Antiphospholipid Antibodies Are Major Risk Factors for Non-Thrombotic Cardiac Complications in Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Laboratory Evaluation
2.3. Statistical Analysis
3. Results
3.1. Main Analyses
3.2. Antiphospholipid Antibodies and Other Laboratory Parameters in APA+ and APA− Patients
3.3. Clinical Manifestations and Steroid Treatment in APA+ and APA− Patients
3.4. Cardiac Manifestations in APA+ and APA− Patients
3.5. Differences between Patients with and without Cardiac Manifestations
3.6. Characteristics of the APA+ Patients
3.7. The Associations between the APAs and aGAPSS Values and the Specific Cardiac Diseases
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zagelbaum Ward, N.K.; Linares-Koloffon, C.; Posligua, A.; Gandrabur, L.; Kim, W.Y.; Sperber, K.; Wasserman, A.; Ash, J. Cardiac Manifestations of Systemic Lupus Erythematous: An Overview of the Incidence, Risk Factors, Diagnostic Criteria, Pathophysiology and Treatment Options. Cardiol. Rev. 2022, 30, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Kim, A.H. Cardiac manifestations of systemic lupus erythematosus. Rheum. Dis. Clin. N. Am. 2014, 40, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Tselios, K.; Urowitz, M.B. Cardiovascular and Pulmonary Manifestations of Systemic Lupus Erythematosus. Curr. Rheumatol. Rev. 2017, 13, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Zen, M.; Salmaso, L.; Barbiellini Amidei, C.; Fedeli, U.; Bellio, S.; Iaccarino, L.; Doria, A.; Saia, M. Mortality and causes of death in systemic lupus erythematosus over the last decade: Data from a large population-based study. Eur. J. Intern. Med. 2023, 112, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Petri, M. Antiphospholipid syndrome. Transl. Res. 2020, 225, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; DEGroot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Nagy, N.; Papp, G.; Gáspár-Kiss, E.; Diószegi, Á.; Tarr, T. Changes in Clinical Manifestations and Course of Systemic Lupus Erythematosus and Secondary Antiphospholipid Syndrome over Three Decades. Biomedicines 2023, 11, 1218. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Ricard, L.; Nguyen, Y.; Boffa, J.J.; Rondeau, E.; Gerotziafas, G.; Elalamy, I.; Deriaz, S.; De Moreuil, C.; Planche, V.; et al. Triple positive profile in antiphospholipid syndrome: Prognosis, relapse and management from a retrospective multicentre study. RMD Open 2023, 9, e002534. [Google Scholar] [CrossRef]
- Tarr, T.; Lakos, G.; Bhattoa, H.P.; Soltesz, P.; Shoenfeld, Y.; Szegedi, G.; Kiss, E. Clinical thrombotic manifestations in SLE patients with and without antiphospholipid antibodies: A 5-year follow-up. Clin. Rev. Allergy Immunol. 2007, 32, 131–137. [Google Scholar] [CrossRef]
- Gartshteyn, Y.; Bhave, N.; Joseph, M.S.; Askanase, A.; Bernstein, E.J. Inflammatory and thrombotic valvulopathies in autoimmune disease. Heart 2023, 109, 583–588. [Google Scholar] [CrossRef]
- Wade, N.S.; Major, A.S. The problem of accelerated atherosclerosis in systemic lupus erythematosus: Insights into a complex co-morbidity. Thromb. Haemost. 2011, 106, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Kolitz, T.; Shiber, S.; Sharabi, I.; Winder, A.; Zandman-Goddard, G. Cardiac Manifestations of Antiphospholipid Syndrome With Focus on Its Primary Form. Front. Immunol. 2019, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Gauto-Mariotti, E.; Cattoni, H.M.; Arif, A.W.; Richardson, C.; Manadan, A.; Yadav, N. A Meta-analysis and Systematic Review of Valvular Heart Disease in Systemic Lupus Erythematosus and Its Association with Antiphospholipid Antibodies. J. Clin. Rheumatol. 2021, 27, e525–e532. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.; Cervera, R. Current treatment of antiphospholipid syndrome: Lights and shadows. Nat. Rev. Rheumatol. 2015, 11, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Xourgia, E.; Tektonidou, M.G. Management of Non-criteria Manifestations in Antiphospholipid Syndrome. Curr. Rheumatol. Rep. 2020, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.; Tektonidou, M. Emerging Therapies in Antiphospholipid Syndrome. Curr. Rheumatol. Rep. 2016, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Sevim, E.; Willis, R.; Erkan, D. Is there a role for immunosuppression in antiphospholipid syndrome? Hematol. Am. Soc. Hematol. Educ. Program. 2019, 2019, 426–432. [Google Scholar] [CrossRef]
- Sciascia, S.; Sanna, G.; Murru, V.; Roccatello, D.; Khamashta, M.A.; Bertolaccini, M.L. The global anti-phospholipid syndrome score in primary APS. Rheumatology 2015, 54, 134–138. [Google Scholar] [CrossRef]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.C.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann. Rheum. Dis. 2023, 82, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Amigo, M.C.; García-Torres, R. Morphology of vascular, renal, and heart lesions in the antiphospholipid syndrome: Relationship to pathogenesis. Curr. Rheumatol. Rep. 2000, 2, 262–270. [Google Scholar] [CrossRef]
- Frostegård, J. Systemic lupus erythematosus and cardiovascular disease. J. Intern. Med. 2023, 293, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.G. Cardiovascular disease risk in antiphospholipid syndrome: Thrombo-inflammation and atherothrombosis. J. Autoimmun. 2022, 128, 102813. [Google Scholar] [CrossRef] [PubMed]
- Khamashta, M.A.; Cervera, R.; Asherson, R.A.; Font, J.; Gil, A.; Coltart, D.J.; Vázquez, J.J.; Paré, C.; Ingelmo, M.; Oliver, J.; et al. Association of antibodies against phospholipids with heart valve disease in systemic lupus erythematosus. Lancet 1990, 335, 1541–1544. [Google Scholar] [CrossRef]
- Nihoyannopoulos, P.; Gomez, P.M.; Joshi, J.; Loizou, S.; Walport, M.J.; Oakley, C.M. Cardiac abnormalities in systemic lupus erythematosus. Association with raised anticardiolipin antibodies. Circulation 1990, 82, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Font, J.; Paré, C.; Azqueta, M.; Pérez-Villa, F.; López-Soto, A.; Ingelmo, M. Cardiac disease in systemic lupus erythematosus: Prospective study of 70 patients. Ann. Rheum. Dis. 1992, 51, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Jouhikainen, T.; Pohjola-Sintonen, S.; Stephansson, E. Lupus anticoagulant and cardiac manifestations in systemic lupus erythematosus. Lupus 1994, 3, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Zuily, S.; Regnault, V.; Selton-Suty, C.; Eschwège, V.; Bruntz, J.F.; Bode-Dotto, E.; De Maistre, E.; Dotto, P.; Perret-Guillaume, C.; Lecompte, T.; et al. Increased risk for heart valve disease associated with antiphospholipid antibodies in patients with systemic lupus erythematosus: Meta-analysis of echocardiographic studies. Circulation 2011, 124, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Turiel, M.; Sarzi-Puttini, P.; Peretti, R.; Bonizzato, S.; Muzzupappa, S.; Atzeni, F.; Rossi, E.; Doria, A. Five-year follow-up by transesophageal echocardiographic studies in primary antiphospholipid syndrome. Am. J. Cardiol. 2005, 96, 574–579. [Google Scholar] [CrossRef]
- Pons, I.; Louro, J.; Sitges, M.; Vidal, B.; Cervera, R.; Espinosa, G. Heart Valve Involvement in Patients with Antiphospholipid Syndrome: A Long-Term Follow-Up Study of a Single Centre. J. Clin. Med. 2023, 12, 2996. [Google Scholar] [CrossRef]
- Djokovic, A.; Stojanovich, L.; Kontic, M.; Stanisavljevic, N.; Radovanovic, S.; Marisavljevic, D. Association between cardiac manifestations and antiphospholipid antibody type and level in a cohort of Serbian patients with primary and secondary antiphospholipid syndrome. Isr. Med. Assoc. J. 2014, 16, 162–167. [Google Scholar]
- Roldan, C.A.; Gelgand, E.A.; Qualls, C.R.; Sibbitt, W.L., Jr. Valvular heart disease as a cause of cerebrovascular disease in patients with systemic lupus erythematosus. Am. J. Cardiol. 2005, 95, 1441–1447. [Google Scholar] [CrossRef]
- Vonk, M.C.; Vandecasteele, E.; van Dijk, A.P. Pulmonary hypertension in connective tissue diseases, new evidence and challenges. Eur. J. Clin. Investig. 2021, 51, e13453. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, I.; Tufano, A.; Lupoli, R.; Iannuzzo, G.; Emmi, G.; Di Minno, M.N.D. Cardiovascular disease and antiphospholipid syndrome: How to predict and how to treat? Pol. Arch. Intern. Med. 2021, 131, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Fan, Y.; Jia, Y.; Li, G.; Liu, M.; Xu, Y.; Zhang, J.; Li, C. A novel aGAPSS-based nomogram for the prediction of ischemic stroke in patients with antiphospholipid syndrome. Front. Immunol. 2022, 13, 930087. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio-Longarela, S.; Martínez-Taboada, V.M.; Blanco-Olavarri, P.; Merino, A.; Riancho-Zarrabeitia, L.; Comins-Boo, A.; López-Hoyos, M.; Hernández, J.L. Does Adjusted Global Antiphospholipid Syndrome Score (aGAPSS) Predict the Obstetric Outcome in Antiphospholipid Antibody Carriers? A Single-Center Study. Clin. Rev. Allergy Immunol. 2022, 63, 297–310. [Google Scholar] [CrossRef]
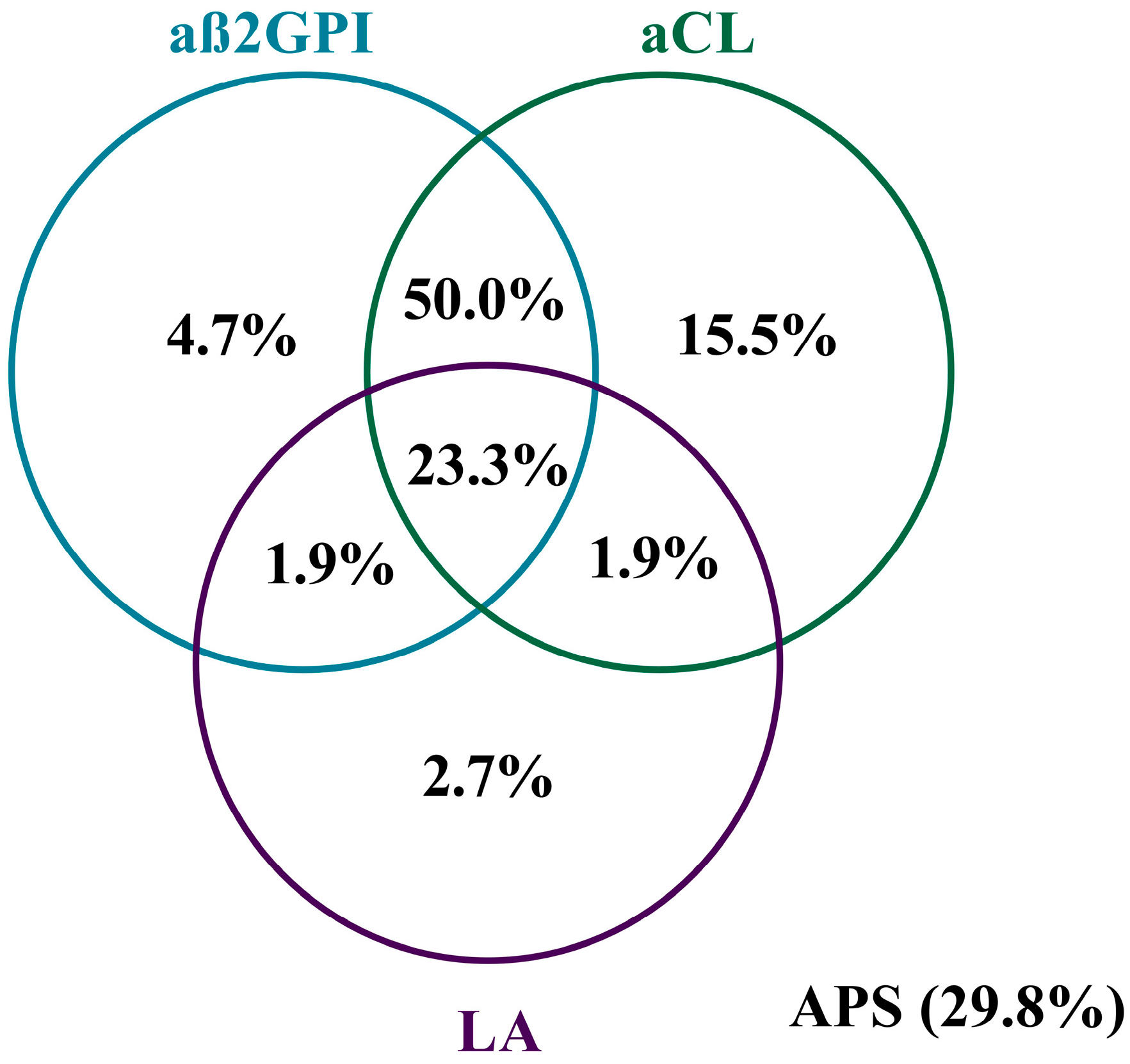
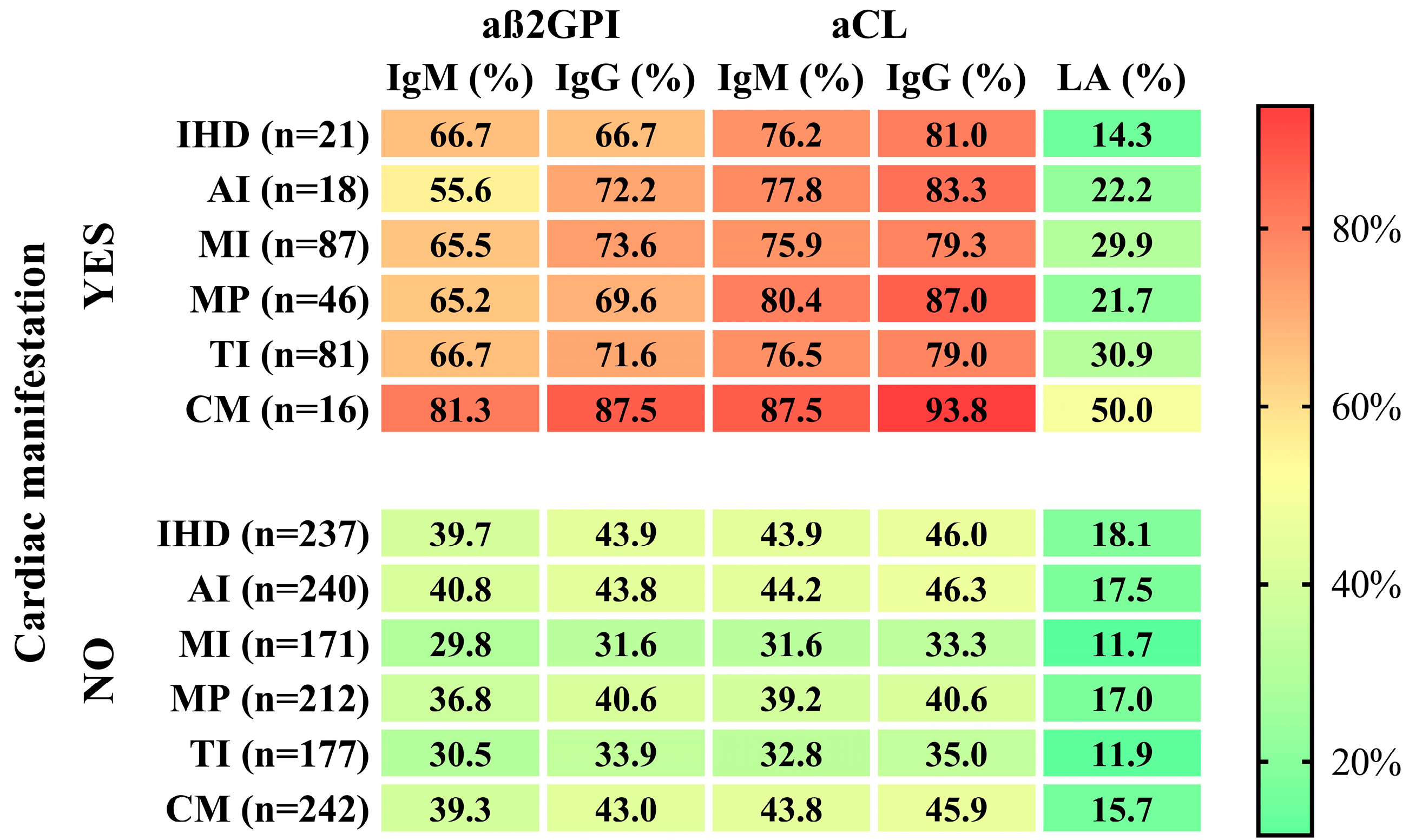
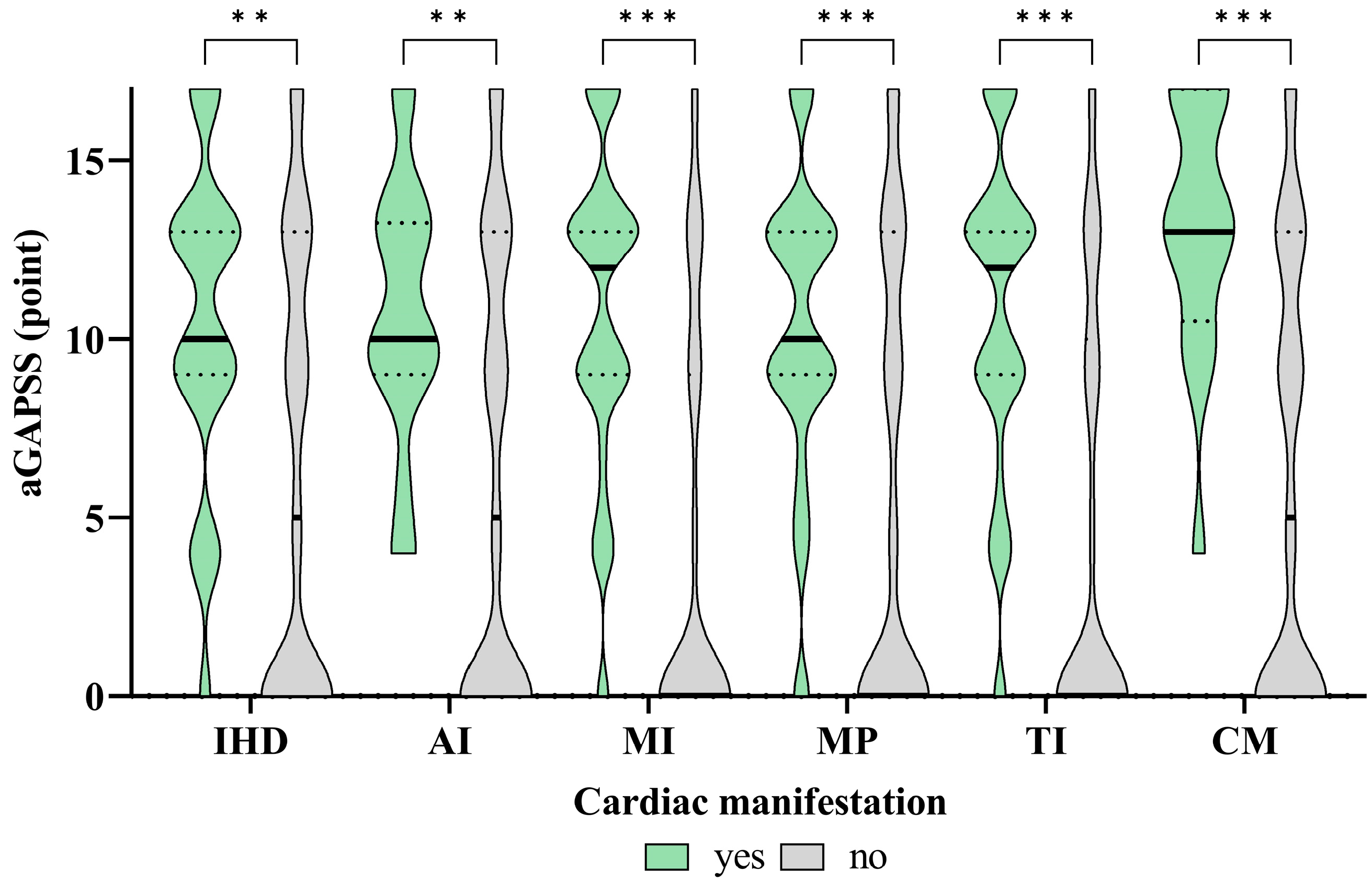

| SLE Cohort (n = 369) | APA− (n = 111) | APA+ (n = 258) | p-Value | |
|---|---|---|---|---|
| Sex (women, %) | 336 (91.1) | 100 (90.1) | 236 (91.5) | 0.669 |
| Age, years | 49.7 ± 13.4 | 48.8 ± 14.3 | 50.1 ± 13.1 | 0.401 |
| Age onset SLE, years | 32.3 ± 11.6 | 32.4 ± 11.9 | 32.3 ± 11.5 | 0.998 |
| Duration of SLE, years | 17.3 ± 10.1 | 16.4 ± 11.8 | 17.1 ± 9.2 | 0.310 |
| SLE Cohort (n = 369) | APA− (n = 111) | APA+ (n = 258) | p-Value | |
|---|---|---|---|---|
| Thrombocytopenia | 145 (39.3) | 34 (30.6) | 111 (43.0) | 0.025 |
| Leukopaenia | 269 (72.9) | 78 (70.3) | 191 (74.0) | 0.456 |
| Anaemia | 284 (77.0) | 72 (64.9) | 212 (82.2) | <0.001 |
| Anti-dsDNA | 338 (91.6) | 91 (82.0) | 247 (95.7) | <0.001 |
| Anti-Sm | 131 (35.5) | 25 (22.5) | 106 (41.1) | 0.001 |
| Anti-RNP | 116 (31.4) | 23 (20.7) | 93 (36.0) | 0.004 |
| Anti-SS-A (Ro) | 247 (66.9) | 66 (59.5) | 181 (70.2) | 0.045 |
| Anti-SS-B (La) | 168 (45.5) | 46 (41.4) | 122 (47.3) | 0.301 |
| ANCA | 38 (10.3) | 2 (1.8) | 36 (14.0) | <0.001 |
| Cryoglobulin | 15 (4.1) | 1 (0.9) | 14 (5.4) | 0.046 |
| Coombs test positivity | 41 (11.1) | 10 (9.0) | 31 (12.0) | 0.399 |
| SLE Cohort (n = 369) | APA− (n = 111) | APA+ (n = 258) | p-Value | |
|---|---|---|---|---|
| Acute skin lesions | 151 (40.9) | 41 (36.9) | 110 (42.6) | 0.307 |
| DLE | 52 (14.1) | 17 (15.3) | 35 (13.6) | 0.658 |
| SCLE | 41 (11.1) | 23 (20.7) | 18 (7.0) | <0.001 |
| Alopecia | 88 (23.8) | 16 (14.4) | 72 (27.9) | 0.005 |
| Photosensitivity | 101 (27.4) | 31 (27.9) | 70 (27.1) | 0.875 |
| Mucous ulcer | 35 (9.5) | 15 (13.5) | 20 (7.8) | 0.083 |
| Pleuritis | 94 (25.5) | 27 (24.3) | 67 (26.0) | 0.739 |
| Pericarditis | 61 (16.5) | 19 (17.1) | 42 (16.3) | 0.842 |
| CNS manifestations | 87 (23.6) | 14 (12.6) | 73 (28.3) | 0.001 |
| PNS manifestations | 38 (10.3) | 6 (5.4) | 32 (12.4) | 0.043 |
| Psychiatric manifestations | 74 (20.1) | 14 (12.6) | 60 (23.3) | 0.019 |
| LN | 114 (30.9) | 30 (27.0) | 84 (32.6) | 0.292 |
| Polyarthritis | 314 (85.1) | 92 (82.9) | 222 (86.0) | 0.434 |
| Cumulative dose of steroid, mg/kg | 17,520 (7300–32,120) | 11,680 (4380–27,740) | 19,710 (8760–35,030) | 0.003 |
| SDI, points | 1 (0-1) | 1 (0-1) | 1 (0-2) | 0.001 |
| SLE Cohort (n = 369) | APA− (n = 111) | APA+ (n = 258) | p-Value | |
|---|---|---|---|---|
| Ischemic heart disease | 29 (7.9) | 8 (7.2) | 21 (8.1) | 0.760 |
| Valvulopathy | 153 (41.5) | 38 (34.2) | 115 (44.6) | 0.064 |
| Aortic insufficiency | 24 (6.5) | 6 (5.4) | 18 (7.0) | 0.575 |
| Aortic stenosis | 4 (1.1) | 0 | 4 (1.6) | 0.320 |
| Pulmonary insufficiency | 3 (0.8) | 0 | 3 (1.2) | 0.557 |
| Mitral insufficiency | 111 (30.1) | 24 (21.6) | 87 (33.7) | 0.020 |
| Mitral prolapse | 61 (16.5) | 15 (13.5) | 46 (17.8) | 0.306 |
| Tricuspid insufficiency | 101 (27.4) | 20 (18.0) | 81 (31.4) | 0.008 |
| Cardiomyopathy | 21 (5.7) | 5 (4.5) | 16 (6.2) | 0.519 |
| Pulmonary hypertension | 5 (1.4) | 0 | 5 (1.9) | 0.328 |
| Libman–Sacks endocarditis | 2 (0.5) | 0 | 2 (0.8) | 1.000 |
| Hyperlipidemia | 118 (32.0) | 35 (31.5) | 83 (32.2) | 0.904 |
| Hypertonia | 152 (41.2) | 52 (46.8) | 100 (38.8) | 0.148 |
| SLE Cohort (n = 369) | Without Cardiac Manifestations (n = 198; 53.7%) | With Cardiac Manifestations (n = 171; 43.6%) | p-Value | |
|---|---|---|---|---|
| Demographic | ||||
| Gender (female) | 336 (91.1) | 180 (90.9) | 156 (91.2) | 0.915 |
| Age at SLE onset (years) | 32.3 ± 11.6 | 31.9 ± 11.7 | 32.8 ± 11.5 | 0.444 |
| Duration of SLE (years) | 17.3 ± 10.1 | 16.4 ± 9.5 | 18.4 ± 10.7 | 0.068 |
| Autoantibodies | ||||
| LA | 77 (20.9) | 39 (19.7) | 38 (22.2) | 0.552 |
| aß2GPI | 206 (55.8) | 103 (52) | 103 (60.2) | 0.113 |
| aß2GPI IgM | 108 (41.9) | 20 (15.5) | 88 (68.2) | <0.001 |
| aß2GPI IgG | 118 (45.7) | 22 (17.1) | 96 (74.4) | <0.001 |
| aCL | 234 (63.4) | 119 (60.1) | 115 (67.3) | 0.155 |
| aCL IgM | 120 (46.5) | 19 (14.7) | 101 (78.3) | <0.001 |
| aCL IgG | 126 (48.8) | 21 (16.3) | 105 (81.4) | <0.001 |
| Single antibody positivity | 59 (16) | 28 (14.1) | 31 (18.1) | 0.297 |
| LA | 7 (1.9) | 3 (1.5) | 4 (2.3) | 0.709 |
| aß2GPI | 12 (3.3) | 5 (2.5) | 7 (4.1) | 0.397 |
| aCL | 40 (10.8) | 20 (10.1) | 20 (11.7) | 0.623 |
| Double antibody positivity | 139 (37.7) | 70 (35.4) | 69 (40.4) | 0.323 |
| LA + aß2GPI | 5 (1.4) | 2 (1.0) | 3 (1.8) | 0.666 |
| LA + aCL | 5 (1.4) | 3 (1.5) | 2 (1.2) | 1.000 |
| aß2GPI + aCL | 129 (35.0) | 65 (32.8) | 64 (37.4) | 0.356 |
| Triple antibody positivity | 60 (16.3) | 31 (15.7) | 29 (17) | 0.735 |
| Cardiovascular risk factors | ||||
| Hyperlipidemia | 118 (32.0) | 33 (16.7) | 85 (49.7) | <0.001 |
| Hypertonia | 152 (41.2) | 44 (22.2) | 108 (63.2) | <0.001 |
| APS | 77 (20.9) | 36 (18.2) | 41 (24.0) | 0.172 |
| Deep vein thrombosis | 68 (18.4) | 39 (19.7) | 29 (17.0) | 0.499 |
| Acute myocardial infarction | 13 (3.5) | 5 (2.5) | 8 (4.7) | 0.263 |
| Pulmonary embolism | 13 (3.5) | 7 (3.5) | 6 (3.5) | 0.989 |
| Stroke | 25 (6.8) | 7 (3.5) | 18 (10.5) | 0.008 |
| Spontaneous abortion | 49 (13.3) | 21 (10.6) | 28 (16.4) | 0.103 |
| Livedo reticularis | 25 (6.8) | 11 (5.6) | 14 (8.2) | 0.316 |
| Thrombocytopenia | 145 (39.3) | 70 (35.4) | 75 (43.9) | 0.095 |
| SLE Cohort (n = 369) | Without Valvulopathy (n = 216; 58.5%) | With Valvulopathy (n = 153; 41.5%) | p-Value | |
|---|---|---|---|---|
| Demographic | ||||
| Gender (female) | 336 (91.1) | 195 (90.3) | 141 (92.2) | 0.533 |
| Age at SLE onset (years) | 32.3 ± 11.6 | 32.2 ± 11.9 | 32.5 ± 11.2 | 0.831 |
| Duration of SLE (years) | 17.3 ± 10.1 | 16.9 ± 9.8 | 17.9 ± 10.5 | 0.377 |
| Autoantibodies | ||||
| LA | 77 (20.9) | 44 (20.4) | 33 (21.6) | 0.780 |
| aß2GPI | 206 (55.8) | 116 (53.7) | 90 (58.8) | 0.329 |
| aß2GPI IgM | 108 (41.9) | 32 (22.4) | 76 (66.1) | <0.001 |
| aß2GPI IgG | 118 (45.7) | 34 (23.8) | 84 (73.0) | <0.001 |
| aCL | 234 (63.4) | 133 (61.6) | 101 (66.0) | 0.383 |
| aCL IgM | 120 (46.5) | 31 (21.7) | 89 (77.4) | <0.001 |
| aCL IgG | 126 (48.8) | 33 (23.1) | 93 (80.9) | <0.001 |
| Single antibody positivity | 59 (16.0) | 28 (13.0) | 31 (20.3) | 0.059 |
| LA | 7 (1.9) | 3 (1.4) | 4 (2.6) | 0.455 |
| aß2GPI | 12 (3.3) | 5 (2.3) | 7 (4.6) | 0.247 |
| aCL | 40 (10.8) | 20 (9.3) | 20 (13.1) | 0.246 |
| Double antibody positivity | 139 (37.7) | 80 (21.7) | 59 (16.0) | 0.766 |
| LA + aß2GPI | 5 (1.4) | 2 (0.9) | 3 (2.0) | 0.653 |
| LA + aCL | 5 (1.4) | 4 (1.9) | 1 (0.7) | 0.408 |
| aß2GPI + aCL | 129 (35.0) | 74 (34.3) | 55 (35.9) | 0.738 |
| Triple antibody positivity | 60 (16.3) | 35 (16.2) | 25 (16.3) | 0.972 |
| Anti-dsDNA | 338 (91.6) | 199 (92.1) | 139 (90.8) | 0.662 |
| Anti-Sm | 131 (35.5) | 77 (35.6) | 54 (35.3) | 0.944 |
| Anti-RNP | 116 (31.4) | 64 (29.6) | 52 (34.0) | 0.374 |
| Anti-SS-A (Ro) | 247 (66.9) | 145 (67.1) | 102 (66.7) | 0.926 |
| Anti-SS-B (La) | 168 (45.5) | 98 (45.4) | 70 (45.8) | 0.942 |
| Cardiac manifestations | ||||
| Ischemic heart disease | 29 (7.9) | 9 (4.2) | 20 (13.1) | 0.002 |
| Cardiomyopathy | 21 (5.7) | 6 (2.8) | 15 (9.8) | 0.004 |
| Carotid stenosis | 5 (1.4) | 1 (0.5) | 4 (2.6) | 0.165 |
| Pulmonary hypertension | 5 (1.4) | 2 (0.9) | 3 (2.0) | 0.653 |
| Libman–Sacks endocarditis | 2 (0.5) | 1 (0.5) | 1 (0.7) | 1.000 |
| Cardiovascular risk factors | ||||
| Hyperlipidemia | 118 (32.0) | 41 (19.0) | 77 (50.3) | <0.001 |
| Hypertonia | 152 (41.2) | 59 (27.3) | 93 (60.8) | <0.001 |
| APS | 77 (20.9) | 43 (19.9) | 34 (22.2) | 0.590 |
| Deep vein thrombosis | 68 (18.4) | 44 (20.4) | 24 (15.7) | 0.253 |
| Acute myocardial infarction | 13 (3.5) | 7 (3.2) | 6 (3.9) | 0.727 |
| Pulmonary embolism | 13 (3.5) | 8 (3.7) | 5 (3.3) | 0.823 |
| Stroke | 25 (6.8) | 10 (4.6) | 15 (9.8) | 0.051 |
| Spontaneous abortion | 49 (13.3) | 24 (11.1) | 25 (16.3) | 0.145 |
| Livedo reticularis | 25 (6.8) | 12 (5.6) | 13 (8.5) | 0.268 |
| Thrombocytopenia | 145 (39.3) | 80 (37.0) | 65 (42.5) | 0.291 |
| Single AB Positivity (n = 59; 22.9%) | Double AB Positivity (n = 139; 53.9%) | p-Value Double vs. Single Pos. | Triple AB Positivity (n = 60; 23.3%) | p-Value Triple vs. Single Pos. | |
|---|---|---|---|---|---|
| Demographic | |||||
| Gender (female) | 57 (96.6) | 127 (91.4) | 0.188 | 52 (86.7) | 0.095 |
| Age at SLE onset (years) | 34 ± 12.0 | 31.8 ± 11.4 | 0.215 | 32.1 ± 11.3 | 0.371 |
| Duration of SLE (years) | 17.7 ± 10.5 | 18.2 ± 8.2 | 0.722 | 16.7 ± 10.2 | 0.606 |
| Cardiac manifestations | |||||
| Ischemic heart disease | 7 (11.9) | 11 (7.9) | 0.376 | 3 (5.0) | 0.204 |
| Valvulopathy | 31 (52.5) | 59 (42.4) | 0.192 | 25 (41.7) | 0.235 |
| Aortic insufficiency | 4 (6.8) | 10 (7.2) | 1.000 | 4 (6.7) | 1.000 |
| Aorta stenosis | 0 (0) | 4 (2.9) | 0.320 | 0 (0) | n.c. |
| Tricuspid insufficiency | 22 (37.3) | 37 (26.6) | 0.133 | 22 (36.7) | 0.944 |
| Mitral insufficiency | 23 (39.0) | 43 (30.9) | 0.272 | 21 (35.0) | 0.653 |
| Pulmonary insufficiency | 1 (1.7) | 2 (1.4) | 1.000 | 0 (0) | 0.496 |
| Mitral prolapse | 13 (22.0) | 24 (17.3) | 0.431 | 9 (15.0) | 0.323 |
| Cardiomyopathy | 1 (1.7) | 9 (6.5) | 0.287 | 6 (10.0) | 0.114 |
| Carotid stenosis | 1 (1.7) | 2 (1.4) | 1.000 | 2 (3.3) | 1.000 |
| Pulmonary hypertension | 1 (1.7) | 1 (0.7) | 0.508 | 3 (5.0) | 0.619 |
| Libman–Sacks endocarditis | 0 (0) | 2 (1.4) | 1.000 | 0 (0) | n.c. |
| Cardiovascular risk factors | |||||
| Hyperlipidemia | 18 (30.5) | 46 (33.1) | 0.722 | 19 (31.7) | 0.891 |
| Hypertonia | 19 (32.2) | 53 (38.1) | 0.428 | 28 (46.7) | 0.107 |
| APS | 9 (15.3) | 31 (22.3) | 0.259 | 37 (61.7) | <0.001 |
| Deep vein thrombosis | 4 (6.8) | 22 (15.8) | 0.085 | 30 (50.0) | <0.001 |
| Acute myocardial infarction | 3 (5.1) | 4 (2.9) | 0.427 | 4 (6.7) | 1.000 |
| Pulmonary embolism | 1 (1.7) | 1 (0.7) | 0.508 | 6 (10.0) | 0.114 |
| Stroke | 4 (6.8) | 8 (5.8) | 0.753 | 10 (16.7) | 0.094 |
| Spontaneous abortion | 8 (13.6) | 16 (11.5) | 0.686 | 14 (23.3) | 0.170 |
| Livedo reticularis | 3 (5.1) | 12 (8.6) | 0.560 | 4 (6.7) | 1.000 |
| Thrombocytopenia | 20 (33.9) | 60 (43.2) | 0.224 | 31 (51.7) | 0.050 |
| AUC | p-Value | aGAPSS Cut-off Value * | Sens. | Spec. | Prev. | PPV ** | NPV ** | |
|---|---|---|---|---|---|---|---|---|
| IHD | 69.28 | 0.003 | >8.5 | 80.95 | 55.27 | 8.1 | 13.76 | 97.05 |
| AI | 71.39 | 0.002 | >8.5 | 83.33 | 55.00 | 7.0 | 12.23 | 97.77 |
| MI # | 78.73 | <0.001 | >8.5 | 79.31 | 68.42 | 33.7 | 56.07 | 86.68 |
| MP | 69.42 | <0.001 | >8.5 | 80.43 | 59.43 | 17.8 | 30.04 | 93.34 |
| TI # | 77.69 | <0.001 | >8.5 | 79.01 | 66.67 | 31.4 | 52.04 | 87.40 |
| CM # | 79.01 | <0.001 | >9.5 | 87.50 | 67.36 | 6.2 | 15.05 | 98.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, N.; Bói, B.; Papp, G.; Fiák, E.; Gáspár-Kiss, E.; Perge, B.; Farmasi, N.; Tarr, T. Antiphospholipid Antibodies Are Major Risk Factors for Non-Thrombotic Cardiac Complications in Systemic Lupus Erythematosus. Biomedicines 2024, 12, 530. https://doi.org/10.3390/biomedicines12030530
Nagy N, Bói B, Papp G, Fiák E, Gáspár-Kiss E, Perge B, Farmasi N, Tarr T. Antiphospholipid Antibodies Are Major Risk Factors for Non-Thrombotic Cardiac Complications in Systemic Lupus Erythematosus. Biomedicines. 2024; 12(3):530. https://doi.org/10.3390/biomedicines12030530
Chicago/Turabian StyleNagy, Nikolett, Bernadett Bói, Gábor Papp, Edit Fiák, Eszter Gáspár-Kiss, Bianka Perge, Nikolett Farmasi, and Tünde Tarr. 2024. "Antiphospholipid Antibodies Are Major Risk Factors for Non-Thrombotic Cardiac Complications in Systemic Lupus Erythematosus" Biomedicines 12, no. 3: 530. https://doi.org/10.3390/biomedicines12030530
APA StyleNagy, N., Bói, B., Papp, G., Fiák, E., Gáspár-Kiss, E., Perge, B., Farmasi, N., & Tarr, T. (2024). Antiphospholipid Antibodies Are Major Risk Factors for Non-Thrombotic Cardiac Complications in Systemic Lupus Erythematosus. Biomedicines, 12(3), 530. https://doi.org/10.3390/biomedicines12030530








