Prospective Observational Study of COVID-19 Vaccination in Patients with Thoracic Malignancies: Adverse Events, Breakthrough Infections and Survival Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Recognition of Adverse Events
2.4. Detection of Breakthrough COVID-19 Infection
2.5. Statistical Analysis and Outcomes
3. Results
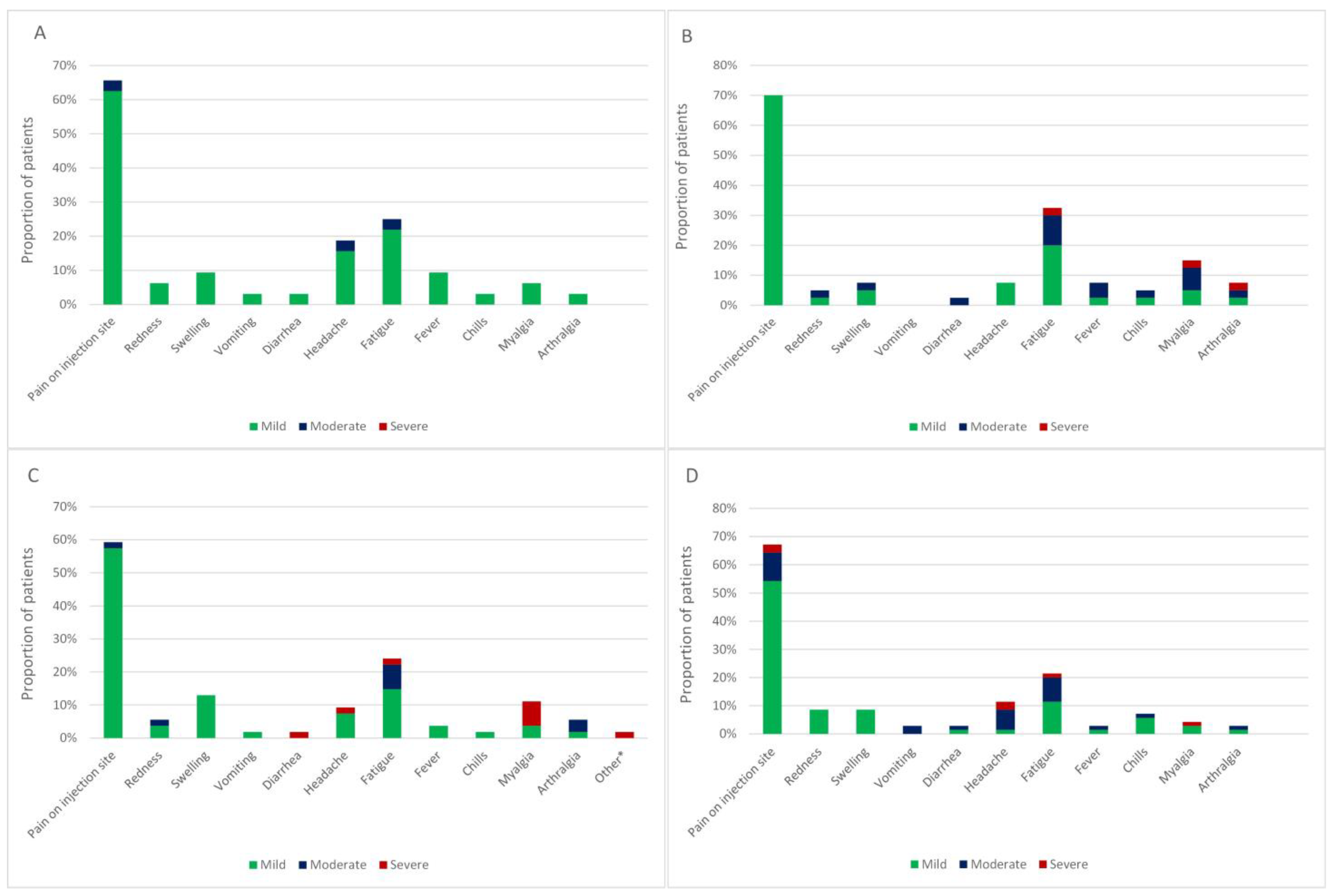
3.1. Adverse Events after the Primary Course of Vaccination
3.2. Breakthrough COVID-19 Infections
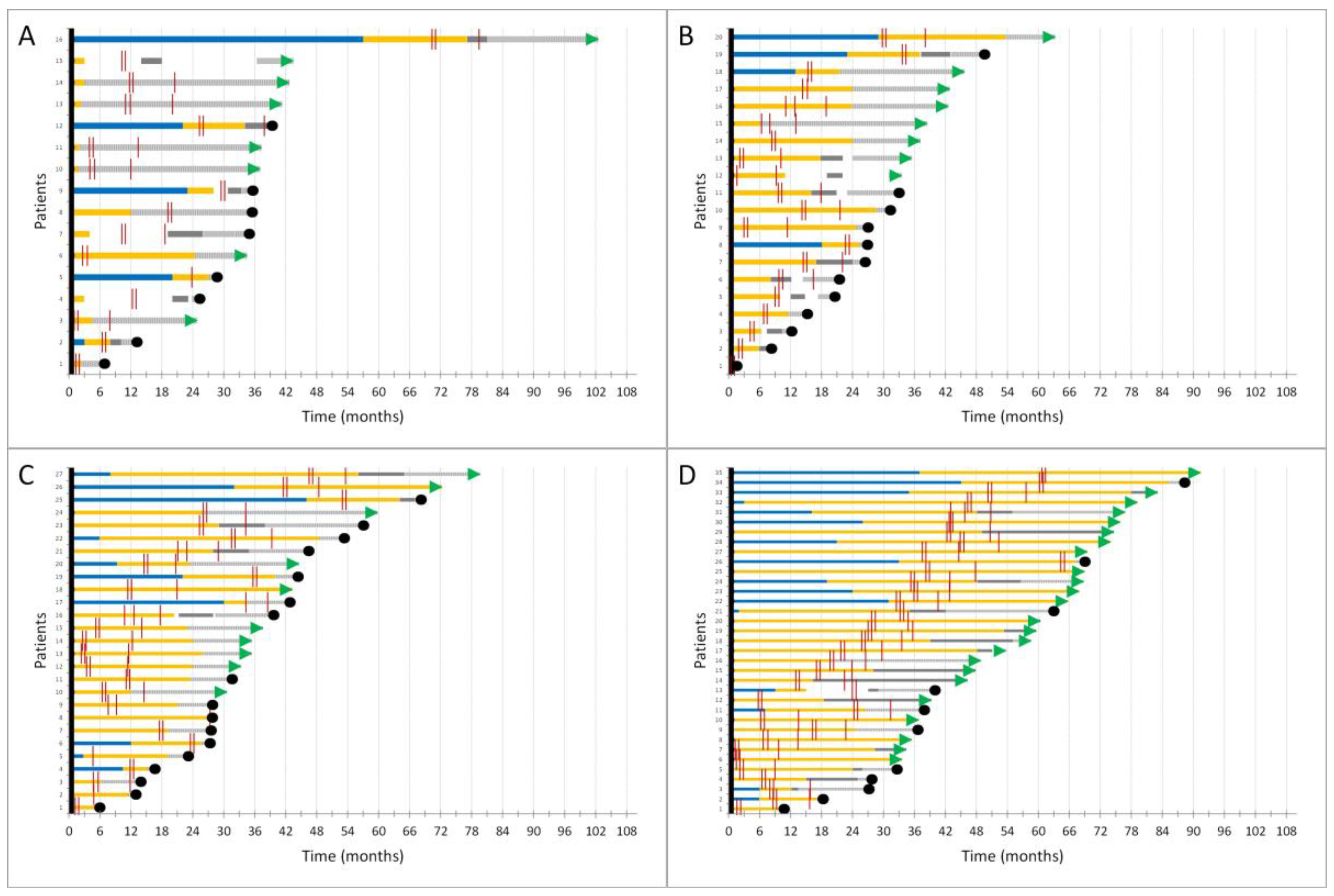
3.3. Thoracic Malignancies Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worldometers.info. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/ (accessed on 2 February 2024).
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A Global Database of COVID-19 Vaccinations. Nat. Hum. Behav. 2021, 5, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Almond, J.; Hacker, J.; Harwood, C.; Pizza, M.; Rappuoli, R.; Ron, E.Z.; Sansonetti, P.; Vanderslott, S.; Wieler, L.H. Development of Vaccines at the Time of COVID-19. microLife 2020, 1, uqaa003. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Rizvi, H.; Preeshagul, I.R.; Egger, J.V.; Hoyos, D.; Bandlamudi, C.; McCarthy, C.G.; Falcon, C.J.; Schoenfeld, A.J.; Arbour, K.C.; et al. COVID-19 in Patients with Lung Cancer. Ann. Oncol. 2020, 31, 1386–1396. [Google Scholar] [CrossRef]
- Bungaro, M.; Passiglia, F.; Scagliotti, G.V. COVID-19 and Lung Cancer: A Comprehensive Overview from Outbreak to Recovery. Biomedicines 2022, 10, 776. [Google Scholar] [CrossRef]
- Garassino, M.C.; Whisenant, J.G.; Huang, L.C.; Trama, A.; Torri, V.; Agustoni, F.; Baena, J.; Banna, G.; Berardi, R.; Bettini, A.C.; et al. COVID-19 in Patients with Thoracic Malignancies (TERAVOLT): First Results of an International, Registry-Based, Cohort Study. Lancet Oncol. 2020, 21, 914–922. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-hogan, N.; Shum, B.; et al. Adaptive Immunity and Neutralizing Antibodies against SARS-CoV-2 Variants of Concern Following Vaccination in Patients with Cancer: The CAPTURE Study. Nat. Cancer 2021, 2, 1305–1320. [Google Scholar] [CrossRef]
- Naranbhai, V.; Pernat, C.A.; Gavralidis, A.; St Denis, K.J.; Lam, E.C.; Spring, L.M.; Isakoff, S.J.; Farmer, J.R.; Zubiri, L.; Hobbs, G.S.; et al. Immunogenicity and Reactogenicity of SARS-CoV-2 Vaccines in Patients With Cancer: The CANVAX Cohort Study. J. Clin. Oncol. 2021, 40, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; Di Marco, M.; Kaklamani, V.; Dietrich, P.Y.; Taylor, B.S.; Simand, P.F.; et al. Immunogenicity of SARS-CoV-2 Messenger RNA Vaccines in Patients with Cancer. Cancer Cell 2021, 39, 1091–1098.e2. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.L.; Labaki, C.; Hsu, C.Y.; Bakouny, Z.; Balanchivadze, N.; Berg, S.A.; Blau, S.; Daher, A.; El Zarif, T.; Friese, C.R.; et al. COVID-19 Vaccination and Breakthrough Infections in Patients with Cancer. Ann. Oncol. 2022, 33, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Janzic, U.; Bidovec-stojkovic, U.; Mohorcic, K.; Mrak, L.; Fokter, N. Solid Cancer Patients Achieve Adequate Immunogenicity and Low Rate of Severe Adverse Events after SARS-CoV-2 Vaccination. Futur. Oncol. 2022, 18, 2537–2550. [Google Scholar] [CrossRef] [PubMed]
- Janzic, U.; Bidovec-Stojkovic, U.; Korosec, P.; Mohorcic, K.; Mrak, L.; Caks, M.; Ravnik, M.; Skof, E.; Rijavec, M. A Three-Dose MRNA COVID-19 Vaccine Regime Produces Both Suitable Immunogenicity and Satisfactory Efficacy in Patients with Solid Cancers. Vaccines 2023, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Grom, A.H.; Rehberger, M.; Lavtar, D.; Šinko, M.; Zaletel, M.; Klanšček, H.J.; Vinko, M.; Korošec, A.; Vitek, M.G.; Učakar, V.; et al. COVID-19 Pandemic in Slovenia. Available online: https://nijz.si/wp-content/uploads/2023/06/SI-PANDA-21.-izvedba_ANG_.pdf (accessed on 10 February 2024).
- WHO. COVID-19 Vaccination: World Data. Available online: https://data.who.int/dashboards/covid19/vaccines (accessed on 1 February 2024).
- Saini, K.S.; Tagliamento, M.; Lambertini, M.; McNally, R.; Romano, M.; Leone, M.; Curigliano, G.; de Azambuja, E. Mortality in Patients with Cancer and Coronavirus Disease 2019: A Systematic Review and Pooled Analysis of 52 Studies. Eur. J. Cancer 2020, 139, 43–50. [Google Scholar] [CrossRef]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A.; et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020, 10, 935–941. [Google Scholar] [CrossRef]
- Cui, W.; Yousaf, N.; Bhosle, J.; Minchom, A.; Lee, R.; Brien, M.O.; Popat, S. Real-World Outcomes in Thoracic Cancer Patients with Severe Acute Respiratory Syndrome Coronavirus 2 (COVID-19): Single UK Institution Experience. Cancer Treat. Res. Commun. 2020, 25, 100261. [Google Scholar] [CrossRef]
- Moujaess, E.; Zeid, N.B.; Samaha, R.; Sawan, J.; Kourie, H.; Labaki, C.; Chebel, R.; Chahine, G.; El Karak, F.; Nasr, F.; et al. Perceptions of the COVID-19 Vaccine among Patients with Cancer: A Single-Institution Survey. Futur. Oncol. 2021, 17, 4071–4079. [Google Scholar] [CrossRef]
- Brko, G.M.; Popovic, M.; Jovic, M.; Radic, J.; Kladar, M.B.; Nikolic, I.; Vidovic, V.; Bjelobrk, I.K.; Kukic, B.; Salma, S.; et al. COVID-19 Vaccines and Cancer Patients: Acceptance, Attitudes and Safety. J. BUON 2021, 26, 2183–2190. [Google Scholar]
- Oosting, S.F.; van der Veldt, A.A.M.; GeurtsvanKessel, C.H.; Fehrmann, R.S.N.; van Binnendijk, R.S.; Dingemans, A.-M.C.; Smit, E.F.; Hiltermann, T.J.N.; den Hartog, G.; Jalving, M.; et al. MRNA-1273 COVID-19 Vaccination in Patients Receiving Chemotherapy, Immunotherapy, or Chemoimmunotherapy for Solid Tumours: A Prospective, Multicentre, Non-Inferiority Trial. Lancet Oncol. 2021, 22, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Pradhan, K.; Jindal, S.; Cui, Z.; Rockwell, B.; Shah, A.P.; Packer, S.; Sica, R.A.; Sparano, J.; Goldstein, D.Y.; et al. Patterns of Seroconversion for SARS-CoV-2 IgG in Patients with Malignant Disease and Association with Anticancer Therapy. Nat. Cancer 2021, 2, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Benda, M.; Mutschlechner, B.; Ulmer, H.; Grabher, C.; Severgnini, L.; Volgger, A.; Reimann, P.; Lang, T.; Atzl, M.; Huynh, M.; et al. Serological SARS-CoV-2 Antibody Response, Potential Predictive Markers and Safety of BNT162b2 MRNA COVID-19 Vaccine in Haematological and Oncological Patients. Br. J. Haematol. 2021, 195, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Ribas, A. At the Crossroads: COVID-19 and Immune-Checkpoint Blockade for Cancer. Cancer Immunol. Res. 2021, 9, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; del Molino del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and Immunogenicity of One versus Two Doses of the COVID-19 Vaccine BNT162b2 for Patients with Cancer: Interim Analysis of a Prospective Observational Study. Lancet Oncol. 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Peeters, M.; Verbruggen, L.; Teuwen, L.; Vanhoutte, G.; Vande Kerckhove, S.; Peeters, B.; Raats, S.; Van der Massen, I.; De Keersmaecker, S.; Debie, Y.; et al. Reduced Humoral Immune Response after BNT162b2 Coronavirus Disease 2019 Messenger RNA Vaccination in Cancer Patients under Antineoplastic Treatment. ESMO Open 2021, 6, 100274. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Abu-salman, A.; Steckbeck, R.; Jacob, B.M.; Massalha, I.; Yakobson, A. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Cancers 2021, 13, 10948. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse Events Following COVID-19 MRNA Vaccines: A Systematic Review of Cardiovascular Complication, Thrombosis, and Thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef]
- Scovino, A.M.; Dahab, E.C.; Vieira, G.F.; Freire-de-Lima, L.; Freire-de-Lima, C.G.; Morrot, A. SARS-CoV-2’s Variants of Concern: A Brief Characterization. Front. Immunol. 2022, 13, 834098. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Ionescu, M.C.; Starkey, T.; Little, M.; Tilby, M.; Tripathy, A.R.; Mckenzie, H.S.; Al-Hajji, Y.; Appanna, N.; Barnard, M.; et al. COVID-19: Third Dose Booster Vaccine Effectiveness against Breakthrough Coronavirus Infection, Hospitalisations and Death in Patients with Cancer: A Population-Based Study. Eur. J. Cancer 2022, 175, 1–10. [Google Scholar] [CrossRef]
- Gong, I.Y.; Vijenthira, A.; Powis, M.; Calzavara, A.; Patrikar, A.; Sutradhar, R.; Hicks, L.K.; Wilton, D.; Singh, S.; Krzyzanowska, M.K.; et al. Association of COVID-19 Vaccination with Breakthrough Infections and Complications in Patients with Cancer. JAMA Oncol. 2023, 9, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Labaki, C.; Bakouny, Z.; Hsu, C.Y.; Schmidt, A.L.; de Lima Lopes, G.; Hwang, C.; Singh, S.R.K.; Jani, C.; Weissmann, L.B.; et al. Breakthrough SARS-CoV-2 Infections among Patients with Cancer Following Two and Three Doses of COVID-19 MRNA Vaccines: A Retrospective Observational Study from the COVID-19 and Cancer Consortium. Lancet Reg. Health–Am. 2023, 19, 100445. [Google Scholar] [CrossRef] [PubMed]
- Agbarya, A.; Sarel, I.; Ziv-Baran, T.; Schwartz, O.; Shechtman, Y.; Kozlener, E.; Khoury, R.; Sheikh-Ahmad, M.; Saiegh, L.; Swaid, F.; et al. Response Rate of the Third and Fourth Doses of the BNT162b2 Vaccine Administered to Cancer Patients Undergoing Active Anti-Neoplastic Treatments. Diseases 2023, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Barrière, J.; Carles, M.; Audigier-Valette, C.; Re, D.; Adjtoutah, Z.; Seitz-Polski, B.; Gounant, V.; Descamps, D.; Zalcman, G. Third Dose of Anti-SARS-CoV-2 Vaccine for Patients with Cancer: Should Humoral Responses Be Monitored? A Position Article. Eur. J. Cancer 2022, 162, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.C.; Thakkar, A.; Campbell, S.T.; Forest, S.K.; Pradhan, K.; Gonzalez-Lugo, J.D.; Quinn, R.; Bhagat, T.D.; Choudhary, G.S.; McCort, M.; et al. Efficacy of Booster Doses in Augmenting Waning Immune Responses to COVID-19 Vaccine in Patients with Cancer. Cancer Cell 2022, 40, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]

| N = 98 | |
|---|---|
| Age in years, median (range) | 63.5 (24–81) |
| Sex, n (%) | |
| Male | 54 (55%) |
| Female | 44 (45%) |
| Cancer type, n (%) | |
| NSCLC * | 87 (89%) |
| SCLC ** | 6 (6%) |
| MPM *** | 5 (5%) |
| Stage, n (%) | |
| Limited | 8 (8%) |
| Locoregionally advanced | 2 (2%) |
| Metastatic | 88 (90%) |
| Anticancer therapy, n (%) | |
| Chemotherapy | 16 (16%) |
| Chemotherapy + ICI | 20 (20%) |
| ICI alone | 27 (28%) |
| Targeted therapy | 35 (36%) |
| Receiving systemic therapy at the time of vaccination, n (%) | |
| Yes | 88 (90%) |
| No | 10 (10%) |
| Positive SARS-CoV-2 IgG antibodies prior to vaccination, n (%) † | |
| No | 79 (81%) |
| Yes | 19 (19%) |
| No. of vaccine doses received | |
| First dose | 98 (100%) |
| Second dose | 95 (97%) |
| Third dose | 57 (58%) |
| Type of primary vaccination received, n (%) | |
| mRNA-based BNT162b2 (Pfizer/BioNTech) | 91 (91%) |
| mRNA-based mRNA-1273 (Moderna) | 3 (4%) |
| Vector-based vaccine AZD1222 (AstraZeneca) | 4 (5%) |
| Type of booster 3rd dose vaccination received, n (%) | |
| mRNA-based BNT162b2 (Pfizer/BioNTech) | 57 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janzic, U.; Janzic, A.; Agbarya, A.; Bidovec-Stojkovic, U.; Mohorcic, K.; Caks, M.; Korosec, P.; Rijavec, M.; Skof, E. Prospective Observational Study of COVID-19 Vaccination in Patients with Thoracic Malignancies: Adverse Events, Breakthrough Infections and Survival Outcomes. Biomedicines 2024, 12, 535. https://doi.org/10.3390/biomedicines12030535
Janzic U, Janzic A, Agbarya A, Bidovec-Stojkovic U, Mohorcic K, Caks M, Korosec P, Rijavec M, Skof E. Prospective Observational Study of COVID-19 Vaccination in Patients with Thoracic Malignancies: Adverse Events, Breakthrough Infections and Survival Outcomes. Biomedicines. 2024; 12(3):535. https://doi.org/10.3390/biomedicines12030535
Chicago/Turabian StyleJanzic, Urska, Andrej Janzic, Abed Agbarya, Urska Bidovec-Stojkovic, Katja Mohorcic, Marina Caks, Peter Korosec, Matija Rijavec, and Erik Skof. 2024. "Prospective Observational Study of COVID-19 Vaccination in Patients with Thoracic Malignancies: Adverse Events, Breakthrough Infections and Survival Outcomes" Biomedicines 12, no. 3: 535. https://doi.org/10.3390/biomedicines12030535
APA StyleJanzic, U., Janzic, A., Agbarya, A., Bidovec-Stojkovic, U., Mohorcic, K., Caks, M., Korosec, P., Rijavec, M., & Skof, E. (2024). Prospective Observational Study of COVID-19 Vaccination in Patients with Thoracic Malignancies: Adverse Events, Breakthrough Infections and Survival Outcomes. Biomedicines, 12(3), 535. https://doi.org/10.3390/biomedicines12030535











