARS-CoV-2-specific CD8+ T cells. Nat. Med. 2021, 27, 78–85. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Garliss, C.C.; Blankson, J.N. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J. Clin. Investig. 2021, 131, 149335. [Google Scholar] [CrossRef] [PubMed]
- Nathan, A.; Rossin, E.J.; Kaseke, C.; Park, R.J.; Khatri, A.; Koundakjian, D.; Urbach, J.M.; Singh, N.K.; Bashirova, A.; Tano-Menka, R.; et al. Structure-guided T cell vaccine design for SARS-CoV-2 variants and sarbecoviruses. Cell 2021, 184, 4401–4413.e10. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, C.I.; Galloway, J.; Chu, H.Y.; Shipley, M.M.; Sung, K.; Itell, H.L.; Wolf, C.R.; Logue, J.K.; Magedson, A.; Garrett, M.E.; et al. Epitope profiling reveals binding signatures of SARS-CoV-2 immune response in natural infection and cross-reactivity with endemic human CoVs. Cell Rep. 2021, 35, 109164. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.M.; Leistritz-Edwards, D.; Dunn, A.; Tarr, C.; Lehman, J.; Dempsey, C.; Hamel, A.; Rayon, V.; Liu, G.; Wang, Y.; et al. Allelic variation in class I HLA determines CD8+ T cell repertoire shape and cross-reactive memory responses to SARS-CoV-2. Sci. Immunol. 2022, 7, eabk3070. [Google Scholar] [PubMed]
- Becerra-Artiles, A.; Calvo-Calle, J.M.; Co, M.D.; Nanaware, P.P.; Cruz, J.; Weaver, G.C.; Lu, L.; Forconi, C.; Finberg, R.W.; Moormann, A.M.; et al. Broadly recognized, cross-reactive SARS-CoV-2 CD4 T cell epitopes are highly conserved across human coronaviruses and presented by common HLA alleles. Cell Rep. 2022, 39, 110952. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Alves, R.P.; Timis, J.; Miller, R.; Valentine, K.; Pinto, P.B.A.; Gonzalez, A.; Regla-Nava, J.A.; Maule, E.; Nguyen, M.N.; Shafee, N.; et al. Human coronavirus OC43-elicited CD4+ T cells protect against SARS-CoV-2 in HLA transgenic mice. Nat. Commun. 2024, 15, 787. [Google Scholar] [CrossRef] [PubMed]
- Minervina, A.A.; Pogorelyy, M.V.; Kirk, A.M.; Crawford, J.C.; Allen, E.K.; Chou, C.H.; Mettelman, R.C.; Allison, K.J.; Lin, C.Y.; Brice, D.C.; et al. SARS-CoV-2 antigen exposure history shapes phenotypes and specificity of memory CD8+ T cells. Nat. Immunol. 2022, 23, 781–790. [Google Scholar] [CrossRef]
- Augusto, D.G.; Murdolo, L.D.; Chatzileontiadou, D.S.M.; Sabatino, J.J., Jr.; Yusufali, T.; Peyser, N.D.; Butcher, X.; Kizer, K.; Guthrie, K.; Murray, V.W.; et al. A common allele of HLA is associated with asymptomatic SARS-CoV-2 infection. Nature 2023, 620, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Srivastava, R.; Coulon, P.G.; Dhanushkodi, N.R.; Chentoufi, A.A.; Tifrea, D.F.; Edwards, R.A.; Figueroa, C.J.; Schubl, S.D.; Hsieh, L.; et al. Genome-Wide B Cell, CD4+, and CD8+ T Cell Epitopes That Are Highly Conserved between Human and Animal Coronaviruses, Identified from SARS-CoV-2 as Targets for Preemptive Pan-Coronavirus Vaccines. J. Immunol. 2021, 206, 2566–2582. [Google Scholar] [CrossRef] [PubMed]
- Habel, J.R.; Nguyen, T.H.O.; van de Sandt, C.E.; Juno, J.A.; Chaurasia, P.; Wragg, K.; Koutsakos, M.; Hensen, L.; Jia, X.; Chua, B.; et al. Suboptimal SARS-CoV-2-specific CD8+ T cell response associated with the prominent HLA-A*02:01 phenotype. Proc. Natl. Acad. Sci. USA 2020, 117, 24384–24391. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Quan, Y.; Xin, Z.T.; Wrammert, J.; Ma, M.J.; Lv, H.; Wang, T.B.; Yang, H.; Richardus, J.H.; Liu, W.; et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: A six-year follow-up study. J. Immunol. 2011, 186, 7264–7268. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.P.; Wang, N.C.; Chang, Y.H.; Tian, X.Y.; Na, D.Y.; Zhang, L.Y.; Zheng, L.; Lan, T.; Wang, L.F.; Liang, G.D. Duration of antibody responses after severe acute respiratory syndrome. Emerg. Infect. Dis. 2007, 13, 1562–1564. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent COVID-19 patients. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mateus, J.; Dan, J.M.; Zhang, Z.; Rydyznski Moderbacher, C.; Lammers, M.; Goodwin, B.; Sette, A.; Crotty, S.; Weiskopf, D. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 2021, 374, eabj9853. [Google Scholar] [CrossRef]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2020, 370, eabd4250. [Google Scholar] [CrossRef]
- Mallajosyula, V.; Ganjavi, C.; Chakraborty, S.; McSween, A.M.; Pavlovitch-Bedzyk, A.J.; Wilhelmy, J.; Nau, A.; Manohar, M.; Nadeau, K.C.; Davis, M.M. CD8+ T cells specific for conserved coronavirus epitopes correlate with milder disease in COVID-19 patients. Sci. Immunol. 2021, 6, eabg5669. [Google Scholar] [CrossRef] [PubMed]
- Ladner, J.T.; Henson, S.N.; Boyle, A.S.; Engelbrektson, A.L.; Fink, Z.W.; Rahee, F.; D’Ambrozio, J.; Schaecher, K.E.; Stone, M.; Dong, W.; et al. Epitope-resolved profiling of the SARS-CoV-2 antibody response identifies cross-reactivity with endemic human coronaviruses. Cell Rep. Med. 2021, 2, 100189. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.K.; Hersby, D.S.; Tamhane, T.; Povlsen, H.R.; Amaya Hernandez, S.P.; Nielsen, M.; Gang, A.O.; Hadrup, S.R. SARS-CoV-2 genome-wide T cell epitope mapping reveals immunodominance and substantial CD8+ T cell activation in COVID-19 patients. Sci. Immunol. 2021, 6, eabf7550. [Google Scholar] [CrossRef]
- Fischer, W.; Giorgi, E.E.; Chakraborty, S.; Nguyen, K.; Bhattacharya, T.; Theiler, J.; Goloboff, P.A.; Yoon, H.; Abfalterer, W.; Foley, B.T.; et al. HIV-1 and SARS-CoV-2: Patterns in the evolution of two pandemic pathogens. Cell Host Microbe 2021, 29, 1093–1110. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef] [PubMed]
- Redd, A.D.; Nardin, A.; Kared, H.; Bloch, E.M.; Abel, B.; Pekosz, A.; Laeyendecker, O.; Fehlings, M.; Quinn, T.C.; Tobian, A.A.R. Minimal Crossover between Mutations Associated with Omicron Variant of SARS-CoV-2 and CD8+ T-Cell Epitopes Identified in COVID-19 Convalescent Individuals. mBio 2022, 13, e0361721. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, D.J.; Fournelle, D.; Grenier, J.C.; Schockaert, J.; Kovalchik, K.A.; Kubiniok, P.; Mostefai, F.; Duquette, J.D.; Saab, F.; Sirois, I.; et al. The mutational landscape of SARS-CoV-2 variants diversifies T cell targets in an HLA-supertype-dependent manner. Cell Syst. 2022, 13, 143–157.e3. [Google Scholar] [CrossRef] [PubMed]
- Agerer, B.; Koblischke, M.; Gudipati, V.; Montano-Gutierrez, L.F.; Smyth, M.; Popa, A.; Genger, J.W.; Endler, L.; Florian, D.M.; Muhlgrabner, V.; et al. SARS-CoV-2 mutations in MHC-I-restricted epitopes evade CD8+ T cell responses. Sci. Immunol. 2021, 6, eabg6461. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, S.; Ren, L.; Zheng, P.; Hu, X.; Jin, T.; Tan, X. Profiling CD8+ T cell epitopes of COVID-19 convalescents reveals reduced cellular immune responses to SARS-CoV-2 variants. Cell Rep. 2021, 36, 109708. [Google Scholar] [CrossRef]
- Jing, L.; Wu, X.; Krist, M.P.; Hsiang, T.Y.; Campbell, V.L.; McClurkan, C.L.; Favors, S.M.; Hemingway, L.A.; Godornes, C.; Tong, D.Q.; et al. T cell response to intact SARS-CoV-2 includes coronavirus cross-reactive and variant-specific components. JCI Insight 2022, 7, e158126. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Effort, C.H.G.; Cobat, A.; Casanova, J.L. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature 2022, 603, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Yu, J.; Liu, J.; Chandrashekar, A.; Borducchi, E.N.; Tostanoski, L.H.; McMahan, K.; Jacob-Dolan, C.; Martinez, D.R.; Chang, A.; et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021, 596, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Geers, D.; Shamier, M.C.; Bogers, S.; den Hartog, G.; Gommers, L.; Nieuwkoop, N.N.; Schmitz, K.S.; Rijsbergen, L.C.; van Osch, J.A.T.; Dijkhuizen, E.; et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021, 6, eabj1750. [Google Scholar] [CrossRef]
- Keeton, R.; Richardson, S.I.; Moyo-Gwete, T.; Hermanus, T.; Tincho, M.B.; Benede, N.; Manamela, N.P.; Baguma, R.; Makhado, Z.; Ngomti, A.; et al. Prior infection with SARS-CoV-2 boosts and broadens Ad26.COV2.S immunogenicity in a variant-dependent manner. Cell Host Microbe 2021, 29, 1611–1619.e5. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.T.; Harding, A.C.; Gilbert-Jaramillo, J.; Knight, M.L.; Longet, S.; Brown, A.; Adele, S.; Adland, E.; Brown, H.; Medawar Laboratory, T.; et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat. Commun. 2021, 12, 5061. [Google Scholar] [CrossRef]
- GeurtsvanKessel, C.H.; Geers, D.; Schmitz, K.S.; Mykytyn, A.Z.; Lamers, M.M.; Bogers, S.; Scherbeijn, S.; Gommers, L.; Sablerolles, R.S.G.; Nieuwkoop, N.N.; et al. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci. Immunol. 2022, 7, eabo2202. [Google Scholar] [CrossRef] [PubMed]
- Team, C.-F. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar]
- Mahajan, S.; Kode, V.; Bhojak, K.; Karunakaran, C.; Lee, K.; Manoharan, M.; Ramesh, A.; Hv, S.; Srivastava, A.; Sathian, R.; et al. Immunodominant T-cell epitopes from the SARS-CoV-2 spike antigen reveal robust pre-existing T-cell immunity in unexposed individuals. Sci. Rep. 2021, 11, 13164. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Netea, M.G. BCG-induced trained immunity: Can it offer protection against COVID-19? Nat. Rev. Immunol. 2020, 20, 335–337. [Google Scholar] [CrossRef]
- Curtis, N.; Sparrow, A.; Ghebreyesus, T.A.; Netea, M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet 2020, 395, 1545–1546. [Google Scholar] [CrossRef]
- Higgins, J.P.; Soares-Weiser, K.; Lopez-Lopez, J.A.; Kakourou, A.; Chaplin, K.; Christensen, H.; Martin, N.K.; Sterne, J.A.; Reingold, A.L. Association of BCG, DTP, and measles containing vaccines with childhood mortality: Systematic review. BMJ 2016, 355, i5170. [Google Scholar] [CrossRef] [PubMed]
- Wardhana; Datau, E.A.; Sultana, A.; Mandang, V.V.; Jim, E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med. Indones. 2011, 43, 185–190. [Google Scholar]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef]
- de Castro, M.J.; Pardo-Seco, J.; Martinon-Torres, F. Nonspecific (Heterologous) Protection of Neonatal BCG Vaccination Against Hospitalization Due to Respiratory Infection and Sepsis. Clin. Infect. Dis. 2015, 60, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Floyd, K.; Wu, S.; Fang, Z.; Tan, T.K.; Froggatt, H.M.; Powers, J.M.; Leist, S.R.; Gully, K.L.; Hubbard, M.L.; et al. BCG vaccination stimulates integrated organ immunity by feedback of the adaptive immune response to imprint prolonged innate antiviral resistance. Nat. Immunol. 2024, 25, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Jalalizadeh, M.; Buosi, K.; Dionato, F.A.V.; Dal Col, L.S.B.; Giacomelli, C.F.; Ferrari, K.L.; Pagliarone, A.C.; Leme, P.A.F.; Maia, C.L.; Yadollahvandmiandoab, R.; et al. Randomized clinical trial of BCG vaccine in patients with convalescent COVID-19: Clinical evolution, adverse events, and humoral immune response. J. Intern. Med. 2022, 292, 654–666. [Google Scholar] [CrossRef]
- Santos, A.P.; Werneck, G.L.; Dalvi, A.P.R.; Dos Santos, C.C.; Tierno, P.; Condelo, H.S.; Macedo, B.; de Medeiros Leung, J.A.; de Souza Nogueira, J.; Malvao, L.; et al. The effect of BCG vaccination on infection and antibody levels against SARS-CoV-2-The results of ProBCG: A multicenter randomized clinical trial in Brazil. Int. J. Infect. Dis. 2023, 130, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Tsilika, M.; Taks, E.; Dolianitis, K.; Kotsaki, A.; Leventogiannis, K.; Damoulari, C.; Kostoula, M.; Paneta, M.; Adamis, G.; Papanikolaou, I.; et al. ACTIVATE-2: A Double-Blind Randomized Trial of BCG Vaccination Against COVID-19 in Individuals at Risk. Front. Immunol. 2022, 13, 873067. [Google Scholar] [CrossRef]
- Moorlag, S.; Taks, E.; Ten Doesschate, T.; van der Vaart, T.W.; Janssen, A.B.; Muller, L.; Ostermann, P.; Dijkstra, H.; Lemmers, H.; Simonetti, E.; et al. Efficacy of BCG Vaccination Against Respiratory Tract Infections in Older Adults during the Coronavirus Disease 2019 Pandemic. Clin Infect Dis 2022, 75, e938–e946. [Google Scholar] [CrossRef]
- Padmanabhan, U.; Mukherjee, S.; Borse, R.; Joshi, S.; Deshmukh, R. Phase II Clinical trial for Evaluation of BCG as potential therapy for COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Kumar, N.P.; Padmapriyadarsini, C.; Rajamanickam, A.; Bhavani, P.K.; Nancy, A.; Jeyadeepa, B.; Selvaraj, N.; Ashokan, D.; Renji, R.M.; Venkataramani, V.; et al. BCG vaccination induces enhanced frequencies of dendritic cells and altered plasma levels of type I and type III interferons in elderly individuals. Int. J. Infect. Dis. 2021, 110, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Ajayababu, A.; Thukral, H.; Gupta, S.; Guha, S.K.; Basu, A.; Gupta, G.; Thakur, P.; Lingaiah, R.; Das, B.K.; et al. Efficacy of Bacillus Calmette-Guerin (BCG) Vaccination in Reducing the Incidence and Severity of COVID-19 in High-Risk Population (BRIC): A Phase III, Multi-centre, Quadruple-Blind Randomised Control Trial. Infect. Dis. Ther. 2022, 11, 2205–2217. [Google Scholar] [CrossRef]
- Rivas, M.N.; Ebinger, J.E.; Wu, M.; Sun, N.; Braun, J.; Sobhani, K.; Van Eyk, J.E.; Cheng, S.; Arditi, M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J. Clin. Investig. 2021, 131, 145157. [Google Scholar] [CrossRef] [PubMed]
- Amirlak, L.; Haddad, R.; Hardy, J.D.; Khaled, N.S.; Chung, M.H.; Amirlak, B. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum. Vaccin. Immunother. 2021, 17, 3913–3915. [Google Scholar] [CrossRef] [PubMed]
- Arslan Gulen, T.; Bayraktar, M.; Yaksi, N.; Kayabas, U. Is the course of COVID-19 associated with tuberculin skin test diameter? A retrospective study. J. Med. Virol. 2022, 94, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.R.; Schaltz-Buchholzer, F.; Nielsen, S.; Benfield, T.; Bjerregaard-Andersen, M.; Dalgaard, L.S.; Dam, C.; Ditlev, S.B.; Faizi, G.; Azizi, M.; et al. Using BCG vaccine to enhance non-specific protection of health care workers during the COVID-19 pandemic: A randomized controlled trial. J. Infect. Dis. 2023, 229, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, L.R.B.; da Costa, A.C.; Cardoso, A.; Guimaraes, R.A.; Rodrigues, R.L.; Ribeiro, K.M.; Borges, K.C.M.; Carvalho, A.C.O.; Dias, C.I.S.; Rezende, A.O.; et al. Efficacy and Safety of BCG Revaccination with M. bovis BCG Moscow to Prevent COVID-19 Infection in Health Care Workers: A Randomized Phase II Clinical Trial. Front. Immunol. 2022, 13, 841868. [Google Scholar] [CrossRef] [PubMed]
- Ten Doesschate, T.; van der Vaart, T.W.; Debisarun, P.A.; Taks, E.; Moorlag, S.; Paternotte, N.; Boersma, W.G.; Kuiper, V.P.; Roukens, A.H.E.; Rijnders, B.J.A.; et al. Bacillus Calmette-Guerin vaccine to reduce healthcare worker absenteeism in COVID-19 pandemic, a randomized controlled trial. Clin. Microbiol. Infect. 2022, 28, 1278–1285. [Google Scholar] [CrossRef]
- Czajka, H.; Zapolnik, P.; Krzych, L.; Kmiecik, W.; Stopyra, L.; Nowakowska, A.; Jackowska, T.; Darmochwal-Kolarz, D.; Szymanski, H.; Radziewicz-Winnicki, I.; et al. A Multi-Center, Randomised, Double-Blind, Placebo-Controlled Phase III Clinical Trial Evaluating the Impact of BCG Re-Vaccination on the Incidence and Severity of SARS-CoV-2 Infections among Symptomatic Healthcare Professionals during the COVID-19 Pandemic in Poland-First Results. Vaccines 2022, 10, 314. [Google Scholar]
- Upton, C.M.; van Wijk, R.C.; Mockeliunas, L.; Simonsson, U.S.H.; McHarry, K.; van den Hoogen, G.; Muller, C.; von Delft, A.; van der Westhuizen, H.M.; van Crevel, R.; et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine 2022, 48, 101414. [Google Scholar] [CrossRef]
- Pittet, L.F.; Messina, N.L.; Orsini, F.; Moore, C.L.; Abruzzo, V.; Barry, S.; Bonnici, R.; Bonten, M.; Campbell, J.; Croda, J.; et al. Randomized Trial of BCG Vaccine to Protect against COVID-19 in Health Care Workers. N. Engl. J. Med. 2023, 388, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Finotti, P. Sequence similarity of HSP65 of Mycobacterium bovis BCG with SARS-CoV-2 spike and nuclear proteins: May it predict an antigen-dependent immune protection of BCG against COVID-19? Cell Stress. Chaperones 2022, 27, 37–43. [Google Scholar] [CrossRef]
- Eggenhuizen, P.J.; Ng, B.H.; Chang, J.; Fell, A.L.; Cheong, R.M.Y.; Wong, W.Y.; Gan, P.Y.; Holdsworth, S.R.; Ooi, J.D. BCG Vaccine Derived Peptides Induce SARS-CoV-2 T Cell Cross-Reactivity. Front. Immunol. 2021, 12, 692729. [Google Scholar] [CrossRef] [PubMed]
- Tarabini, R.F.; Rigo, M.M.; Faustino Fonseca, A.; Rubin, F.; Belle, R.; Kavraki, L.E.; Ferreto, T.C.; Amaral Antunes, D.; de Souza, A.P.D. Large-Scale Structure-Based Screening of Potential T Cell Cross-Reactivities Involving Peptide-Targets from BCG Vaccine and SARS-CoV-2. Front. Immunol. 2021, 12, 812176. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Adiga, V.; Ahmed, A.; Parthiban, C.; Chetan Kumar, N.; Dwarkanath, P.; Shivalingaiah, S.; Rao, S.; D’Souza, G.; Dias, M.; et al. Evidence for the heterologous benefits of prior BCG vaccination on COVISHIELD vaccine-induced immune responses in SARS-CoV-2 seronegative young Indian adults. Front. Immunol. 2022, 13, 985938. [Google Scholar] [CrossRef]
- Messina, N.L.; Germano, S.; McElroy, R.; Rudraraju, R.; Bonnici, R.; Pittet, L.F.; Neeland, M.R.; Nicholson, S.; Subbarao, K.; Curtis, N.; et al. Off-target effects of bacillus Calmette-Guerin vaccination on immune responses to SARS-CoV-2: Implications for protection against severe COVID-19. Clin. Transl. Immunology 2022, 11, e1387. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Padmapriyadarsini, C.; Rajamanickam, A.; Bhavani, P.K.; Nancy, A.; Jayadeepa, B.; Selvaraj, N.; Asokan, D.; Renji, R.M.; Venkataramani, V.; et al. BCG vaccination induces enhanced frequencies of memory T cells and altered plasma levels of common gammac cytokines in elderly individuals. PLoS ONE 2021, 16, e0258743. [Google Scholar] [CrossRef]
- Cocco, P.; Meloni, F.; Coratza, A.; Schirru, D.; Campagna, M.; De Matteis, S. Vaccination against seasonal influenza and socio-economic and environmental factors as determinants of the geographic variation of COVID-19 incidence and mortality in the Italian elderly. Prev. Med. 2021, 143, 106351. [Google Scholar] [CrossRef] [PubMed]
- Marin-Hernandez, D.; Schwartz, R.E.; Nixon, D.F. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J. Med. Virol. 2021, 93, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Zanettini, C.; Omar, M.; Dinalankara, W.; Imada, E.L.; Colantuoni, E.; Parmigiani, G.; Marchionni, L. Influenza Vaccination and COVID-19 Mortality in the USA: An Ecological Study. Vaccines 2021, 9, 427. [Google Scholar] [CrossRef]
- Amato, M.; Werba, J.P.; Frigerio, B.; Coggi, D.; Sansaro, D.; Ravani, A.; Ferrante, P.; Veglia, F.; Tremoli, E.; Baldassarre, D. Relationship between Influenza Vaccination Coverage Rate and COVID-19 Outbreak: An Italian Ecological Study. Vaccines 2020, 8, 535. [Google Scholar] [CrossRef]
- Lopez-Martin, I.; Andres Esteban, E.; Garcia-Martinez, F.J. Relationship between MMR vaccination and severity of COVID-19 infection. Survey among primary care physicians. Med. Clin. (Engl. Ed.) 2021, 156, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.E.; Baumgartl, W.H.; Okyay, R.A.; Licht, W.E.; Fidel, P.L., Jr.; Noverr, M.C.; Tilley, L.P.; Hurley, D.J.; Rada, B.; Ashford, J.W. Analysis of Measles-Mumps-Rubella (MMR) Titers of Recovered COVID-19 Patients. mBio 2020, 11, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Neumann, B.; Mendez, R.F.; Reyahi, A.; Joannides, A.; Modis, Y.; Franklin, R.J.M. Homologous protein domains in SARS-CoV-2 and measles, mumps and rubella viruses: Preliminary evidence that MMR vaccine might provide protection against COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Ahmadi, E.; Zabihi, M.R.; Hosseinzadeh, R.; Mohamed Khosroshahi, L.; Noorbakhsh, F. SARS-CoV-2 spike protein displays sequence similarities with paramyxovirus surface proteins; a bioinformatics study. PLoS ONE 2021, 16, e0260360. [Google Scholar] [CrossRef] [PubMed]
- Eggenhuizen, P.J.; Ng, B.H.; Chang, J.; Cheong, R.M.Y.; Yellapragada, A.; Wong, W.Y.; Ting, Y.T.; Monk, J.A.; Gan, P.Y.; Holdsworth, S.R.; et al. Heterologous Immunity between SARS-CoV-2 and Pathogenic Bacteria. Front. Immunol. 2022, 13, 821595. [Google Scholar] [CrossRef] [PubMed]
- Bartolo, L.; Afroz, S.; Pan, Y.G.; Xu, R.; Williams, L.; Lin, C.F.; Tanes, C.; Bittinger, K.; Friedman, E.S.; Gimotty, P.A.; et al. SARS-CoV-2-specific T cells in unexposed adults display broad trafficking potential and cross-react with commensal antigens. Sci. Immunol. 2022, 7, eabn3127. [Google Scholar] [CrossRef] [PubMed]
| Registry Number | Study Title | Phase/Country/Participant Group | Outcome |
|---|---|---|---|
| NCT04659941 | Use of BCG Vaccine as a Preventive Measure for COVID-19 in Health Care Workers (ProBCG) | Phase 2 Brazil Healthcare workers | BCG could protect from COVID-19 [109] |
| RBR-4kjqtg | BCG revaccination of health care professionals working in the COVID-19 pandemic, a preventive strategy to improve innate immune response | Phase 2 Brazil Healthcare workers | BCG could not protect from COVID-19 [119] |
| NCT04373291 | Using BCG Vaccine to Protect Health Care Workers in the COVID-19 Pandemic | Phase 3 Denmark Healthcare workers | BCG could not protect from COVID-19 [118] |
| NCT04414267 | Bacillus Calmette-guérin Vaccination to Prevent COVID-19 (ACTIVATEII) | Phase 4 Greece Adults ≥ 50 years with comorbidities | BCG could protect from COVID-19 [110] |
| NCT04328441 | Reducing Health Care Workers Absenteeism in COVID-19 Pandemic Through BCG Vaccine (BCG-CORONA) | Phase 3 Netherlands Healthcare workers | BCG could not protect from COVID-19 [120] |
| NCT04417335 | Reducing COVID-19 Related Hospital Admission in Elderly by BCG Vaccination | Phase 4 Netherlands Adults ≥ 60 years | BCG could protect from COVID-19. [111] |
| NCT04537663 | Prevention Of Respiratory Tract Infection And COVID-19 Through BCG Vaccination In Vulnerable Older Adults (BCG-PRIME) | Phase 4 Netherlands Adults ≥ 60 years with comorbidities | BCG could not protect |
| NCT04648800 | Clinical Trial Evaluating the Effect of BCG Vaccination on the Incidence and Severity of SARS-CoV-2 Infections Among Healthcare Professionals During the COVID-19 Pandemic in Poland | Phase 3 Poland Healthcare workers | BCG could not protect from COVID-19 [121] |
| CTRI/2020/05/025013 | Phase 2 Clinical Trial for the Evaluation of BCG as potential therapy for COVID-I9 | Phase 2 India Adults with COVID-19 | BCG could protect from COVID-19 [112] |
| NCT04475302 | BCG Vaccine in Reducing Morbidity and Mortality in Elderly Individuals in COVID-19 Hotspots | Phase 3 India Adults 60–80 years | BCG could protect from COVID-19 [113] |
| CTRI/2020/07/026668 | To study the effect of BCG vaccine in Reducing the Incidence and severity of COVID-19 in the high-risk population | Phase N/A India High-risk groups of adults 18–60 years | BCG could protect from COVID-19 [114] |
| NCT04379336 | BCG Vaccination for Healthcare Workers in COVID-19 Pandemic | Phase 3 South Africa Healthcare workers | BCG could not protect from COVID-19 [122] |
| NCT04327206 | BCG Vaccination to Protect Healthcare Workers Against COVID-19 (BRACE) | Phase 3 Australia and Netherlands Healthcare workers | BCG could not protect from COVID-19 [123] |
| NCT04369794 | COVID-19: BCG As Therapeutic Vaccine, Transmission Limitation, and Immunoglobulin Enhancement (BATTLE) | Phase 4 Brazil | BCG could protect from COVID-19 [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggenhuizen, P.J.; Ooi, J.D. The Influence of Cross-Reactive T Cells in COVID-19. Biomedicines 2024, 12, 564. https://doi.org/10.3390/biomedicines12030564
Eggenhuizen PJ, Ooi JD. The Influence of Cross-Reactive T Cells in COVID-19. Biomedicines. 2024; 12(3):564. https://doi.org/10.3390/biomedicines12030564
Chicago/Turabian StyleEggenhuizen, Peter J., and Joshua D. Ooi. 2024. "The Influence of Cross-Reactive T Cells in COVID-19" Biomedicines 12, no. 3: 564. https://doi.org/10.3390/biomedicines12030564
APA StyleEggenhuizen, P. J., & Ooi, J. D. (2024). The Influence of Cross-Reactive T Cells in COVID-19. Biomedicines, 12(3), 564. https://doi.org/10.3390/biomedicines12030564






