Drug-Induced Anaphylaxis in Children
Abstract
1. Introduction
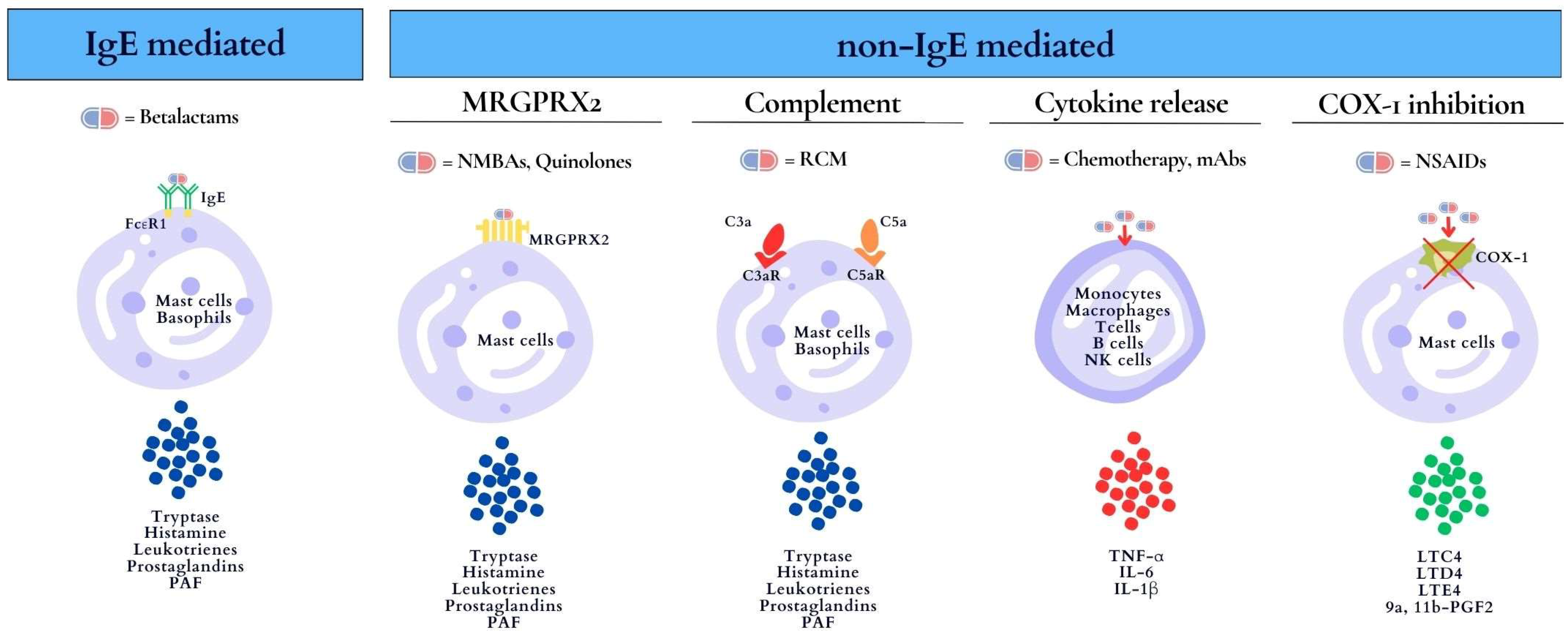
2. Mechanisms of Drug-Induced Anaphylaxis
3. Diagnosis
4. In Vitro Test and In Vivo Test
5. Epidemiology
6. Risk Factors
7. Management
7.1. Acute Phase
- Recognize anaphylaxis symptoms and signs, suspend the culprit drug, and optimize patient posture.
- Immediately administer intramuscular adrenaline in the mid-thigh area as the first-line management of anaphylaxis. The EAACI Task Force suggests a dose of 0.01 mg/kg up to a maximum of 0.5 mg in healthcare settings, or 0.15 mg (for children from 7.5 kg to 25–30 kg), 0.3 mg (for children from 25 to 30 kg), and 0.5 mg (for adolescents when the patient is overweight or has experienced a previous episode of life-threatening anaphylaxis) in community settings.
- Give high-flow oxygen at 10 liters/minute if there are circulatory/severe respiratory symptoms: i.v. fluid—crystalloid bolus 10 mL per kg of patient’s weight (in children < 25–30 kg) or 500 mL (in children > 25–30 kg). If no improvement in 5–10 min is observed, repeat the adrenaline dose and give intravenous fluids.
- Call the emergency team, including critical care experts, to provide advanced treatment, including adrenaline infusion; in case of cardiac arrest, follow the guidelines.
- Monitor the cerebral status, pulse oximetry, blood pressure, and ECG.
- When the patient is stabilized, measure serum tryptase 30 min to 2 h after the reaction onset and consider additional treatment (antihistamines, corticosteroids).
7.2. Following the Resolution of the Acute Phase
- Educate the child and their caregivers about anaphylaxis, including triggers, signs, and symptoms, and the importance of avoiding allergens.
- Ensure the child has access to self-injectable epinephrine and properly teach them or their caregivers how to use it.
- Develop an emergency action plan that outlines steps to take in the event of another anaphylactic reaction, including when and how to administer epinephrine and when to seek medical help.
- Ensure regular follow-up appointments with healthcare providers to monitor the child’s allergies, assess any potential triggers or changes in allergens, and adjust treatment plans accordingly.
- Assess the need for allergy testing to identify specific allergens and potential avoidance strategies.
- Provide appropriate counseling and support to help the child and their caregivers cope with the emotional and psychological impacts of living with severe allergies.
- Collaborate with personnel at school or in other educational settings to ensure a safe environment for the child, including staff training on recognizing and managing anaphylactic reactions.
8. Desensitization Protocols
9. Culprit Drugs
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muraro, A.; Worm, M.; Alviani, C.; Cardona, V.; DunnGalvin, A.; Garvey, L.H.; Riggioni, C.; de Silva, D.; Angier, E.; Arasi, S.; et al. EAACI guidelines: Anaphylaxis (2021 update). Allergy 2022, 77, 357–377. [Google Scholar] [CrossRef]
- Cardinale, F.; Amato, A.; Mastrototaro, M.F.; Caffarelli, C.; Crisafulli, G.; Franceschini, F.; Liotti, L.; Caimmi, S.; Bottau, P.; Saretta, F.; et al. Drug-induced anaphylaxis in children. Acta Biomed. 2019, 90 (Suppl. 3), 30–35. [Google Scholar] [PubMed]
- Olabarri, M.; Vazquez, P.; Gonzalez-Posada, A.; Sanz, N.; Gonzalez-Peris, S.; Diez, N.; Vinuesa, A.; Martinez-Indart, L.; Benito, J.; Mintegi, S. Risk Factors for Severe Anaphylaxis in Children. J. Pediatr. 2020, 225, 193–197. [Google Scholar] [CrossRef]
- Regateiro, F.S.; Marques, M.L.; Rebelo Gomes, E. Drug-Induced Anaphylaxis: An Update on Epidemiology and Risk Factors. Int. Arch. Allergy Immunol. 2020, 181, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Atanaskovic-Markovic, M.; Gomes, E.; Rodrigues Cernadas, J.; Du Toit, G.; Kidon, M.; Kuyucu, S.; Mori, F.; Ponvert, C.; Terreehorst, I.; Caubet, J.C. Diagnosis and management of drug-induced anaphylaxis in children: An EAACI position paper. Pediatr. Allergy Immunol. 2019, 30, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Lemanske, R.F., Jr.; Castells, M.; Torres, M.J.; Khan, D.; Simon, H.U.; Bindslev-Jensen, C.; Burks, W.; Poulsen, L.K.; Sampson, H.A.; et al. Precision medicine in allergic disease-food allergy, drug allergy, and anaphylaxis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and Immunology. Allergy 2017, 72, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J. Delayed drug hypersensitivity reactions. Ann. Intern. Med. 2003, 139, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Labella, M.; Garcia-Neuer, M.; Castells, M. Application of precision medicine to the treatment of anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 190–197. [Google Scholar] [CrossRef]
- Kumar, M.; Duraisamy, K.; Chow, B.K. Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2). Cells 2021, 10, 1033. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Gilfillan, A.M. G protein-coupled receptors and the modification of FcepsilonRI-mediated mast cell activation. Immunol. Lett. 2007, 113, 59–69. [Google Scholar] [CrossRef]
- Caimmi, S.; Crisafulli, G.; Franceschini, F.; Liotti, L.; Bianchi, A.; Bottau, P.; Mori, F.; Triggiano, P.; Paglialunga, C.; Saretta, F.; et al. Hypersensitivity to Intravenous Iron Preparations. Children 2022, 9, 1473. [Google Scholar] [CrossRef]
- Mori, F.; Saretta, F.; Bianchi, A.; Crisafulli, G.; Caimmi, S.; Liotti, L.; Bottau, P.; Franceschini, F.; Paglialunga, C.; Ricci, G.; et al. Hypersensitivity Reactions to monoclonal Antibodies in Children. Medicina 2020, 56, 232. [Google Scholar] [CrossRef]
- Shimabukuro-Vornhage, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Doña, I.; Jurado-Escobar, R.; Perkins, J.R.; Ayuso, P.; Plaza-Serón, M.C.; Pérez-Sánchez, N.; Campo, P.; Bogas-Herrera, G.; Bartra, J.; Torres, M.J.; et al. Eicosanoid mediator profiles in different phenotypes of nonsteroidal anti-inflammatory drug-induced urticaria. Allergy 2019, 74, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Muñoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F., Jr.; Bock, S.A.; Branum, A.; Brown, S.G.; Camargo, C.A., Jr.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and management of anaphylaxis: Summary report-Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J. Allergy Clin. Immunol. 2006, 117, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Fernandez Rivas, M.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Sanchez Borges, M.; et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef] [PubMed]
- Castells, M. Diagnosis and management of anaphylaxis in precision medicine. J. Allergy Clin. Immunol. 2017, 140, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Messmer, K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet 1977, 1, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G. Clinical features and severity grading of anaphylaxis. J. Allergy Clin. Immunol. 2004, 114, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Isabwe, G.A.C.; Garcia Neuer, M.; de Las Vecillas Sanchez, L.; Lynch, D.M.; Marquis, K.; Castells, M. Hypersensitivity reactions to therapeutic monoclonal antibodies: Phenotypes and endotypes. J. Allergy Clin. Immunol. 2018, 142, 159–170. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, H.; Sun, S.; Ma, X.; Pleasants, R.A.; Tang, H.; Zheng, H.; Zhai, S.; Wang, T. Clinical features and treatment of pediatric patients with drug-induced anaphylaxis: A study based on pharmacovigilance data. Eur. J. Pediatr. 2018, 177, 145–154. [Google Scholar] [CrossRef]
- Hsin, Y.C.; Hsin, Y.C.; Huang, J.L.; Yeh, K.W. Clinical features of adult and pediatric anaphylaxis in Taiwan. Asian Pac. J. Allergy Immunol. 2011, 29, 307–312. [Google Scholar]
- Jares, E.J.; Baena-Cagnani, C.E.; Sánchez-Borges, M.; Ensina, L.F.; Arias-Cruz, A.; Gómez, M.; Cuello, M.N.; Morfin-Maciel, B.M.; De Falco, A.; Barayazarra, S.; et al. Drug-induced anaphylaxis in Latin American countries. J. Allergy Clin. Immunol. Pract. 2015, 3, 780–788. [Google Scholar] [CrossRef]
- Hanschmann, T.; Francuzik, W.; Dölle-Bierke, S.; Hofmeier, K.S.; Grabenhenrich, L.; Ruëff, F.; Renaudin, J.M.; Pföhler, C.; Treudler, R.; Bilò, M.B.; et al. Different phenotypes of drug-induced anaphylaxis-Data from the European Anaphylaxis Registry. Allergy 2023, 78, 1615–1627. [Google Scholar] [CrossRef]
- Gold, M.S.; MacDonald, N.E.; McMurtry, C.M.; Balakrishnan, M.R.; Heininger, U.; Menning, L.; Benes, O.; Pless, R.; Zuber, P.L.F. Immunization stress-related response—Redefining immunization anxiety-related reaction as an adverse event following immunization. Vaccine 2020, 38, 3015–3020. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Strauss, B.; De Serres, G.; MacDonald, D.; Marion, S.A.; Naus, M.; Patrick, D.M.; Kendall, P. Oculo-respiratory syndrome: A new influenza vaccine- associated adverse event? Clin. Infect. Dis. 2003, 36, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.R.; Brockow, K.; Kuyucu, S.; Saretta, F.; Mori, F.; Blanca-Lopez, N.; Ott, H.; Atanaskovic-Markovic, M.; Kidon, M.; Caubet, J.C.; et al. ENDA/EAACI Drug Allergy Interest Group. Drug hypersensitivity in children: Report from the pediatric task force of the EAACI Drug Allergy Interest Group. Allergy 2016, 71, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Erkoçoğlu, M.; Kaya, A.; Civelek, E.; Ozcan, C.; Cakır, B.; Akan, A.; Toyran, M.; Ginis, T.; Kocabas, C.N. Prevalence of confirmed immediate type drug hypersensitivity reactions among school children. Pediatr. Allergy Immunol. 2013, 24, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Romano, A.; Blanca, M.; Ring, J.; Pichler, W.; Demoly, P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy 2002, 57, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Saretta, F.; Tomei, L.; Mori, F.; Mayorga, C. In vitro diagnostic testing for drug allergy in children. Pediatr. Allergy Immunol. 2023, 34, e13955. [Google Scholar] [CrossRef]
- Romano, A.; Atanaskovic-Markovic, M.; Barbaud, A.; Bircher, A.J.; Brockow, K.; Caubet, J.C.; Celik, G.; Cernadas, J.; Chiriac, A.M.; Demoly, P.; et al. Towards a more precise diagnosis of hypersensitivity to beta-lactams—An EAACI position paper. Allergy 2020, 75, 1300–1315. [Google Scholar] [CrossRef]
- Hoffmann, H.J.; Santos, A.F.; Mayorga, C.; Nopp, A.; Eberlein, B.; Ferrer, M.; Rouzaire, P.; Ebo, D.G.; Sabato, V.; Sanz, M.L.; et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy 2015, 70, 1393–1405. [Google Scholar] [CrossRef]
- Mayorga, C.; Celik, G.; Rouzaire, P.; Whitaker, P.; Bonadonna, P.; Rodrigues-Cernadas, J.; Vultaggio, A.; Brockow, K.; Caubet, J.C.; Makowska, J.; et al. In vitro tests for Drug Allergy Task Force of EAACI Drug Interest Group. In vitro tests for Drug Allergy Task Force of EAACI Drug Interest Group. In vitro tests for drug hypersensitivity reactions: An ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2016, 71, 1103–1134. [Google Scholar] [CrossRef]
- Barbaud, A.; Romano, A. Skin Testing Approaches for Immediate and Delayed Hypersensitivity Reactions. Immunol. Allergy Clin. N. Am. 2022, 42, 307–322. [Google Scholar] [CrossRef]
- Brockow, K.; Garvey, L.H.; Aberer, W.; Atanaskovic-Markovic, M.; Barbaud, A.; Bilo, M.B.; Bircher, A.; Blanca, M.; Bonadonna, B.; Campi, P.; et al. Skin test concentrations for systemically administered drugs—An ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2013, 68, 702–712. [Google Scholar] [CrossRef]
- Bruusgaard-Mouritsen, M.A.; Jensen, B.M.; Poulsen, L.K.; Duus Johansen, J.; Garvey, L.H. In vitro tests for Drug Allergy Task Force of EAACI Drug Interest Group. Optimizing investigation of suspected allergy to polyethylene glycols. J. Allergy Clin. Immunol. 2022, 149, 168–175. [Google Scholar] [CrossRef]
- Torres, M.J.; Romano, A.; Celik, G.; Demoly, P.; Khan, D.A.; Macy, E.; Park, M.; Blumenthal, K.; Aberer, W.; Castells, M.; et al. Approach to the diagnosis of drug hypersensitivity reactions: Similarities and differences between Europe and North America. Clin. Transl. Allergy 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Ebo, D.G.; Lang, D.M.; Pichler, W.J.; Sabato, V.; Park, M.A.; Makowska, J.; Atanaskovic-Markovic, M.; Bonadonna, P.; Jares, E. Controversies in drug allergy: In vitro testing. J. Allergy Clin. Immunol. 2019, 143, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Leysen, J.; Bridts, C.H.; De Clerck, L.S.; Vercauteren, M.; Lambert, J.; Weyler, J.J.; Stevens, W.J.; Ebo, D.G. Allergy to rocuronium: From clinical suspicion to correct diagnosis. Allergy 2011, 66, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, A.; Brockow, K.; Stoevesandt, J. Metamizole-induced reactions as a paradigm of drug hypersensitivity: Non-allergic reactions, anaphylaxis, and delayed-type allergy. Clin. Exp. Allergy 2020, 50, 1103–1106. [Google Scholar] [CrossRef]
- Bavbek, S.; Pagani, M.; Alvarez-Cuesta, E.; Castells, M.; Dursun, A.B.; Hamadi, S.; Madrigal-Burgaleta, R.; Sanchez-Sanchez, S.; Vultaggio, A. Hypersensitivity reactions to biologicals: An EAACI position paper. Allergy 2022, 77, 39–54. [Google Scholar] [CrossRef]
- Tejedor Alonso, M.; Moro Moro, M.; Múgica García, M.V. Epidemiology of anaphylaxis. Clin. Exp. Allergy 2015, 45, 1027–1039. [Google Scholar] [CrossRef]
- Yu, J.E.; Lin, R.Y. The Epidemiology of Anaphylaxis. Clin. Rev. Allergy Immunol. 2018, 54, 366–374. [Google Scholar] [CrossRef]
- Mullins, R.J.; Wainstein, B.K.; Barnes, E.H.; Liew, W.K.; Campbell, D.E. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin. Exp. Allergy 2016, 46, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Jerschow, E.; Lin, R.Y.; Scaperotti, M.M.; McGinn, A.P. Fatal anaphylaxis in the United States, 1999–2010: Temporal patterns and demographic associations. J. Allergy Clin. Immunol. 2014, 134, 1318–1328. [Google Scholar] [CrossRef]
- Pouessel, G.; Tanno, L.K.; Claverie, C.; Lejeune, S.; Labreuche, J.; Dorkenoo, A.; Renaudin, J.M.; Eb, M.; Leteurtre, S.; Deschildre, A. Fatal anaphylaxis in children in France: Analysis of national data. Pediatr. Allergy Immunol. 2018, 29, 101–104. [Google Scholar] [CrossRef]
- Pouessel, G.; Claverie, C.; Labreuche, J.; Dorkenoo, A.; Renaudin, J.M.; Eb, M.; Lejeune, S.; Deschildre, A.; Leteurtre, S. Fatal anaphylaxis in France: Analysis of national anaphylaxis data, 1979–2011. J. Allergy Clin. Immunol. 2017, 140, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Alonso, M.A.; Martínez-Fernandez, P.; Vallejo-de-Torres, G.; Navarro-Escayola, E.; Moro-Moro, M.; Alberti-Masgrau, N. Clinical and demographic characteristics of fatal anaphylaxis in Spain (1998–2011): A comparison between a series from the hospital system and a national forensic series. Clin. Exp. Allergy 2019, 49, 82–91. [Google Scholar] [CrossRef]
- Pouessel, G.; Alonzo, S.; Divaret-Chauveau, A.; Dumond, P.; Bradatan, E.; Liabeuf, V.; Beaumont, P.; Tscheiller, S.; Diesnis, R.; Renaudin, J.M.; et al. Allergy-Vigilance® Network. Fatal and near-fatal anaphylaxis: The Allergy-Vigilance® Network data (2002–2020). Allergy 2023, 78, 1628–1638. [Google Scholar] [CrossRef]
- Wang, Y.; Allen, K.J.; Suaini, N.H.A.; McWilliam, V.; Peters, R.L.; Koplin, J.J. The global incidence and prevalence of anaphylaxis in children in the general population: A systematic review. Allergy 2019, 74, 1063–1080. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, N.B.; Guffey, D.; Anagnostou, K.; Coleman, N.E.; Davis, C.M. Epidemiology of Anaphylaxis in Critically Ill Children in the United States and Canada. J. Allergy Clin. Immunol. Pract. 2019, 7, 2241–2249. [Google Scholar] [CrossRef]
- Grabenhenrich, L.B.; Dölle, S.; Moneret-Vautrin, A.; Köhli, A.; Lange, L.; Spindler, T.; Ruëff, F.; Nemat, K.; Maris, I.; Roumpedaki, E.; et al. Anaphylaxis in children and adolescents: The European Anaphylaxis Registry. J. Allergy Clin. Immunol. 2016, 137, 1128–1137. [Google Scholar] [CrossRef]
- Orhan, F.; Canitez, Y.; Bakirtas, A.; Yilmaz, O.; Boz, A.B.; Can, D.; Kuyucu, S.; Harmanci, K.; Tahan, F.; Reisli, I.; et al. Anaphylaxis in Turkish children: A multi-centre, retrospective, case study. Clin. Exp. Allergy 2011, 41, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Thomson, H.; Seith, R.; Craig, S. Downstream consequences of diagnostic error in pediatric anaphylaxis. BMC Pediatr. 2018, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Pinto, B.; Fonseca, J.A.; Gomes, E.R. Frequency of self-reported drug allergy: A systematic review and meta-analysis with meta-regression. Ann. Allergy Asthma Immunol. 2017, 119, 362–373. [Google Scholar] [CrossRef]
- Cavkaytar, O.; Karaatmaca, B.; Cetinkaya, P.G.; Esenboga, S.; Arik Yilmaz, E.; Sahiner, U.M.; Sekerel, B.E.; Soyer, O. Characteristics of drug-induced anaphylaxis in children and adolescents. Allergy Asthma Proc. 2017, 38, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Topal, E.; Bakirtas, A.; Yilmaz, O.; Ertoy Karagol, I.H.; Arga, M.; Demirsoy, M.S. Anaphylaxis in infancy compared with older children. Allergy Asthma Proc. 2013, 34, 233–238. [Google Scholar] [CrossRef]
- Muraro, A.; Roberts, G.; Clark, A.; Eigenmann, P.A.; Halken, S.; Lack, G.; Moneret-Vautrin, A.; Niggemann, B.; Rancé, F.; EAACI Task Force on Anaphylaxis in Children. The management of anaphylaxis in childhood: Position paper of the European academy of allergology and clinical immunology. Allergy 2007, 62, 857–871. [Google Scholar] [CrossRef]
- Gonzalez-Perez, A.; Aponte, Z.; Vidaurre, C.F.; Rodriguez, L.A. Anaphylaxis epidemiology in patients with and patients without asthma: A United Kingdom database review. J. Allergy Clin. Immunol. 2010, 25, 1098–1104. [Google Scholar] [CrossRef]
- Gabrielli, S.; Clarke, A.E.; Eisman, H.; Morris, J.; Joseph, L.; La Vieille, S.; Small, P.; Lim, R.; Enarson, P.; Zelcer, M.; et al. Disparities in rate, triggers, and management in pediatric and adult cases of suspected drug-induced anaphylaxis in Canada. Immun. Inflamm. Dis. 2018, 6, 3–12. [Google Scholar] [CrossRef]
- Matito, A.; Morgado, J.M.; Sánchez-López, P.; Álvarez-Twose, I.; Sánchez-Muñoz, L.; Orfao, A.; Escribano, L. Management of Anesthesia in Adult and Pediatric Mastocytosis: A Study of the Spanish Network on Mastocytosis (REMA) Based on 726 Anesthetic Procedures. Int. Arch. Allergy Immunol. 2015, 167, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Crisafulli, G.; Bianchi, A.; Bottau, P.; Caimmi, S.; Franceschini, F.; Liotti, L.; Paglialunga, C.; Saretta, F.; Caffarelli, C. Drugs and Vaccines Hypersensitivity in Children with Mastocytosis. J. Clin. Med. 2022, 11, 3153. [Google Scholar] [CrossRef] [PubMed]
- Cernadas, J.; Vasconcelos, M.J.; Carneiro-Leão, L. Carneiro-Leão, L. Desensitization in children allergic to drugs: Indications, protocols, and limits. Pediatr. Allergy Immunol. 2023, 34, e13965. [Google Scholar] [CrossRef] [PubMed]
- Burrows, J.A.; Nissen, L.M.; Kirkpatrick, C.M.; Bell, S.C. Beta-lactam allergy in adults with cystic fibrosis. J. Cyst. Fibros. 2007, 6, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Parmar, J.S.; Nasser, S. Antibiotic allergy in cystic fibrosis. Thorax 2005, 60, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Dilley, M.A.; Lee, J.P.; Platt, C.D.; Broyles, A.D. Rituximab desensitization in pediatric patients: Results of a case series. Pediat. Allergy Immunol. Pulmonol. 2016, 29, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, M.; Yologlu, N.; Gacar, G.; Uyan, Z.S.; Eser, I.; Karaoz, E. Successful rapid rituximab desensitization in an adolescent patient with nephrotic syndrome: Increase in number of T-reg cells after desensitization. J. Allergy Clin. Immunol. 2013, 132, 478–480. [Google Scholar] [CrossRef]
- Caimmi, S.M.; Caimmi, D.; Riscassi, S.; Marseglia, G.L. A new pediatric protocol for rapid desensitization to monoclonal antibodies. Int. Arch. Allergy Immunol 2014, 165, 214–218. [Google Scholar] [CrossRef]
- Puchner, T.C.; Kugathasan, S.; Kelly, K.J.; Binion, D.G. Successful desensitization and therapeutic use of infliximab in adult and pediatric Crohn’s disease patients with prior anaphylactic reaction. Inflamm. Bowel Dis. 2001, 7, 34–37. [Google Scholar] [CrossRef]
- Justet, A.; Neukirch, C.; Arrault, X.; Borie, R.; Dombret, M.C.; Crestani, B. Successful rapid tocilizumab desensitization in a patient with Still disease. J. Allergy Clin. Immunol. Pract. 2014, 2, 631–632. [Google Scholar] [CrossRef]
- Castells, M.C.; Tennant, N.M.; Sloane, D.E.; Hsu, F.I.; Barrett, N.A.; Hong, D.I.; Laidlaw, T.M.; Legere, H.J.; Nallamshetty, S.N.; Palis, R.I.; et al. Hypersensitivity reactions to chemotherapy: Outcomes and safety of rapid desensitization in 413 cases. J. Allergy Clin. Immunol. 2008, 122, 574–580. [Google Scholar] [CrossRef]
- Del Carmen Sancho, M.; Breslow, R.; Sloane, D.; Castells, M. Desensitization for hypersensitivity reactions to medications. Chem. Immunol. Allergy 2012, 97, 217–233. [Google Scholar] [PubMed]
- Su, Y.; Wen, J.; Zhang, H.; Zou, Z.; Cai, Y.; Zhang, C. Clinical Characteristics of Anaphylaxis in Children Aged 0–16 Years in Xi'an, China. Int. Arch. Allergy Immunol. 2023, 184, 220–227. [Google Scholar] [CrossRef]
- Blanca-Lopez, N.; Atanaskovic-Markovic, M.; Gomes, E.R.; Kidon, M.; Kuyucu, S.; Mori, F.; Soyer, O.; Caubet, J.C. An EAACI Task Force report on allergy to beta-lactams in children: Clinical entities and diagnostic procedures. Pediatr. Allergy Immunol. 2021, 32, 1426–1436. [Google Scholar] [CrossRef]
- Süleyman, A.; Yücel, E.; Tamay, Z.Ü.; Güler, N. Evaluation of Suspected Macrolide Allergies in Children. Turk. Arch. Pediatr. 2022, 57, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Cavkaytar, O.; Arga, M. NSAID Hypersensitivity in the Pediatric Population:Classification and Diagnostic Strategies. J. Asthma Allergy 2022, 15, 1383–1399. [Google Scholar] [CrossRef]
- Kidon, M.; Blanca-Lopez, N.; Gomes, E.; Terreehorst, I.; Tanno, L.; Ponvert, C.; Chin, C.W.; Caubet, J.C.; Soyer, O.; Mori, F.; et al. EAACI/ENDA Position Paper: Diagnosis and management of hypersensitivity reactions to non- steroidal anti-inflammatory drugs (NSAIDs) in children and adolescents. Pediatr. Allergy Immunol. 2018, 29, 469–480. [Google Scholar] [CrossRef]
- Kowalski, M.L.; Asero, R.; Bavbek, S.; Blanca, M.; Blanca-Lopez, N.; Bochenek, G.; Brockow, K.; Campo, P.; Celik, G.; Cernadas, J.; et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy 2013, 68, 1219–1232. [Google Scholar] [CrossRef]
- Broyles, A.D.; Banerji, A.; Barmettler, S.; Biggs, C.M.; Blumenthal, K.; Brennan, P.J.; Breslow, R.G.; Brockow, K.; Buchheit, K.M.; Cahill, K.N.; et al. Practical Guidance for the Evaluation and Management of Drug Hypersensitivity: Specific Drugs. J. Allergy Clin. Immunol. Pract. 2020, 8, S16–S116. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.A.; Banerji, A.; Phillips, E.J.; Solensky, R.; White, A.A.; Bernstein, J.A.; Chu, D.K.; Ellis, A.K.; Golden, D.B.K.; Greenhawt, M.J.; et al. Drug allergy: A 2022 practice parameter update. J. Allergy Clin. Immunol. 2022, 150, 1333–1393. [Google Scholar] [CrossRef]
- Cavkaytar, O.; Arik Yilmaz, E.; Karaatmaca, B.; Buyuktiryaki, B.; Sackesen, C.; Sekerel, B.E.; Soyer, O. Different Phenotypes of Non-Steroidal Anti-Inflammatory Drug Hypersensitivity during Childhood. Int. Arch. Allergy Immunol. 2015, 167, 211–221. [Google Scholar] [CrossRef]
- Cousin, M.; Chiriac, A.; Molinari, N.; Demoly, P.; Caimmi, D. Phenotypical characterization of children with hypersensitivity reactions to NSAIDs. Pediatr. Allergy Immunol. 2016, 27, 743–748. [Google Scholar] [CrossRef]
- Yilmaz Topal, O.; Kulhas Celik, I.; Turgay Yagmur, I.; Toyran, M.; Civelek, E.; Karaatmaca, B.; Kocabas, C.N.; Dibek Misirlioglu, E. Results of NSAID provocation tests and difficulties in the classification of children with nonsteroidal anti-inflammatory drug hypersensitivity. Ann. Allergy Asthma Immunol. 2020, 125, 202–207. [Google Scholar] [CrossRef]
- Caffarelli, C.; Franceschini, F.; Caimmi, D.; Mori, F.; Diaferio, L.; Di Mauro, D.; Mastrorilli, C.; Arasi, S.; Barni, S.; Bottau, P.; et al. SIAIP position paper: Provocation challenge to antibiotics and non-steroidal anti-inflammatory drugs in children. Ital. J. Pediatr. 2018, 44, 147. [Google Scholar] [CrossRef]
- Heath, J.L.; Heath, R.D.; Tamboli, C.; Johnson, L.; Wilson, A.S.; Chervinskiy, S.; Bell, M.C.; Kennedy, J.L. Mesalamine desensitization in a patient with treatment refractory ulcerative colitis and aspirin and nonsteroidal anti-inflammatory drug hypersensitivity. Ann. Allergy Asthma Immunol. 2017, 118, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Gaeta, F.; Caruso, C.; Fiocchi, A.; Valluzzi, R.L. Evaluation and Updated Classification of Acute Hypersensitivity Reactions to Nonsteroidal Anti- Inflammatory Drugs (NSAIDs): NSAID-Exacerbated or -Induced Food Allergy. J. Allergy Clin. Immunol. Pract. 2023, 11, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Karaatmaca, B.; Sahiner, U.M.; Sekerel, B.E.; Soyer, O. Perioperative hypersensitivity reactions during childhood and outcomes of subsequent anesthesia. Paediatr. Anaesth. 2021, 31, 436–443. [Google Scholar] [CrossRef]
- Dewachter, P.; Mouton-Faivre, C. Allergic risk during paediatric anaesthesia. Ann. Fr. Anesth. Reanim. 2010, 29, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Karila, C.; Brunet-Langot, D.; Labbez, F.; Jacqmarcq, O.; Ponvert, C.; Paupe, J.; Scheinmann, P.; de Blic, J. Anaphylaxis during anesthesia: Results of a 12-year survey at a French pediatric center. Allergy 2005, 60, 828–834. [Google Scholar] [CrossRef]
- Harper, N.J.N.; Cook, T.M.; Garcez, T.; Farmer, L.; Floss, K.; Marinho, S.; Torevell, H.; Warner, A.; Ferguson, K.; Hitchman, J.; et al. Anaesthesia, surgery, and life-threatening allergic reactions: Epidemiology and clinical features of perioperative anaphylaxis in the 6th National Audit Project (NAP6). Br. J. Anaesth. 2018, 121, 159–171. [Google Scholar] [CrossRef]
- Reitter, M.; Petitpain, N.; Latarche, C.; Cottin, J.; Massy, N.; Demoly, P. Fatal anaphylaxis with neuromuscular blocking agents: A risk factor and management analysis. Allergy 2014, 69, 954–959. [Google Scholar] [CrossRef]
- Spoerl, D.; Nigolian, H.; Czarnetzki, C.; Harr, T. Reclassifying anaphylaxis to neuromuscular blocking agents based on the presumed patho-mechanism: IgE-mediated, pharmacological adverse reaction or “Innate Hypersensitivity”? Int. J. Mol. Sci. 2017, 18, 1223. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, B.; Sommerfield, D.; Lucas, M.; von Ungern-Sternberg, B.S. An update on allergy and anaphylaxis in pediatric anesthesia. Paediatr. Anaesth. 2019, 29, 892–900. [Google Scholar] [CrossRef] [PubMed]
- de Las Vecillas, L.; Caimmi, D.; Isabwe, G.A.C.; Madrigal-Burgaleta, R.; Soyer, O.; Tanno, L.; Vultaggio, A.; Giovannini, M.; Mori, F. Hypersensitivity reactions to biologics in children. Expert Opin. Biol. Ther. 2023, 23, 61–72. [Google Scholar] [CrossRef] [PubMed]
| NIAID/FAAN Criteria [15] | WAO Criteria [16] |
|---|---|
| Anaphylaxis is highly likely when any of the following three criteria are fulfilled: | Anaphylaxis is highly likely when any of the following two criteria are fulfilled: |
|
|
| |
|
adults and children over 10 years: systolic BP less than <90 mmHg. |
| Symptoms | Ring and Messmer’s Severity Index [18] | Brown’s Severity Index [19] |
|---|---|---|
| Skin lesions | Grade 1 | Grade 1 |
| Gastrointestinal and respiratory disturbances | Grade 2 | Grade 2 |
| Non-life-threatening cardiovascular symptoms (tachycardia or hypotension) | Grade 2 | Grade 3 |
| Shock and life-threatening smooth muscle spams | Grade 3 | Grade 3 |
| Cardiac and/or respiratory arrest | Grade 4 | Not applicable |
| Specific IgE Determination by FEIA | |
|---|---|
| Antibiotics | Amoxycilloyl, ampicilloyl, penicilloyl G, penicilloyl V, cefaclor |
| Hormones | Human insulin; pancreatin (research only) |
| Gelatin | Bovine gelatin |
| Opioids | Pholcodine |
| Perioperative drugs | Morphine, chlorhexidine, suxamethonium; rocuronium (research only) |
| Intradermal Tests | Skin Prick Tests | |
|---|---|---|
| Beta-Lactams | ||
| Amoxicillin, ampicillin, and other semisynthetic penicillins | 20 mg/mL | 20 mg/mL |
| Aztreonam | 2–20 mg/mL | 2–20 mg/mL |
| Benzylpenicilloyl-poly-L-lysine | 6 × 10−5 mol/L | 6 × 10−5 mol/L |
| Benzylpenicilloyl-octa-L-lysine | 8.64 × 10−5 mol/L | 8.64 × 10−5 mol/L |
| Sodium benzylpenilloate | 1.5 × 10−3 mol/L | 1.5 × 10−3 mol/L |
| Benzylpenicillin | 10,000 IU/mL | 10,000 IU/mL |
| Cefepime | 2 mg/mL | 2 mg/mL |
| Cephalosporins other than cefepime | 20 mg/mL | 20 mg/mL |
| Clavulanic acid | 20 mg/mL | 20 mg/mL |
| Imipenem-cilastatin | 0.5 mg/mL–0.5 mg/mL | 0.5 mg/mL–0.5 mg/mL |
| Ertapenem and meropenem | 1 mg/mL | 1 mg/mL |
| Quinolones | ||
| Ciprofloxacin | 0.006 mg/mL | 0.006 mg/mL |
| Levofloxacin | 0.025 mg/mL | 0.025 mg/mL |
| Ofloxacin | 0.05 mg/mL | 0.05 mg/mL |
| Pefloxacin | None | 0.32 mg/mL |
| Rifampicin | 2 mcg/mL | 2 mcg/mL |
| Macrolides | ||
| Azithromycin | 0.01 mg/mL | 0.01 mg/mL |
| Clarithromycin | 0.05 mg/mL | 0.05 mg/mL |
| Erythromycin | 0.01–0.05 mg/mL | 5 mg/mL |
| Rovamycin | 37.5 IU/mL | 37.5 IU/mL |
| Others | ||
| Clindamycin | 15 mg/mL | 15 mg/mL |
| Cotrimoxazole | 0.8 mg/mL | 0.8 mg/mL |
| Gentamycin | 4 mg/mL | 4 mg/mL |
| Rifampicin | 0.002 mg/mL | 0.002 mg/mL |
| Tobramycin | 4 mg/mL | 4 mg/mL |
| Vancomycin | 0.005–0.05 mg/mL | 0.005–0.05 mg/mL |
| Nonsteroidal Anti-Inflammatory Drugs | ||
| Diclofenac | 2.5 mg/mL | 2.5 mg/mL |
| Ketoprofen | 2 mg/mL | 2 mg/mL |
| Piroxicam | 2 mg/mL | 2 mg/mL |
| Pyrazolones and other injectable NSAIDs | 0.1 mg/mL | 0.1 mg/mL |
| Paracetamol/Acetaminophen | 1 mg/mL | 1 mg/mL |
| Neuromuscular Blocking Agents | ||
| Atracurium | 0.01 mg/mL | 1 mg/mL |
| Cisatracurium | 0.02 mg/mL | 2 mg/mL |
| Mivacurium | 0.002 mg/mL | 0.2 mg/mL |
| Pancuronium | 0.02 mg/mL | 2 mg/mL |
| Rocuronium | 0.05 mg/mL | 10 mg/mL |
| Suxamethonium | 0.1 mg/mL | 10 mg/mL |
| Vecuronium | 0.04 mg/mL | 4 mg/mL |
| Monoclonal Antibodies | ||
| Adalimumab | 50 mg/mL | 50 mg/mL |
| Etanercept | 5 mg/mL | 5 mg/mL |
| Infliximab | 2 mg/mL | 2 mg/mL |
| Infliximab | 10 mg/mL | 10 mg/mL |
| Omalizumab | 1.25 mcg/mL | 1.25 mcg/mL |
| Rituximab | 10 mg/mL (7 negative controls) | 10 mg/mL (7 negative controls) |
| Tocilizumab | 0.2 mg/mL or 20 mg/mL (10 negative controls) 1.62 mg/mL | 0.2 mg/mL or 20 mg/mL (10 negative controls) 1.62 mg/mL |
| Culprit Drugs | Specific IgE | Basophil Activation Test | Skin Tests |
|---|---|---|---|
| Beta-lactams | 0–85% [38] | 44–63% [38] | 70% [35] |
| NMBA | |||
| - Rocuronium | 74–89% [39] | 73–83% [39] | 92–99% [39] |
| NSAIDs | |||
| - Dipyrone | nv | 42–65% [38] | >90% * [40] |
| mAb | 26–68% [38] | nv [41] | nv [41] |
| Volume (mL) | Drug per Bag (mg) | Concentration (mg/mL) | ||||
|---|---|---|---|---|---|---|
| Solutions 1 | 250 | 2.06 | 0.008 | |||
| Solutions 2 | 250 | 20.6 | 0.082 | |||
| Solutions 3 | 250 | 205.189 | 0.821 | |||
| Step n. | Solution n. | Rate (ml/h) | Rate (mg/kg/h) | Time (min) | Dose per step (mg) | Cumulative dose (mg) |
| 1 | 1 | 1 | 0.0006 | 15 | 0.0021 | 0.0021 |
| 2 | 1 | 2.5 | 0.002 | 15 | 0.0052 | 0.0073 |
| 3 | 1 | 5 | 0.003 | 15 | 0.0103 | 0.0176 |
| 4 | 1 | 10 | 0.006 | 15 | 0.0206 | 0.0382 |
| 5 | 2 | 2.5 | 0.02 | 15 | 0.0515 | 0.0897 |
| 6 | 2 | 5 | 0.03 | 15 | 0.103 | 0.1927 |
| 7 | 2 | 10 | 0.07 | 15 | 0.206 | 0.3987 |
| 8 | 2 | 20 | 0.1 | 15 | 0.412 | 0.8107 |
| 9 | 3 | 5 | 0.3 | 15 | 1.0259 | 1.8366 |
| 10 | 3 | 10 | 0.7 | 15 | 2.0519 | 3.8885 |
| 11 | 3 | 20 | 1.3 | 15 | 4.1038 | 7.9923 |
| 12 | 3 | 30 | 2 | 482.5 | 198.0078 | 206.0001 |
| Therapeutic dose 206 mg | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, A.; Valluzzi, R.; Crisafulli, G.; Bottau, P.; Caimmi, S.; Franceschini, F.; Liotti, L.; Mori, F.; Riscassi, S.; Saretta, F.; et al. Drug-Induced Anaphylaxis in Children. Biomedicines 2024, 12, 527. https://doi.org/10.3390/biomedicines12030527
Bianchi A, Valluzzi R, Crisafulli G, Bottau P, Caimmi S, Franceschini F, Liotti L, Mori F, Riscassi S, Saretta F, et al. Drug-Induced Anaphylaxis in Children. Biomedicines. 2024; 12(3):527. https://doi.org/10.3390/biomedicines12030527
Chicago/Turabian StyleBianchi, Annamaria, Rocco Valluzzi, Giuseppe Crisafulli, Paolo Bottau, Silvia Caimmi, Fabrizio Franceschini, Lucia Liotti, Francesca Mori, Sara Riscassi, Francesca Saretta, and et al. 2024. "Drug-Induced Anaphylaxis in Children" Biomedicines 12, no. 3: 527. https://doi.org/10.3390/biomedicines12030527
APA StyleBianchi, A., Valluzzi, R., Crisafulli, G., Bottau, P., Caimmi, S., Franceschini, F., Liotti, L., Mori, F., Riscassi, S., Saretta, F., Scavone, S., & Caffarelli, C. (2024). Drug-Induced Anaphylaxis in Children. Biomedicines, 12(3), 527. https://doi.org/10.3390/biomedicines12030527







