Role of Insulin-like Growth Factor-1 Receptor in Tobacco Smoking-Associated Lung Cancer Development
Abstract
1. Introduction
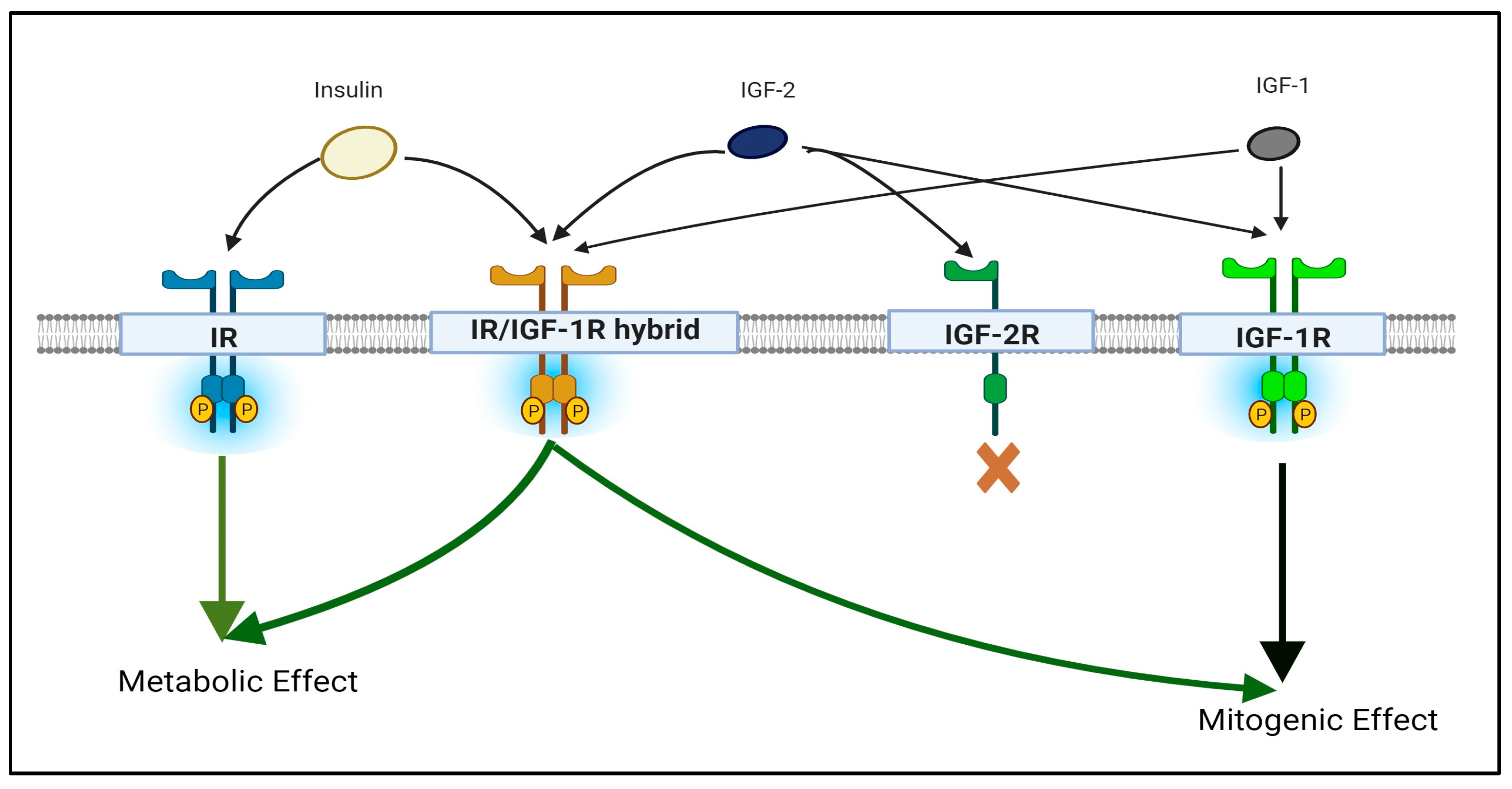
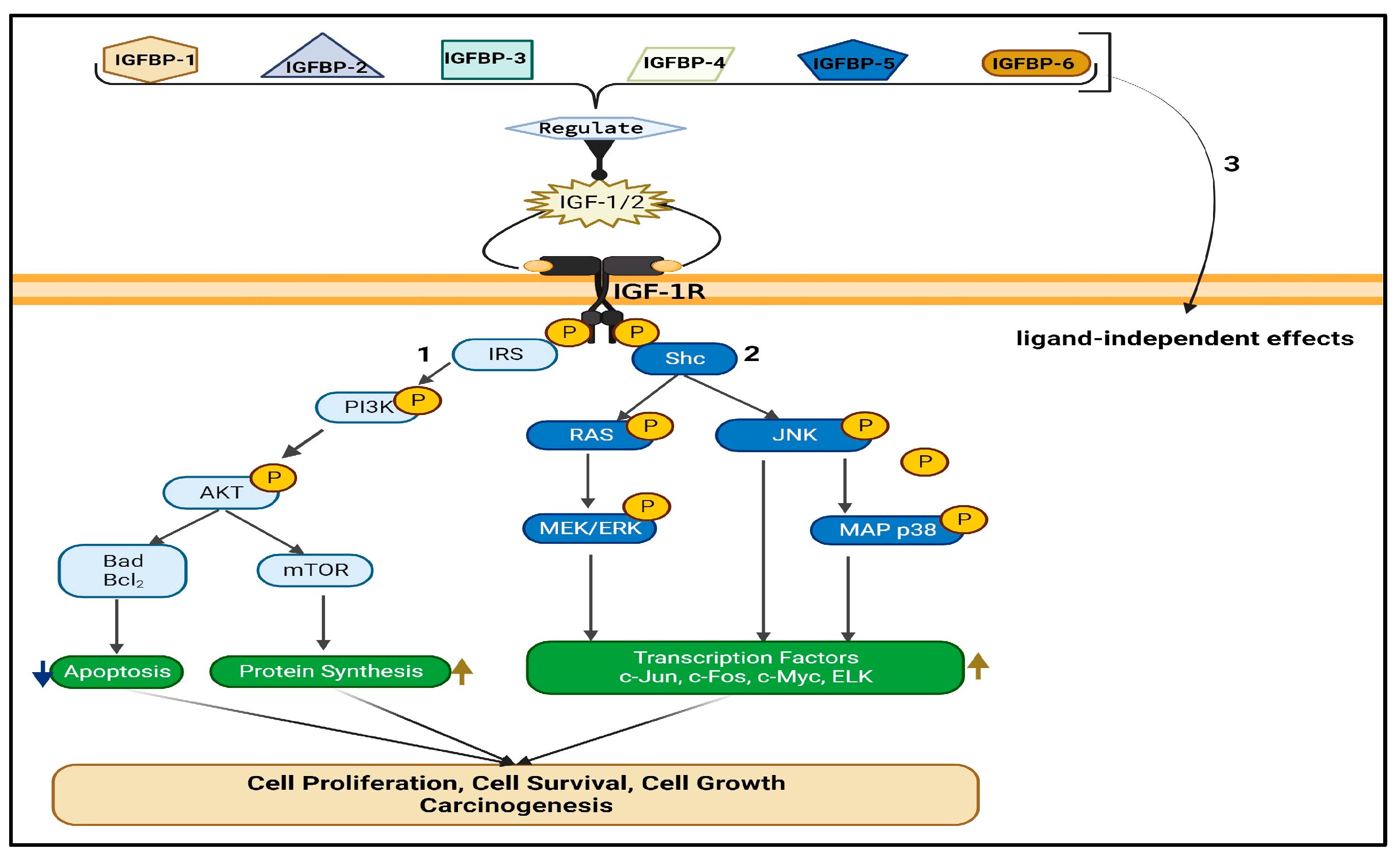
2. What Is the IGF/IGF-R Signaling Axis?
3. Structure and Functions of IGF-1R and its Components
4. IGF-1R Overexpression and Activation in Lung Cancer Initiation and Development
5. Implication of IGF-1R Signaling Pathways in Tobacco Smoke-Associated Carcinogenesis
5.1. Overactivation of IGF-1R Increases Proliferation and Metastasis of Lung Cancer
5.2. Overactivation of IGF-1R Promotes Epithelial–Mesenchymal Transition (EMT) and Stemness of Cancer Cells
6. IGF-1R and Anti-Cancer Drug Resistance in Lung Cancer
7. The IGF/IGF-1R Signaling Axis Is a Potential Target for Cancer Therapy
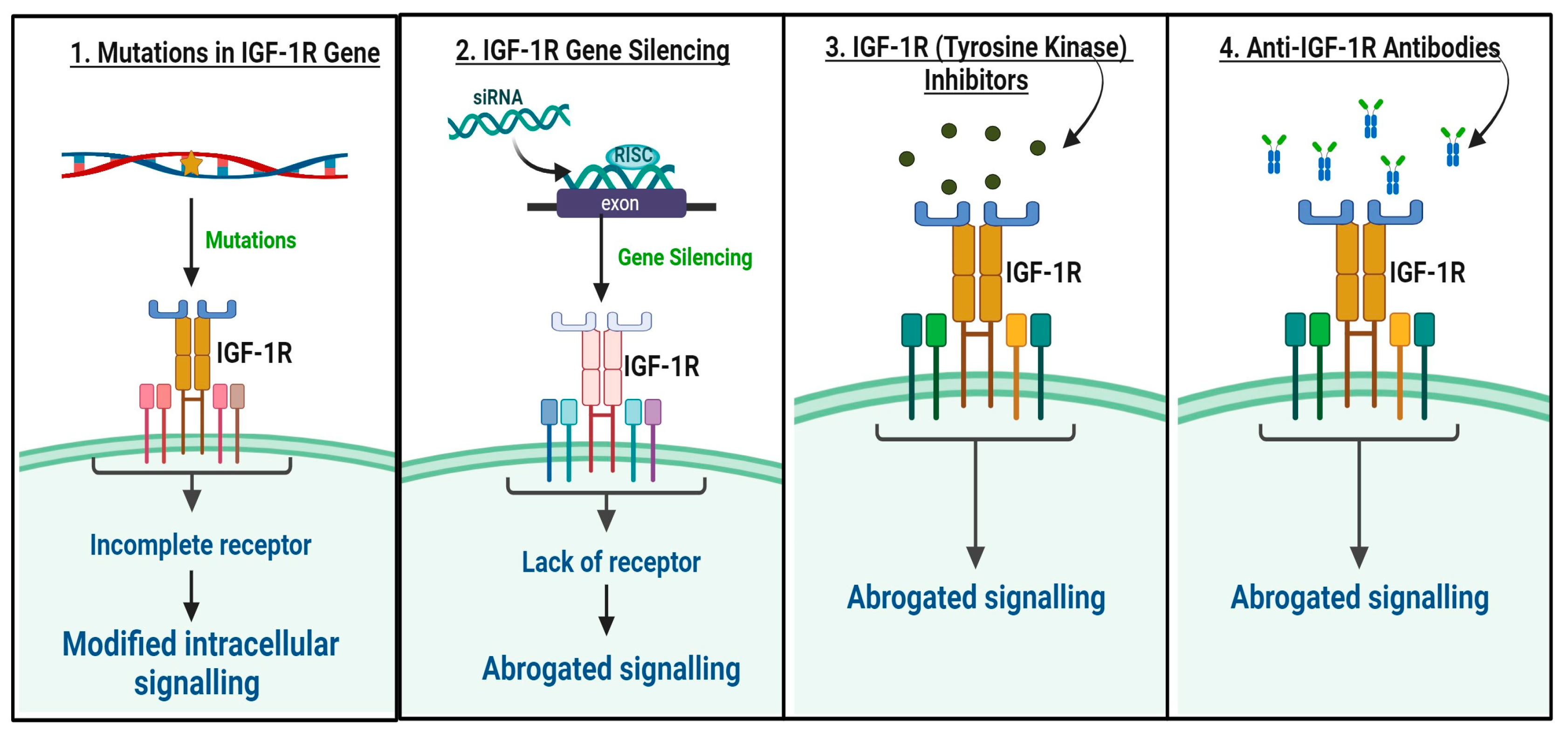
8. Challenges in Targeting the IGF/IGF-1R Signaling Axis
9. Conclusions
10. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wang, W.; Hao, Y.; Liu, Y.; Li, R.; Huang, D.B.; Pan, Y.Y. Nanomedicine in lung cancer: Current states of overcoming drug resistance and improving cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1654. [Google Scholar] [CrossRef]
- Rawal, S.; Patel, M. Bio-Nanocarriers for Lung Cancer Management: Befriending the Barriers. Nanomicro Lett. 2021, 13, 142. [Google Scholar] [CrossRef]
- Walter, J.E.; Heuvelmans, M.A.; de Bock, G.H.; Yousaf-Khan, U.; Groen, H.J.M.; van der Aalst, C.M.; Nackaerts, K.; van Ooijen, P.M.A.; de Koning, H.J.; Vliegenthart, R.; et al. Relationship between the number of new nodules and lung cancer probability in incidence screening rounds of CT lung cancer screening: The NELSON study. Lung Cancer 2018, 125, 103–108. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Lin, C.C.; Suen, K.M.; Lidster, J.; Ladbury, J.E. The emerging role of receptor tyrosine kinase phase separation in cancer. Trends. Cell Biol. 2023. [Google Scholar] [CrossRef]
- Girnita, L.; Worrall, C.; Takahashi, S.; Seregard, S.; Girnita, A. Something old, something new and something borrowed: Emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol. Life Sci. 2014, 71, 2403–2427. [Google Scholar] [CrossRef] [PubMed]
- Larsson, O.; Girnita, A.; Girnita, L. Role of Insulin-like Growth Factor 1 Receptor signalling in cancer. Br. J. Cancer 2005, 92, 2097–2101. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yin, Z.; Tao, K.; Wang, G.; Gao, J. Function of Insulin-like Growth Factor 1 Receptor in cancer resistance to chemotherapy. Oncol. Lett. 2018, 15, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Moschos, S.J.; Mantzoros, C.S. The role of the IGF system in cancer: From basic to clinical studies and clinical applications. Oncology 2002, 63, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, A. Targeting the insulin-like growth factor-1 receptor in human cancer. Front. Pharmacol. 2013, 4, 30. [Google Scholar] [CrossRef]
- Li, R.; Pourpak, A.; Morris, S.W. Inhibition of the insulin-like growth factor-1 receptor (IGF1R) tyrosine kinase as a novel cancer therapy approach. J. Med. Chem. 2009, 52, 4981–5004. [Google Scholar] [CrossRef] [PubMed]
- Ekyalongo, R.C.; Yee, D. Revisiting the IGF-1R as a breast cancer target. NPJ Precis Oncol. 2017, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A.; Varewijck, A.J. IGF-IR Targeted Therapy: Past, Present and Future. Front. Endocrinol. 2014, 5, 224. [Google Scholar] [CrossRef]
- Warren, G.W.; Alberg, A.J.; Kraft, A.S.; Cummings, K.M. The 2014 Surgeon General’s report: "The health consequences of smoking--50 years of progress": A paradigm shift in cancer care. Cancer 2014, 120, 1914–1916. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Progress and challenges in selected areas of tobacco carcinogenesis. Chem. Res. Toxicol. 2008, 21, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Reducing Tobacco-Related Cancer Incidence and Mortality: Workshop Summary; National Academies Press: Washington, DC, USA, 2013.
- Available online: https://www.cancer.org/cancer/risk-prevention/tobacco/carcinogens-found-in-tobacco-products.html (accessed on 28 October 2020).
- Hecht, S.S. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999, 91, 1194–1210. [Google Scholar] [CrossRef]
- Li, Y.; Hecht, S.S. Carcinogenic components of tobacco and tobacco smoke: A 2022 update. Food Chem. Toxicol. 2022, 165, 113179. [Google Scholar] [CrossRef] [PubMed]
- Witsch, E.; Sela, M.; Yarden, Y. Roles for growth factors in cancer progression. Physiology 2010, 25, 85–101. [Google Scholar] [CrossRef]
- Pillai, R.N.; Ramalingam, S.S. Inhibition of insulin-like growth factor receptor: End of a targeted therapy? Transl. Lung Cancer Res. 2013, 2, 14–22. [Google Scholar] [CrossRef]
- Velcheti, V.; Govindan, R. Insulin-like growth factor and lung cancer. J. Thorac. Oncol. 2006, 1, 607–610. [Google Scholar]
- Le Roith, D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N. Engl. J. Med. 1997, 336, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Daughaday, W.H.; Kapadia, M.; Yanow, C.E.; Fabrick, K.; Mariz, I.K. Insulin-like growth factors I and II of nonmammalian sera. Gen. Comp. Endocrinol. 1985, 59, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Rotwein, P. Insulinlike Growth Factor 1 Gene Variation in Vertebrates. Endocrinology 2018, 159, 2288–2305. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, K.; Wallenius, K.; Liu, J.L.; Bohlooly, Y.M.; Pacini, G.; Svensson, L.; Tornell, J.; Isaksson, O.G.; Ahren, B.; Jansson, J.O.; et al. Liver-derived IGF-I is of importance for normal carbohydrate and lipid metabolism. Diabetes 2001, 50, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Talia, C.; Connolly, L.; Fowler, P.A. The insulin-like growth factor system: A target for endocrine disruptors? Environ. Int. 2021, 147, 106311. [Google Scholar] [CrossRef]
- Itoh, K.; Balch, C.M.; Platsoucas, C.D. Monocyte-independent interleukin-2 production and proliferation of human T cells in response to murine hybridomas expressing the OKT3 monoclonal antibody: Interleukin-1 is not required for T-cell proliferation. Cell Immunol. 1988, 115, 36–56. [Google Scholar] [CrossRef]
- Wood, A.W.; Duan, C.; Bern, H.A. Insulin-like growth factor signaling in fish. Int. Rev. Cytol. 2005, 243, 215–285. [Google Scholar] [CrossRef]
- Rotwein, P. The insulin-like growth factor 2 gene and locus in nonmammalian vertebrates: Organizational simplicity with duplication but limited divergence in fish. J. Biol. Chem. 2018, 293, 15912–15932. [Google Scholar] [CrossRef]
- Randhawa, R.; Cohen, P. The role of the insulin-like growth factor system in prenatal growth. Mol. Genet. Metab. 2005, 86, 84–90. [Google Scholar] [CrossRef]
- Adams, T.E.; Epa, V.C.; Garrett, T.P.; Ward, C.W. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol. Life Sci. 2000, 57, 1050–1093. [Google Scholar] [CrossRef]
- Cao, J.; Yee, D. Disrupting Insulin and IGF Receptor Function in Cancer. Int. J. Mol. Sci. 2021, 22, 555. [Google Scholar] [CrossRef]
- Al-Samerria, S.; Radovick, S. The Role of Insulin-like Growth Factor-1 (IGF-1) in the Control of Neuroendocrine Regulation of Growth. Cells 2021, 10, 2664. [Google Scholar] [CrossRef]
- Lou, M.; Garrett, T.P.; McKern, N.M.; Hoyne, P.A.; Epa, V.C.; Bentley, J.D.; Lovrecz, G.O.; Cosgrove, L.J.; Frenkel, M.J.; Ward, C.W. The first three domains of the insulin receptor differ structurally from the Insulin-like Growth Factor 1 Receptor in the regions governing ligand specificity. Proc. Natl. Acad. Sci. USA 2006, 103, 12429–12434. [Google Scholar] [CrossRef]
- Hall, C.; Yu, H.; Choi, E. Insulin receptor endocytosis in the pathophysiology of insulin resistance. Exp. Mol. Med. 2020, 52, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef]
- Choi, E.; Bai, X.C. The Activation Mechanism of the Insulin Receptor: A Structural Perspective. Annu. Rev. Biochem. 2023, 92, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Turvey, S.J.; McPhillie, M.J.; Kearney, M.T.; Muench, S.P.; Simmons, K.J.; Fishwick, C.W.G. Recent developments in the structural characterisation of the IR and IGF1R: Implications for the design of IR-IGF1R hybrid receptor modulators. RSC Med. Chem. 2022, 13, 360–374. [Google Scholar] [CrossRef]
- Iams, W.T.; Lovly, C.M. Molecular Pathways: Clinical Applications and Future Direction of Insulin-like Growth Factor-1 Receptor Pathway Blockade. Clin. Cancer Res. 2015, 21, 4270–4277. [Google Scholar] [CrossRef] [PubMed]
- Leffler, M.; Puusepp, S.; Zilina, O.; Zhu, Y.; Kuuse, K.; Bain, N.; Burgess, T.; Ounap, K.; Field, M. Two familial microduplications of 15q26.3 causing overgrowth and variable intellectual disability with normal copy number of IGF1R. Eur. J. Med. Genet. 2016, 59, 257–262. [Google Scholar] [CrossRef]
- Hebert, E. Mannose-6-phosphate/insulin-like growth factor II receptor expression and tumor development. Biosci. Rep. 2006, 26, 7–17. [Google Scholar] [CrossRef]
- Torrente, Y.; Bella, P.; Tripodi, L.; Villa, C.; Farini, A. Role of Insulin-Like Growth Factor Receptor 2 across Muscle Homeostasis: Implications for Treating Muscular Dystrophy. Cells 2020, 9, 441. [Google Scholar] [CrossRef]
- Dziadziuszko, R.; Camidge, D.R.; Hirsch, F.R. The insulin-like growth factor pathway in lung cancer. J. Thorac. Oncol. 2008, 3, 815–818. [Google Scholar] [CrossRef]
- Haywood, N.J.; Slater, T.A.; Matthews, C.J.; Wheatcroft, S.B. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol. Metab. 2019, 19, 86–96. [Google Scholar] [CrossRef]
- Clemmons, D.R. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol. Cell Endocrinol. 1998, 140, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, C.; Borai, A. Insulin-like growth factor-II: Its role in metabolic and endocrine disease. Clin. Endocrinol. 2014, 80, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, S.E.; Willett, W.C.; Colditz, G.A.; Hunter, D.J.; Michaud, D.S.; Deroo, B.; Rosner, B.; Speizer, F.E.; Pollak, M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998, 351, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Forbes, B.E.; Blyth, A.J.; Wit, J.M. Disorders of IGFs and IGF-1R signaling pathways. Mol. Cell Endocrinol. 2020, 518, 111035. [Google Scholar] [CrossRef] [PubMed]
- Baserga, R.; Resnicoff, M.; Dews, M. The IGF-I receptor and cancer. Endocrine 1997, 7, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Baserga, R.; Peruzzi, F.; Reiss, K. The IGF-1 receptor in cancer biology. Int. J. Cancer 2003, 107, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qiu, Z.; He, J.; Li, L.; Li, W. Insulin-like growth factor receptor 1 (IGF1R) expression and survival in non-small cell lung cancer patients: A meta-analysis. Int. J. Clin. Exp. Pathol. 2014, 7, 6694–6704. [Google Scholar] [PubMed]
- Garcia de la Serrana, D.; Macqueen, D.J. Insulin-Like Growth Factor-Binding Proteins of Teleost Fishes. Front. Endocrinol. 2018, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Xu, Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen. Comp. Endocrinol. 2005, 142, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Dozmorov, M.; Oh, Y. IGFBP-3/IGFBP-3 Receptor System as an Anti-Tumor and Anti-Metastatic Signaling in Cancer. Cells 2020, 9, 1261. [Google Scholar] [CrossRef]
- Yu, H.; Spitz, M.R.; Mistry, J.; Gu, J.; Hong, W.K.; Wu, X. Plasma levels of insulin-like growth factor-I and lung cancer risk: A case-control analysis. J. Natl. Cancer Inst. 1999, 91, 151–156. [Google Scholar] [CrossRef]
- London, S.J.; Yuan, J.M.; Travlos, G.S.; Gao, Y.T.; Wilson, R.E.; Ross, R.K.; Yu, M.C. Insulin-like growth factor I, IGF-binding protein 3, and lung cancer risk in a prospective study of men in China. J. Natl. Cancer Inst. 2002, 94, 749–754. [Google Scholar] [CrossRef]
- Buckbinder, L.; Talbott, R.; Velasco-Miguel, S.; Takenaka, I.; Faha, B.; Seizinger, B.R.; Kley, N. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature 1995, 377, 646–649. [Google Scholar] [CrossRef]
- Hanafusa, T.; Shinji, T.; Shiraha, H.; Nouso, K.; Iwasaki, Y.; Yumoto, E.; Ono, T.; Koide, N. Functional promoter upstream p53 regulatory sequence of IGFBP3 that is silenced by tumor specific methylation. BMC Cancer 2005, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; Liu, B.; Bannerman, P.; El-Deiry, W.S.; Cohen, P. IGFBP-3 mediates p53-induced apoptosis during serum starvation. Int. J. Oncol. 2002, 21, 327–335. [Google Scholar] [CrossRef][Green Version]
- Ludwig, R.L.; Bates, S.; Vousden, K.H. Differential activation of target cellular promoters by p53 mutants with impaired apoptotic function. Mol. Cell. Biol. 1996, 16, 4952–4960. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A. P53 and IGFBP-3: Apoptosis and cancer protection. Mol. Genet Metab. 2000, 70, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Kim, M.J.; Moon, H.; Yuan, P.; Kim, J.S.; Woo, J.K.; Zhang, G.; Suh, Y.A.; Feng, L.; Behrens, C.; et al. Differential impacts of insulin-like growth factor-binding protein-3 (IGFBP-3) in epithelial IGF-induced lung cancer development. Endocrinology 2011, 152, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, T.; Cai, R.; Wangpaichitr, M.; Mirsaeidi, M.; Schally, A.V.; Jackson, R.M. Growth Hormone-Releasing Hormone in Lung Physiology and Pulmonary Disease. Cells 2020, 9, 2331. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Blair, L.A.; Salinas, G.D.; Needleman, L.A.; Marshall, J. Insulin-like growth factor-1 modulation of CaV1.3 calcium channels depends on Ca2+ release from IP3-sensitive stores and calcium/calmodulin kinase II phosphorylation of the alpha1 subunit EF hand. J. Neurosci. 2006, 26, 6259–6268. [Google Scholar] [CrossRef]
- Linnerth, N.M.; Baldwin, M.; Campbell, C.; Brown, M.; McGowan, H.; Moorehead, R.A. IGF-II induces CREB phosphorylation and cell survival in human lung cancer cells. Oncogene 2005, 24, 7310–7319. [Google Scholar] [CrossRef]
- Zha, J.; Lackner, M.R. Targeting the insulin-like growth factor receptor-1R pathway for cancer therapy. Clin. Cancer Res. 2010, 16, 2512–2517. [Google Scholar] [CrossRef]
- Bohula, E.A.; Playford, M.P.; Macaulay, V.M. Targeting the type 1 insulin-like growth factor receptor as anti-cancer treatment. Anticancer Drugs 2003, 14, 669–682. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jin, Q.; Oh, S.H.; Kim, E.S.; Yang, Y.J.; Lee, D.H.; Feng, L.; Behrens, C.; Prudkin, L.; Miller, Y.E.; et al. Elevated epithelial insulin-like growth factor expression is a risk factor for lung cancer development. Cancer Res. 2009, 69, 7439–7448. [Google Scholar] [CrossRef]
- Moorehead, R.A.; Sanchez, O.H.; Baldwin, R.M.; Khokha, R. Transgenic overexpression of IGF-II induces spontaneous lung tumors: A model for human lung adenocarcinoma. Oncogene 2003, 22, 853–857. [Google Scholar] [CrossRef][Green Version]
- Rajski, M.; Zanetti-Dallenbach, R.; Vogel, B.; Herrmann, R.; Rochlitz, C.; Buess, M. IGF-I induced genes in stromal fibroblasts predict the clinical outcome of breast and lung cancer patients. BMC Med. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xiao, F.; Li, B.; Xie, B.; Zhou, J.; Zheng, J.; Zhang, W. The role of epithelial-mesenchymal transition and IGF-1R expression in prediction of gefitinib activity as the second-line treatment for advanced nonsmall-cell lung cancer. Cancer Investig. 2013, 31, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Bielen, A.; Perryman, L.; Box, G.M.; Valenti, M.; de Haven Brandon, A.; Martins, V.; Jury, A.; Popov, S.; Gowan, S.; Jeay, S.; et al. Enhanced efficacy of IGF1R inhibition in pediatric glioblastoma by combinatorial targeting of PDGFRalpha/beta. Mol. Cancer Ther. 2011, 10, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Bhatavdekar, J.M.; Patel, D.D.; Shah, N.G.; Karelia, N.H.; Vora, H.H.; Ghosh, N.; Suthar, T.P.; Balar, D.B. Prognostic value of insulin-like growth factor-1 receptors in patients with colon/rectal cancer: Correlation with plasma prolactin. Eur. J. Surg. Oncol. 1995, 21, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Mountzios, G.; Kostopoulos, I.; Kotoula, V.; Sfakianaki, I.; Fountzilas, E.; Markou, K.; Karasmanis, I.; Leva, S.; Angouridakis, N.; Vlachtsis, K.; et al. Insulin-like Growth Factor 1 Receptor (IGF1R) expression and survival in operable squamous-cell laryngeal cancer. PLoS ONE 2013, 8, e54048. [Google Scholar] [CrossRef]
- Ozkan, E.E. Plasma and tissue insulin-like growth factor-I receptor (IGF-IR) as a prognostic marker for prostate cancer and anti-IGF-IR agents as novel therapeutic strategy for refractory cases: A review. Mol. Cell Endocrinol. 2011, 344, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Obr, A.E.; Kumar, S.; Chang, Y.J.; Bulatowicz, J.J.; Barnes, B.J.; Birge, R.B.; Lazzarino, D.A.; Gallagher, E.; LeRoith, D.; Wood, T.L. Insulin-like growth factor receptor signaling in breast tumor epithelium protects cells from endoplasmic reticulum stress and regulates the tumor microenvironment. Breast. Cancer Res. 2018, 20, 138. [Google Scholar] [CrossRef]
- Yan, S.; Jiao, X.; Li, K.; Li, W.; Zou, H. The impact of IGF-1R expression on the outcomes of patients with breast cancer: A meta-analysis. OncoTargets Ther. 2015, 8, 279–287. [Google Scholar] [CrossRef]
- Xu, J.; Bie, F.; Wang, Y.; Chen, X.; Yan, T.; Du, J. Prognostic value of IGF-1R in lung cancer: A PRISMA-compliant meta-analysis. Medicine 2019, 98, e15467. [Google Scholar] [CrossRef]
- Badzio, A.; Wynes, M.W.; Dziadziuszko, R.; Merrick, D.T.; Pardo, M.; Rzyman, W.; Kowalczyk, A.; Singh, S.; Ranger-Moore, J.; Manriquez, G.; et al. Increased Insulin-like Growth Factor 1 Receptor protein expression and gene copy number in small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1905–1911. [Google Scholar] [CrossRef]
- Boo, H.J.; Min, H.Y.; Hwang, S.J.; Lee, H.J.; Lee, J.W.; Oh, S.R.; Park, C.S.; Park, J.S.; Lee, Y.M.; Lee, H.Y. The tobacco-specific carcinogen NNK induces pulmonary tumorigenesis via nAChR/Src/STAT3-mediated activation of the renin-angiotensin system and IGF-1R signaling. Exp. Mol. Med. 2023, 55, 1131–1144. [Google Scholar] [CrossRef]
- Min, H.Y.; Boo, H.J.; Lee, H.J.; Jang, H.J.; Yun, H.J.; Hwang, S.J.; Smith, J.K.; Lee, H.J.; Lee, H.Y. Smoking-associated lung cancer prevention by blockade of the beta-adrenergic receptor-mediated insulin-like growth factor receptor activation. Oncotarget 2016, 7, 70936–70947. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.J.; Min, H.Y.; Jang, H.J.; Yun, H.J.; Smith, J.K.; Jin, Q.; Lee, H.J.; Liu, D.; Kweon, H.S.; Behrens, C.; et al. The tobacco-specific carcinogen-operated calcium channel promotes lung tumorigenesis via IGF2 exocytosis in lung epithelial cells. Nat. Commun. 2016, 7, 12961. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev. 2006, 17, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Peruzzi, F.; Prisco, M.; Dews, M.; Salomoni, P.; Grassilli, E.; Romano, G.; Calabretta, B.; Baserga, R. Multiple signaling pathways of the Insulin-like Growth Factor 1 Receptor in protection from apoptosis. Mol. Cell Biol. 1999, 19, 7203–7215. [Google Scholar] [CrossRef] [PubMed]
- Ravid, D.; Maor, S.; Werner, H.; Liscovitch, M. Caveolin-1 inhibits cell detachment-induced p53 activation and anoikis by upregulation of insulin-like growth factor-I receptors and signaling. Oncogene 2005, 24, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Uttamsingh, S.; Zong, C.S.; Wang, L.H. Matrix-independent activation of phosphatidylinositol 3-kinase, Stat3, and cyclin A-associated Cdk2 Is essential for anchorage-independent growth of v-Ros-transformed chicken embryo fibroblasts. J. Biol. Chem. 2003, 278, 18798–18810. [Google Scholar] [CrossRef]
- Zhang, W.; Zong, C.S.; Hermanto, U.; Lopez-Bergami, P.; Ronai, Z.; Wang, L.H. RACK1 recruits STAT3 specifically to insulin and Insulin-like Growth Factor 1 Receptor for activation, which is important for regulating anchorage-independent growth. Mol. Cell Biol. 2006, 26, 413–424. [Google Scholar] [CrossRef]
- Alfaro-Arnedo, E.; Lopez, I.P.; Pineiro-Hermida, S.; Canalejo, M.; Gotera, C.; Sola, J.J.; Roncero, A.; Peces-Barba, G.; Ruiz-Martinez, C.; Pichel, J.G. IGF1R acts as a cancer-promoting factor in the tumor microenvironment facilitating lung metastasis implantation and progression. Oncogene 2022, 41, 3625–3639. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Hu, Z.L.; Liao, T.Y.; Li, Y.; Pan, Y.L. LncRNA SND1-IT1 facilitates TGF-beta1-induced epithelial-to-mesenchymal transition via miR-124/COL4A1 axis in gastric cancer. Cell. Death Discov. 2022, 8, 73. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Roche, J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Zeng, Y.; Zhang, X.; Hu, Q.; Zheng, J.; Chen, B.; Xie, B.; Zhang, W.M. Implication of epithelial-mesenchymal transition in IGF1R-induced resistance to EGFR-TKIs in advanced non-small cell lung cancer. Oncotarget 2015, 6, 44332–44345. [Google Scholar] [CrossRef]
- Li, H.; Xu, L.; Zhao, L.; Ma, Y.; Zhu, Z.; Liu, Y.; Qu, X. Insulin-like growth factor-I induces epithelial to mesenchymal transition via GSK-3beta and ZEB2 in the BGC-823 gastric cancer cell line. Oncol. Lett. 2015, 9, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.R.; Zhau, H.E.; Odero-Marah, V.A.; Osunkoya, A.O.; Kimbro, K.S.; Tighiouart, M.; Liu, T.; Simons, J.W.; O’Regan, R.M. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008, 68, 2479–2488. [Google Scholar] [CrossRef]
- Li, H.; Batth, I.S.; Qu, X.; Xu, L.; Song, N.; Wang, R.; Liu, Y. IGF-IR signaling in epithelial to mesenchymal transition and targeting IGF-IR therapy: Overview and new insights. Mol. Cancer 2017, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Cevenini, A.; Orru, S.; Mancini, A.; Alfieri, A.; Buono, P.; Imperlini, E. Molecular Signatures of the Insulin-like Growth Factor 1-mediated Epithelial-Mesenchymal Transition in Breast, Lung and Gastric Cancers. Int. J. Mol. Sci. 2018, 19, 2411. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Yin, J.; Zhang, J.; Jiang, Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: A challenge for cancer therapy. J. Hematol. Oncol. 2020, 13, 64. [Google Scholar] [CrossRef]
- Sivakumar, R.; Koga, H.; Selvendiran, K.; Maeyama, M.; Ueno, T.; Sata, M. Autocrine loop for IGF-I receptor signaling in SLUG-mediated epithelial-mesenchymal transition. Int. J. Oncol. 2009, 34, 329–338. [Google Scholar]
- Kim, H.J.; Litzenburger, B.C.; Cui, X.; Delgado, D.A.; Grabiner, B.C.; Lin, X.; Lewis, M.T.; Gottardis, M.M.; Wong, T.W.; Attar, R.M.; et al. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol. Cell Biol. 2007, 27, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Taliaferro-Smith, L.; Oberlick, E.; Liu, T.; McGlothen, T.; Alcaide, T.; Tobin, R.; Donnelly, S.; Commander, R.; Kline, E.; Nagaraju, G.P.; et al. FAK activation is required for IGF1R-mediated regulation of EMT, migration, and invasion in mesenchymal triple negative breast cancer cells. Oncotarget 2015, 6, 4757–4772. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Shi, W.J.; Gao, J.B. Molecular mechanisms of chemoresistance in gastric cancer. World J. Gastrointest Oncol. 2016, 8, 673–681. [Google Scholar] [CrossRef]
- Nwabo Kamdje, A.H.; Seke Etet, P.F.; Kipanyula, M.J.; Vecchio, L.; Tagne Simo, R.; Njamnshi, A.K.; Lukong, K.E.; Mimche, P.N. Insulin-like growth factor-1 signaling in the tumor microenvironment: Carcinogenesis, cancer drug resistance, and therapeutic potential. Front. Endocrinol. 2022, 13, 927390. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, S.; Torossian, A.; Speirs, C.K.; Schleicher, S.; Giacalone, N.J.; Carbone, D.P.; Zhao, Z.; Lu, B. Role of insulin-like growth factor-1 signaling pathway in cisplatin-resistant lung cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e563–e572. [Google Scholar] [CrossRef]
- Ibanez de Caceres, I.; Cortes-Sempere, M.; Moratilla, C.; Machado-Pinilla, R.; Rodriguez-Fanjul, V.; Manguan-Garcia, C.; Cejas, P.; Lopez-Rios, F.; Paz-Ares, L.; de CastroCarpeno, J.; et al. IGFBP-3 hypermethylation-derived deficiency mediates cisplatin resistance in non-small-cell lung cancer. Oncogene 2010, 29, 1681–1690. [Google Scholar] [CrossRef]
- Pao, W.; Miller, V.; Zakowski, M.; Doherty, J.; Politi, K.; Sarkaria, I.; Singh, B.; Heelan, R.; Rusch, V.; Fulton, L.; et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. USA 2004, 101, 13306–13311. [Google Scholar] [CrossRef]
- Murakami, A.; Takahashi, F.; Nurwidya, F.; Kobayashi, I.; Minakata, K.; Hashimoto, M.; Nara, T.; Kato, M.; Tajima, K.; Shimada, N.; et al. Hypoxia increases gefitinib-resistant lung cancer stem cells through the activation of Insulin-like Growth Factor 1 Receptor. PLoS ONE 2014, 9, e86459. [Google Scholar] [CrossRef]
- Hussmann, D.; Madsen, A.T.; Jakobsen, K.R.; Luo, Y.; Sorensen, B.S.; Nielsen, A.L. IGF1R depletion facilitates MET-amplification as mechanism of acquired resistance to erlotinib in HCC827 NSCLC cells. Oncotarget 2017, 8, 33300–33315. [Google Scholar] [CrossRef] [PubMed]
- Hurbin, A.; Wislez, M.; Busser, B.; Antoine, M.; Tenaud, C.; Rabbe, N.; Dufort, S.; de Fraipont, F.; Moro-Sibilot, D.; Cadranel, J.; et al. Insulin-like growth factor-1 receptor inhibition overcomes gefitinib resistance in mucinous lung adenocarcinoma. J. Pathol. 2011, 225, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Wang, Y.; James, M.; Jeong, J.H.; You, M. Inhibition of IGF1R signaling abrogates resistance to afatinib (BIBW2992) in EGFR T790M mutant lung cancer cells. Mol. Carcinog. 2016, 55, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Manabe, T.; Yasuda, H.; Terai, H.; Kagiwada, H.; Hamamoto, J.; Ebisudani, T.; Kobayashi, K.; Masuzawa, K.; Ikemura, S.; Kawada, I.; et al. IGF2 Autocrine-Mediated IGF1R Activation Is a Clinically Relevant Mechanism of Osimertinib Resistance in Lung Cancer. Mol. Cancer Res. 2020, 18, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Lovly, C.M.; McDonald, N.T.; Chen, H.; Ortiz-Cuaran, S.; Heukamp, L.C.; Yan, Y.; Florin, A.; Ozretic, L.; Lim, D.; Wang, L.; et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat. Med. 2014, 20, 1027–1034. [Google Scholar] [CrossRef]
- Isoyama, S.; Dan, S.; Nishimura, Y.; Nakamura, N.; Kajiwara, G.; Seki, M.; Irimura, T.; Yamori, T. Establishment of phosphatidylinositol 3-kinase inhibitor-resistant cancer cell lines and therapeutic strategies for overcoming the resistance. Cancer Sci. 2012, 103, 1955–1960. [Google Scholar] [CrossRef]
- Langer, C.J.; Novello, S.; Park, K.; Krzakowski, M.; Karp, D.D.; Mok, T.; Benner, R.J.; Scranton, J.R.; Olszanski, A.J.; Jassem, J. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2014, 32, 2059–2066. [Google Scholar] [CrossRef]
- Yeh, J.; Litz, J.; Hauck, P.; Ludwig, D.L.; Krystal, G.W. Selective inhibition of SCLC growth by the A12 anti-IGF-1R monoclonal antibody correlates with inhibition of Akt. Lung Cancer 2008, 60, 166–174. [Google Scholar] [CrossRef]
- Gong, Y.; Yao, E.; Shen, R.; Goel, A.; Arcila, M.; Teruya-Feldstein, J.; Zakowski, M.F.; Frankel, S.; Peifer, M.; Thomas, R.K.; et al. High expression levels of total IGF-1R and sensitivity of NSCLC cells in vitro to an anti-IGF-1R antibody (R1507). PLoS ONE 2009, 4, e7273. [Google Scholar] [CrossRef]
- Liang, S.; Galluzzo, P.; Sobol, A.; Skucha, S.; Rambo, B.; Bocchetta, M. Multimodality Approaches to Treat Hypoxic Non-Small Cell Lung Cancer (NSCLC) Microenvironment. Genes Cancer 2012, 3, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Okamoto, I.; Suzuki, M.; Hatashita, E.; Yamada, Y.; Fukuoka, M.; Ono, K.; Nakagawa, K. Inhibition of Insulin-like Growth Factor 1 Receptor by CP-751,871 radiosensitizes non-small cell lung cancer cells. Clin. Cancer Res. 2009, 15, 5117–5125. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, M.J.; Cooke, A.; Rosenfeld-Franklin, M.; Buck, E.; Foreman, K.; Landfair, D.; O’Connor, M.; Pirritt, C.; Sun, Y.; Yao, Y.; et al. Discovery of OSI-906: A selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med. Chem. 2009, 1, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- McKinley, E.T.; Bugaj, J.E.; Zhao, P.; Guleryuz, S.; Mantis, C.; Gokhale, P.C.; Wild, R.; Manning, H.C. 18FDG-PET predicts pharmacodynamic response to OSI-906, a dual IGF-1R/IR inhibitor, in preclinical mouse models of lung cancer. Clin. Cancer Res. 2011, 17, 3332–3340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Pan, J.; Lubet, R.A.; Wang, Y.; You, M. Targeting the insulin-like growth factor-1 receptor by picropodophyllin for lung cancer chemoprevention. Mol. Carcinog. 2015, 54 (Suppl. 1), E129–E137. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, L.B.L.; Lima, K.; Coelho-Silva, J.L.; Traina, F.; Kobayashi, S.S.; Machado-Neto, J.A. NT157 exerts antineoplastic activity by targeting JNK and AXL signaling in lung cancer cells. Sci. Rep. 2022, 12, 17092. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.E.; Jones, R.A.; Briah, R.; Murray, P.; Moorehead, R.A. BMS-754807 is cytotoxic to non-small cell lung cancer cells and enhances the effects of platinum chemotherapeutics in the human lung cancer cell line A549. BMC Res. Notes 2016, 9, 134. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Wang, Z.; Gong, T.; Zhao, H.; Zhang, D.; Niu, Y.; Li, X.; Zhao, X.; Li, G.; et al. Dasatinib in combination with BMS-754807 induce synergistic cytotoxicity in lung cancer cells through inhibiting lung cancer cell growth, and inducing autophagy as well as cell cycle arrest at the G1 phase. Investig. New. Drugs 2023, 41, 438–452. [Google Scholar] [CrossRef]
- Friedbichler, K.; Hofmann, M.H.; Kroez, M.; Ostermann, E.; Lamche, H.R.; Koessl, C.; Borges, E.; Pollak, M.N.; Adolf, G.; Adam, P.J. Pharmacodynamic and antineoplastic activity of BI 836845, a fully human IGF ligand-neutralizing antibody, and mechanistic rationale for combination with rapamycin. Mol. Cancer Ther. 2014, 13, 399–409. [Google Scholar] [CrossRef]
- Gao, J.; Chesebrough, J.W.; Cartlidge, S.A.; Ricketts, S.A.; Incognito, L.; Veldman-Jones, M.; Blakey, D.C.; Tabrizi, M.; Jallal, B.; Trail, P.A.; et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011, 71, 1029–1040. [Google Scholar] [CrossRef]
- Lee, Y.J.; Imsumran, A.; Park, M.Y.; Kwon, S.Y.; Yoon, H.I.; Lee, J.H.; Yoo, C.G.; Kim, Y.W.; Han, S.K.; Shim, Y.S.; et al. Adenovirus expressing shRNA to IGF-1R enhances the chemosensitivity of lung cancer cell lines by blocking IGF-1 pathway. Lung Cancer 2007, 55, 279–286. [Google Scholar] [CrossRef]
- Akla, B.; Broussas, M.; Loukili, N.; Robert, A.; Beau-Larvor, C.; Malissard, M.; Boute, N.; Champion, T.; Haeuw, J.F.; Beck, A.; et al. Efficacy of the Antibody-Drug Conjugate W0101 in Preclinical Models of IGF-1 Receptor Overexpressing Solid Tumors. Mol. Cancer Ther. 2020, 19, 168–177. [Google Scholar] [CrossRef]
- Weickhardt, A.; Doebele, R.; Oton, A.; Lettieri, J.; Maxson, D.; Reynolds, M.; Brown, A.; Jackson, M.K.; Dy, G.; Adjei, A.; et al. A phase I/II study of erlotinib in combination with the anti-insulin-like growth factor-1 receptor monoclonal antibody IMC-A12 (cixutumumab) in patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 2012, 7, 419–426. [Google Scholar] [CrossRef]
- Argiris, A.; Lee, J.W.; Stevenson, J.; Sulecki, M.G.; Hugec, V.; Choong, N.W.; Saltzman, J.N.; Song, W.; Hansen, R.M.; Evans, T.L.; et al. Phase II randomized trial of carboplatin, paclitaxel, bevacizumab with or without cixutumumab (IMC-A12) in patients with advanced non-squamous, non-small-cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E3508). Ann. Oncol. 2017, 28, 3037–3043. [Google Scholar] [CrossRef]
- Murakami, H.; Doi, T.; Yamamoto, N.; Watanabe, J.; Boku, N.; Fuse, N.; Yoshino, T.; Ohtsu, A.; Otani, S.; Shibayama, K.; et al. Phase 1 study of ganitumab (AMG 479), a fully human monoclonal antibody against the insulin-like growth factor receptor type I (IGF1R), in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2012, 70, 407–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soria, J.C.; Massard, C.; Lazar, V.; Ozoux, M.L.; Mery-Mignard, D.; Deslandes, A.; Tolcher, A.W. A dose finding, safety and pharmacokinetic study of AVE1642, an anti-insulin-like growth factor-1 receptor (IGF-1R/CD221) monoclonal antibody, administered as a single agent and in combination with docetaxel in patients with advanced solid tumours. Eur. J. Cancer 2013, 49, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Spigel, D.R.; Chen, D.; Steins, M.B.; Engelman, J.A.; Schneider, C.P.; Novello, S.; Eberhardt, W.E.; Crino, L.; Habben, K.; et al. Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2011, 29, 4574–4580. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.M.; Shepherd, F.A.; Laurie, S.A.; Goss, G.D.; Olivo, M.; Powers, J.; Seymour, L.; Bradbury, P.A. NCIC CTG IND.190 phase I trial of dalotuzumab (MK-0646) in combination with cisplatin and etoposide in extensive-stage small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.; Felip, E.; Keedy, V.; Borghaei, H.; Shepherd, F.A.; Insa, A.; Brown, H.; Fitzgerald, T.; Sathyanarayanan, S.; Reilly, J.F.; et al. Activity of dalotuzumab, a selective anti-IGF1R antibody, in combination with erlotinib in unselected patients with Non-small-cell lung cancer: A phase I/II randomized trial. Exp. Hematol. Oncol. 2014, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Williamson, S.K.; Neupane, P.; Taylor, S.A.; Allen, A.; Smart, N.J.; Uypeckcuat, A.M.; Spencer, S.; Wick, J.; Smith, H.; et al. Impact Study: MK-0646 (Dalotuzumab), Insulin Growth Factor 1 Receptor Antibody Combined with Pemetrexed and Cisplatin in Stage IV Metastatic Non-squamous Lung Cancer. Front. Oncol. 2015, 5, 301. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.V.; Bondarenko, I.; Blackhall, F.; Barlesi, F.; Hsia, T.C.; Jassem, J.; Milanowski, J.; Popat, S.; Sanchez-Torres, J.M.; Novello, S.; et al. Randomized, phase III trial of figitumumab in combination with erlotinib versus erlotinib alone in patients with nonadenocarcinoma nonsmall-cell lung cancer. Ann. Oncol. 2015, 26, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, M.; Scagliotti, G.V. The lesson learned from figitumumab clinical program and the hope for better results in squamous lung cancer. Transl. Lung Cancer Res. 2015, 4, 15–17. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Britten, C.D.; Pieslor, P.; Saville, W.; Vassos, A.; Harris, S.; Galluppi, G.R.; Darif, M.; Wainberg, Z.A.; Cohen, R.B.; et al. A phase 1, open-label, dose-escalation study of BIIB022 (anti-IGF-1R monoclonal antibody) in subjects with relapsed or refractory solid tumors. Investig. New Drugs 2014, 32, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Chiappori, A.A.; Otterson, G.A.; Dowlati, A.; Traynor, A.M.; Horn, L.; Owonikoko, T.K.; Ross, H.J.; Hann, C.L.; Abu Hejleh, T.; Nieva, J.; et al. A Randomized Phase II Study of Linsitinib (OSI-906) Versus Topotecan in Patients With Relapsed Small-Cell Lung Cancer. Oncologist 2016, 21, 1163–1164. [Google Scholar] [CrossRef]
- Ciuleanu, T.E.; Ahmed, S.; Kim, J.H.; Mezger, J.; Park, K.; Thomas, M.; Chen, J.; Poondru, S.; VanTornout, J.M.; Whitcomb, D.; et al. Randomised Phase 2 study of maintenance linsitinib (OSI-906) in combination with erlotinib compared with placebo plus erlotinib after platinum-based chemotherapy in patients with advanced non-small cell lung cancer. Br. J. Cancer 2017, 117, 757–766. [Google Scholar] [CrossRef]
- Leighl, N.B.; Rizvi, N.A.; de Lima, L.G., Jr.; Arpornwirat, W.; Rudin, C.M.; Chiappori, A.A.; Ahn, M.J.; Chow, L.Q.; Bazhenova, L.; Dechaphunkul, A.; et al. Phase 2 Study of Erlotinib in Combination With Linsitinib (OSI-906) or Placebo in Chemotherapy-Naive Patients With Non-Small-Cell Lung Cancer and Activating Epidermal Growth Factor Receptor Mutations. Clin. Lung Cancer 2017, 18, 34–42.e32. [Google Scholar] [CrossRef]
- Ekman, S.; Harmenberg, J.; Frodin, J.E.; Bergstrom, S.; Wassberg, C.; Eksborg, S.; Larsson, O.; Axelson, M.; Jerling, M.; Abrahmsen, L.; et al. A novel oral insulin-like growth factor-1 receptor pathway modulator and its implications for patients with non-small cell lung carcinoma: A phase I clinical trial. Acta Oncol. 2016, 55, 140–148. [Google Scholar] [CrossRef]
- Park, K.; Tan, D.S.W.; Su, W.C.; Cho, B.C.; Kim, S.W.; Lee, K.H.; Wang, C.C.; Seto, T.; Huang, D.C.; Jung, H.H.; et al. Phase 1b Open-Label Trial of Afatinib Plus Xentuzumab (BI 836845) in Patients With EGFR Mutation-Positive NSCLC After Progression on EGFR Tyrosine Kinase Inhibitors. JTO Clin. Res. Rep. 2021, 2, 100206. [Google Scholar] [CrossRef]
- Haluska, P.; Menefee, M.; Plimack, E.R.; Rosenberg, J.; Northfelt, D.; LaVallee, T.; Shi, L.; Yu, X.Q.; Burke, P.; Huang, J.; et al. Phase I dose-escalation study of MEDI-573, a bispecific, antiligand monoclonal antibody against IGFI and IGFII, in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 4747–4757. [Google Scholar] [CrossRef]
- O’Flanagan, C.H.; O’Shea, S.; Lyons, A.; Fogarty, F.M.; McCabe, N.; Kennedy, R.D.; O’Connor, R. IGF-1R inhibition sensitizes breast cancer cells to ATM-related kinase (ATR) inhibitor and cisplatin. Oncotarget 2016, 7, 56826–56841. [Google Scholar] [CrossRef]
- de Lint, K.; Poell, J.B.; Soueidan, H.; Jastrzebski, K.; Vidal Rodriguez, J.; Lieftink, C.; Wessels, L.F.; Beijersbergen, R.L. Sensitizing Triple-Negative Breast Cancer to PI3K Inhibition by Cotargeting IGF1R. Mol. Cancer Ther. 2016, 15, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Kurmasheva, R.T.; Dudkin, L.; Billups, C.; Debelenko, L.V.; Morton, C.L.; Houghton, P.J. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009, 69, 7662–7671. [Google Scholar] [CrossRef]
- Chakraborty, C.; Doss, C.G.; Bandyopadhyay, S.; Agoramoorthy, G. Influence of miRNA in insulin signaling pathway and insulin resistance: Micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip. Rev. RNA 2014, 5, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Alizadeh, E.; Bernhard, W.; Makhlouf, A.; Hartimath, S.V.; Hill, W.; El-Sayed, A.; Barreto, K.; Geyer, C.R.; Fonge, H. Development and preclinical evaluation of cixutumumab drug conjugates in a model of insulin growth factor receptor I (IGF-1R) positive cancer. Sci. Rep. 2020, 10, 18549. [Google Scholar] [CrossRef]
- Burtrum, D.; Zhu, Z.; Lu, D.; Anderson, D.M.; Prewett, M.; Pereira, D.S.; Bassi, R.; Abdullah, R.; Hooper, A.T.; Koo, H.; et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003, 63, 8912–8921. [Google Scholar]
- Thomson, R.J.; Moshirfar, M.; Ronquillo, Y. Tyrosine Kinase Inhibitors. In StatPearls; StatPearls: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Fuentes-Baile, M.; Ventero, M.P.; Encinar, J.A.; Garcia-Morales, P.; Poveda-Deltell, M.; Perez-Valenciano, E.; Barbera, V.M.; Gallego-Plazas, J.; Rodriguez-Lescure, A.; Martin-Nieto, J.; et al. Differential Effects of IGF-1R Small Molecule Tyrosine Kinase Inhibitors BMS-754807 and OSI-906 on Human Cancer Cell Lines. Cancers 2020, 12, 3717. [Google Scholar] [CrossRef] [PubMed]
- Girnita, A.; Girnita, L.; del Prete, F.; Bartolazzi, A.; Larsson, O.; Axelson, M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004, 64, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Joaquim, A.; Janez, N.M.; Morales, S.; Diaz-Redondo, T.; Blau, S.; Neven, P.; Lemieux, J.; Garcia-Saenz, J.A.; et al. XENERA-1: A randomised double-blind Phase II trial of xentuzumab in combination with everolimus and exemestane versus everolimus and exemestane in patients with hormone receptor-positive/HER2-negative metastatic breast cancer and non-visceral disease. Breast Cancer Res. 2023, 25, 67. [Google Scholar] [CrossRef]
- de Bono, J.; Lin, C.C.; Chen, L.T.; Corral, J.; Michalarea, V.; Rihawi, K.; Ong, M.; Lee, J.H.; Hsu, C.H.; Yang, J.C.; et al. Two first-in-human studies of xentuzumab, a humanised insulin-like growth factor (IGF)-neutralising antibody, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Sharon, E. IGF-1R as an anti-cancer target--trials and tribulations. Chin. J. Cancer 2013, 32, 242–252. [Google Scholar] [CrossRef]
- Crudden, C.; Girnita, A.; Girnita, L. Targeting the IGF-1R: The Tale of the Tortoise and the Hare. Front. Endocrinol. 2015, 6, 64. [Google Scholar] [CrossRef]
- Kim, W.Y.; Prudkin, L.; Feng, L.; Kim, E.S.; Hennessy, B.; Lee, J.S.; Lee, J.J.; Glisson, B.; Lippman, S.M.; Wistuba, I.I.; et al. Epidermal growth factor receptor and K-Ras mutations and resistance of lung cancer to Insulin-like Growth Factor 1 Receptor tyrosine kinase inhibitors. Cancer 2012, 118, 3993–4003. [Google Scholar] [CrossRef] [PubMed]
- Habben, K.; Delmar, P.; Brownstein, C.M.; Koehler, W.; Kuenkele, K.; Splesis, O.; Ramalingam, S.S.; Engelman, J.A.; Chen, D. Investigation of predictive biomarkers for R1507, an anti-IGF1R antibody, in patients with advanced non-small cell lung cancer with progression after first-line chemotherapy. J. Clin. Oncol. 2011, 29. [Google Scholar] [CrossRef]
- Dolgin, E. IGF-1R drugs travel from cancer cradle to Graves’. Nat. Biotechnol. 2020, 38, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Toretsky, J.A.; Gorlick, R. IGF-1R targeted treatment of sarcoma. Lancet Oncol. 2010, 11, 105–106. [Google Scholar] [CrossRef]
- Beckwith, H.; Yee, D. Minireview: Were the IGF Signaling Inhibitors All Bad? Mol. Endocrinol. 2015, 29, 1549–1557. [Google Scholar] [CrossRef]
| Drug Types | Compounds | Lung Cancer Models | Outcomes | References |
|---|---|---|---|---|
| Monoclonal antibody | Cixutumumab (IMC-A12) | SCLC cell lines | Increased sensitivity to chemotherapies, decreased cell growth | [120] |
| R1507 | NSCLC cell lines | The addition of R1507 to erlotinib inhibited cell growth and increased apoptosis | [121] | |
| Dalotuzumab (MK-0646) | NSCLC xenograft tumors in mice | Increased median survival of treated mice compared with controls, increased sensitivity to erlotinib | [122] | |
| Figitumumab (CP-751, 871) | NSCLC cell lines | Increased sensitivity to radiation therapy | [123] | |
| NSCLC xenograft tumors in mice | Addition of CP-751, 871 to radiation therapy delayed tumor growth in vivo | |||
| Tyrosine kinase inhibitor | Linsitinib (OSI-906) | NSCLC cell lines | Inhibited IGF-1/IGF-2-mediated proliferation, increased apoptosis | [124] |
| NSCLC xenograft tumors in mice | Inhibited tumor growth in cells expressing IGF-1R | [125] | ||
| Picropodophyllin | B(a)P-induced lung tumors in mice | Decreased tumor multiplicity and load | [126] | |
| NSCLC cell lines | Decreased cell viability and in vitro invasive capacity | |||
| NT157 | Lung cancer cell lines | Decreased cell viability and oncogene expression, creating a tumor-suppressive signaling network | [127] | |
| BMS-754807 | NSCLC cell lines | Decreased cell survival, increased apoptosis, enhanced cytotoxic effects of platinum chemotherapies | [128] | |
| Lung cancer cell lines | The addition of BMS-754807 to dasatinib inhibited cell growth, and induced autophagy and cell cycle arrest | [129] | ||
| Ligand neutralizing monoclonal antibody | Xentuzumab (BI 836845) | NSCLC and SCLC cell lines | Decreased cell proliferation, enhanced anti-tumor efficacy of rapamycin | [130] |
| Dusigitumab (MEDI-573) | Solid xenograft tumors in mice, including NSCLC | Inhibited IGF signaling pathways in tumors driven by autocrine IGF production | [131] | |
| shRNA-mediated Gene Silencing | shIGF-1R (601, 801 and 3425) | NSCLC cell lines | Increased sensitivity to chemotherapies, decreased cell colony formation | [132] |
| Antibody–drug conjugates | W0101 (IGF-1R antibody-drug conjugate) | Lung cancer cell lines | Potent cytotoxic activity, inhibited tumor growth in cells expressing high levels of IGF-1R | [133] |
| Drug Types | Compounds | Phase | Lung Cancer Types | Outcomes | References |
|---|---|---|---|---|---|
| Monoclonal antibody | Cixutumumab (IMC-A12) | II | NSCLC | The addition of cixutumumab to other therapies increases toxicity without improving efficacy outcomes | [134,135] |
| Ganitumab (AMG-479) | I | Advanced solid tumors | SD > 6 weeks in 7 patients, including 2 with NSCLC | [136] | |
| AVE1642 | I | Advanced solid tumors | SD > 4 months in 11 patients, including 1 with NSCLC | [137] | |
| Teprotumumab (RV 001, R1507) | II | NSCLC | The addition of R1507 to erlotinib did not improve efficacy outcomes | [138] | |
| II | NSCLC | Terminated due to program termination (NCT00760929) | [22,138] | ||
| Dalotuzumab (MK-0646) | I | SCLC | The addition of dalotuzumab to chemotherapies did not improve efficacy outcomes | [139] | |
| II | NSCLC | The addition of dalotuzumab to erlotinib did not improve efficacy outcomes | [140] | ||
| II | Non-squamous lung cancer | The addition of dalotuzumab to chemotherapies did not improve efficacy outcomes | [141] | ||
| Figitumumab (CP-751, 871) | III | NSCLC | The addition of figitumumab to chemotherapies did not improve efficacy outcomes | [119] | |
| III | NSCLC | Discontinued due to futile HR, lack of improved efficacy outcomes, and serious adverse events | [22,142,143] | ||
| BIIB022 | I | Advanced solid tumors | Preliminary evidence of biological activity in select patients; SD > 6 weeks in 20 patients | [144] | |
| Tyrosine kinase inhibitor | Linsitinib (OSI-906) | II | SCLC | Linsitinib did not improve efficacy outcomes | [145] |
| NSCLC | The addition of linsitinib to erlotinib did not improve efficacy outcomes | [146,147] | |||
| Picropodophyllin (AXL 1717) | I | Advanced solid tumors | Median PFS of 31 weeks and OS of 60 weeks in 15 patients with NSCLC | [148] | |
| Ligand neutralizing monoclonal antibody | Xentuzumab (BI 836845) | I | NSCLC | The addition of xentuzumab to afatinib did not substantially improve efficacy outcomes | [149] |
| Dusigitumab (MEDI-573) | I | Advanced solid tumors | Preliminary evidence (from 43 patients, including 1 with NSCLC) warrants further clinical evaluation | [150] |
| Molecule Type | Associated Target(s) | Examples | Outcomes | Intended Anti-Cancer Effects | References |
|---|---|---|---|---|---|
| Monoclonal antibody | Extracellular ligand-binding α-subunit domain of IGF-1R | Cixutumumab (IMC-A12), R1507, Dalotuzumab (MK-0646), Figitumumab (CP-751, 871) | Inhibits ligand binding to receptor and promotes receptor internalization and degradation | Anti-proliferative, anti-growth, anti-metastatic, prevention of EMT transition, pro-apoptotic, increased sensitization to chemotherapy | [121,155,156] |
| Tyrosine kinase inhibitor | ATP pocket of IGF-1R (ATP-competitive) | Linsitinib (OSI-906), BMS-754807, AG1024 | Inhibits auto-phosphorylation of IGF-1R upon ligand binding, preventing recruitment of signaling proteins such as IRS and Shc | Anti-proliferative, anti-growth, anti-metastatic, pro-apoptotic, increased sensitization to chemotherapy | [127,157,158,159] |
| Allosteric site on IGF-1R (non-ATP competitive) | XL228, Picropodophyllin, NT157 | Decreased tumor multiplicity and load | [126] | ||
| Ligand-neutralizing monoclonal antibody | IGF-I/II | Xentuzumab (BI 836845), Dusigitumab (MEDI-573) | Prevents ligand binding to IGF-1R | Anti-proliferative, anti-growth, pro-apoptotic, increased sensitization to chemotherapy | [160,161,162] |
| shRNA-mediated gene silencing | IGF-1R mRNA | shIGF-1R(601, 801 and 3425) | Prevents translation of IGF-1R mRNA, silencing gene expression | Anti-tumorigenic, pro-apoptotic, increased sensitization to chemotherapy | [132] |
| Antibody–drug conjugates | Extracellular domain of IGF-1R | W0101 (IGF-1R antibody-drug conjugate) | Promotes internalization of receptor and conjugated cytotoxic drug in cells expressing high levels of IGF-1R | Anti-mitotic, anti-tumorigenic, cytotoxic | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, A.; Santos, S.G.; Lin, C.; Huang, Y. Role of Insulin-like Growth Factor-1 Receptor in Tobacco Smoking-Associated Lung Cancer Development. Biomedicines 2024, 12, 563. https://doi.org/10.3390/biomedicines12030563
Shahid A, Santos SG, Lin C, Huang Y. Role of Insulin-like Growth Factor-1 Receptor in Tobacco Smoking-Associated Lung Cancer Development. Biomedicines. 2024; 12(3):563. https://doi.org/10.3390/biomedicines12030563
Chicago/Turabian StyleShahid, Ayaz, Shaira Gail Santos, Carol Lin, and Ying Huang. 2024. "Role of Insulin-like Growth Factor-1 Receptor in Tobacco Smoking-Associated Lung Cancer Development" Biomedicines 12, no. 3: 563. https://doi.org/10.3390/biomedicines12030563
APA StyleShahid, A., Santos, S. G., Lin, C., & Huang, Y. (2024). Role of Insulin-like Growth Factor-1 Receptor in Tobacco Smoking-Associated Lung Cancer Development. Biomedicines, 12(3), 563. https://doi.org/10.3390/biomedicines12030563







