A Novel Bionebulizer Approach to Study the Effects of Natural Mineral Water on a 3D In Vitro Nasal Model from Allergic Rhinitis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sulfurous Thermal Water and Isotonic Sodium Chloride Solution
2.2. 3D Organotypic In Vitro Human Nasal Epithelial Model
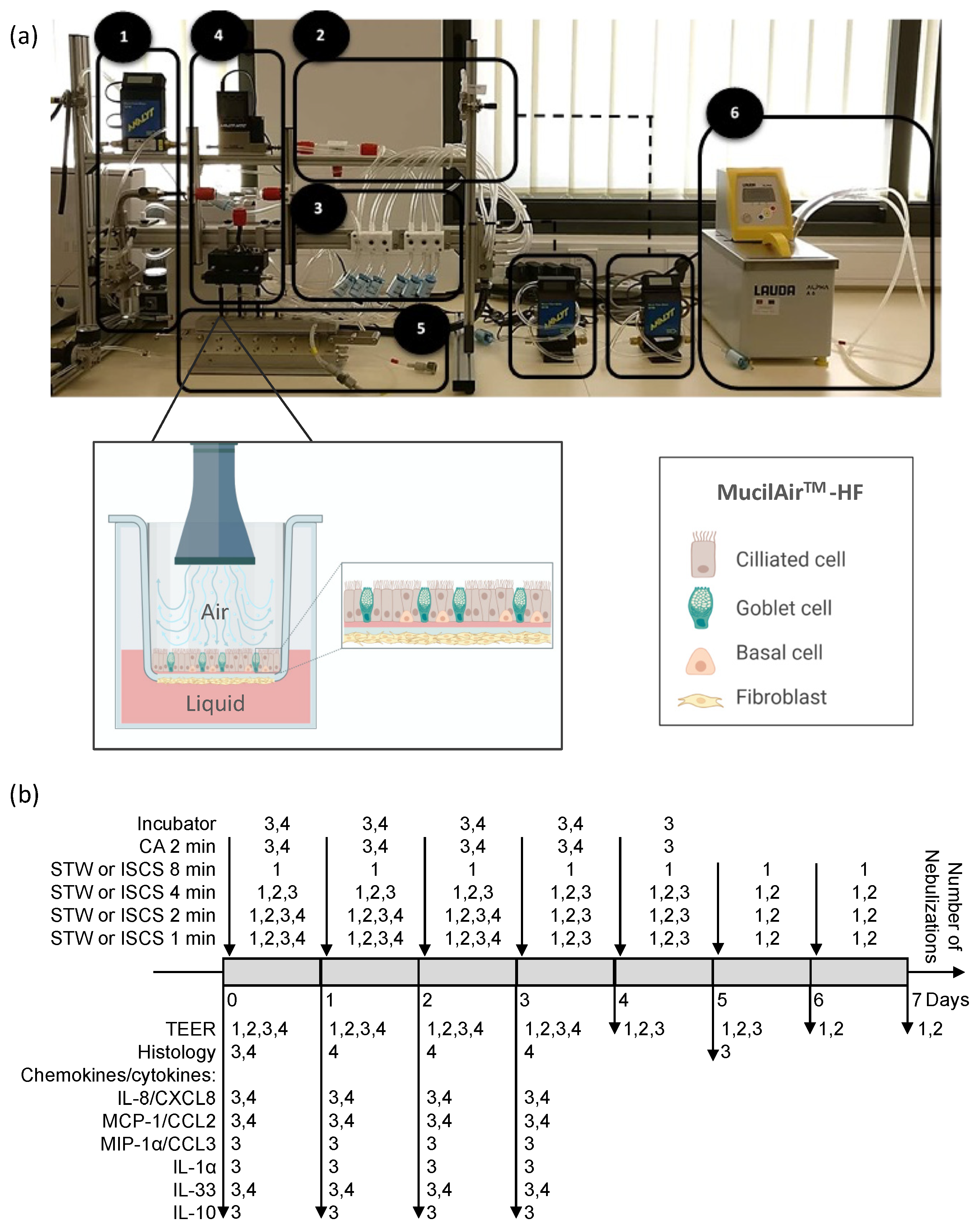
2.3. Aerosol Generation and Exposure
2.4. Experimental Design
2.5. Tissue Integrity Monitoring—Transepithelial Electrical Resistance
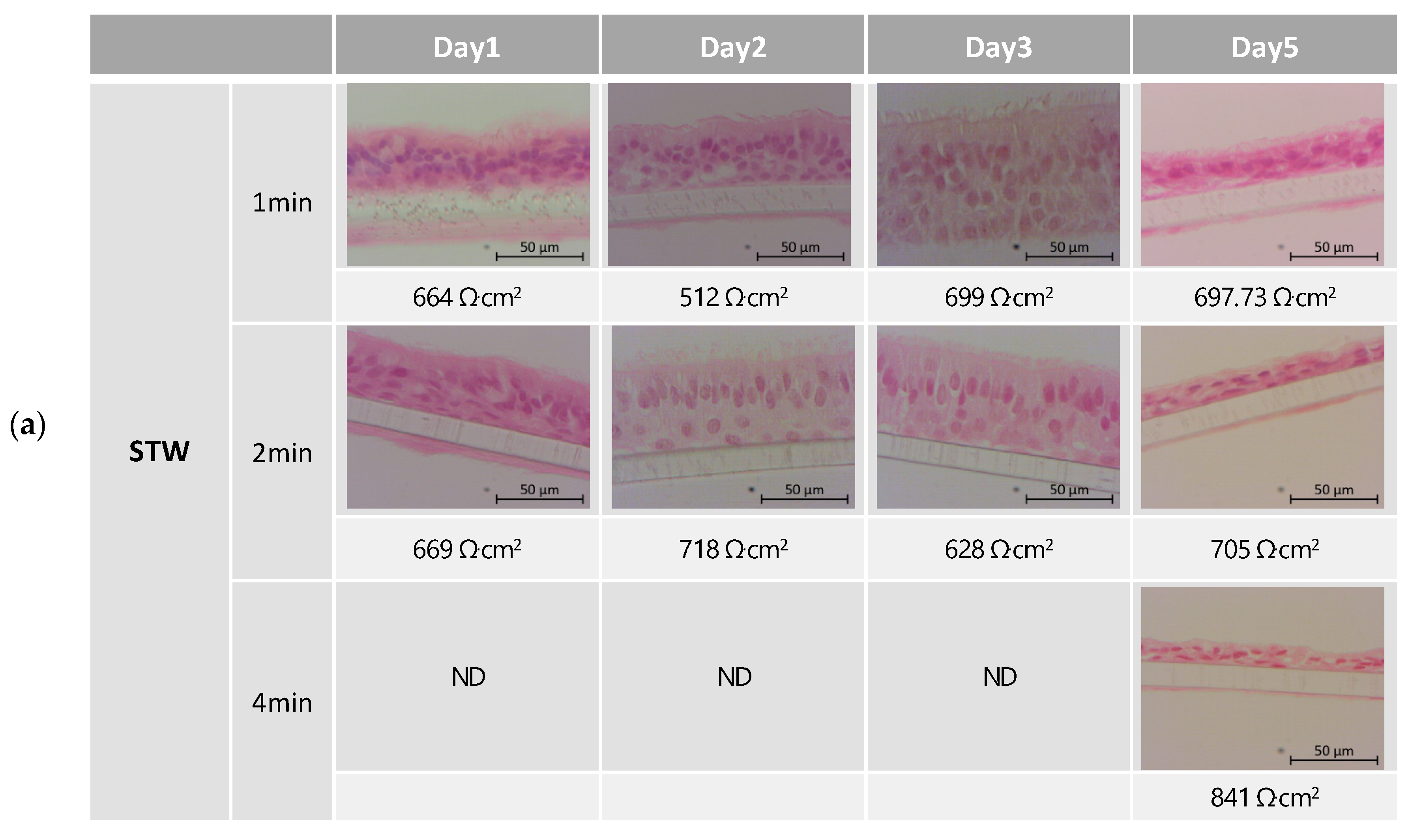
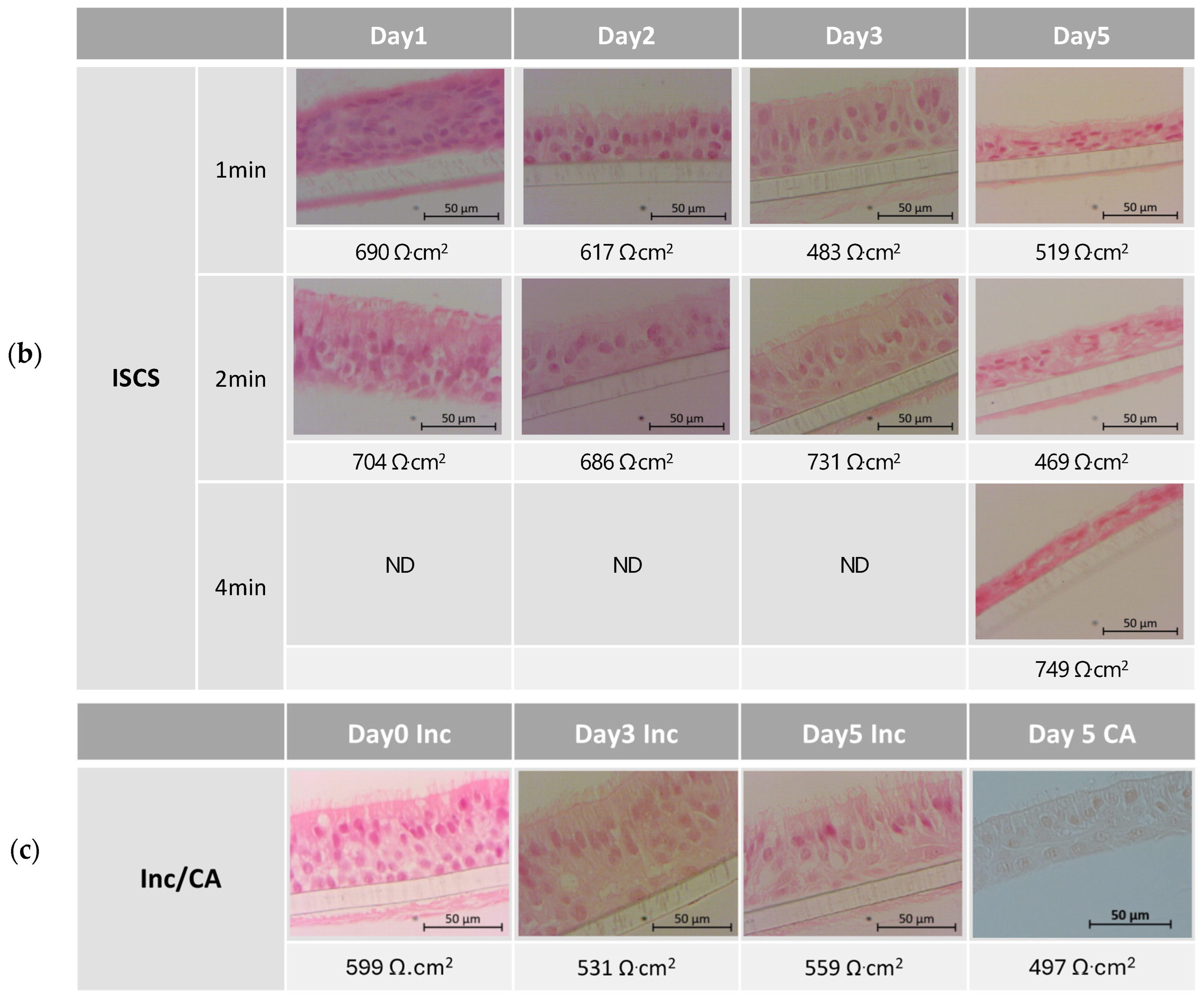
2.6. Morphology Monitoring—Histological Evaluation
2.7. Determination of Chemokine/Cytokine Levels
2.8. Statistical Analysis
3. Results
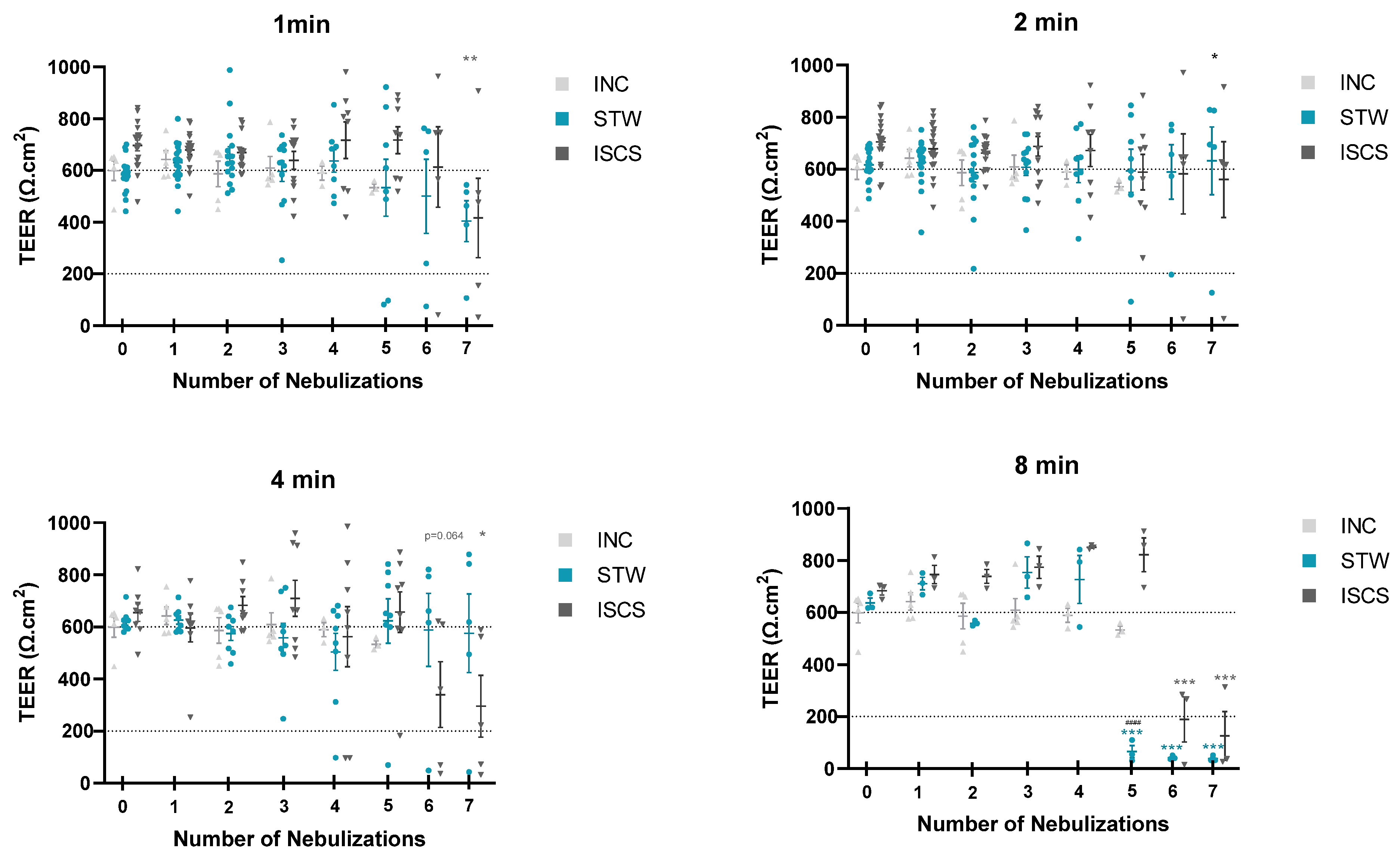
3.1. Tissue Integrity Monitoring
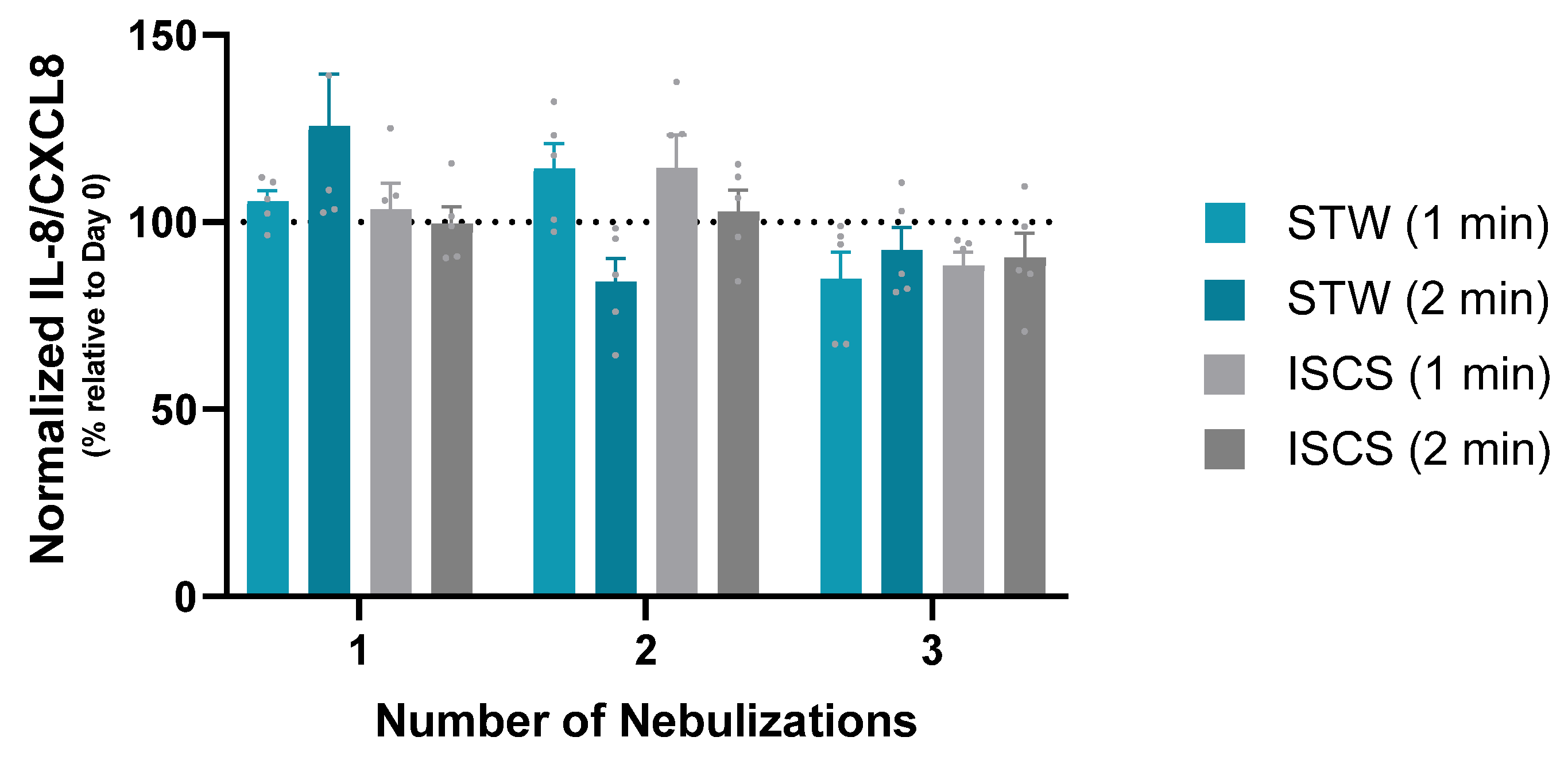
3.2. Chemokines/Cytokines’ Release into the Basal Supernatant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Walter Canonica, G.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic Rhinitis. Nat. Rev. Dis. Prim. 2020, 6, 95. [Google Scholar] [CrossRef]
- Hellings, P.W.; Fokkens, W.J.; Akdis, C.; Bachert, C.; Cingi, C.; Dietz de Loos, D.; Gevaert, P.; Hox, V.; Kalogjera, L.; Lund, V.; et al. Uncontrolled Allergic Rhinitis and Chronic Rhinosinusitis: Where Do We Stand Today? Allergy 2013, 68, 1–7. [Google Scholar] [CrossRef]
- Antonino, M.; Nicolò, M.; Jerome Renee, L.; Federico, M.; Chiara, V.; Stefano, S.; Maria, S.; Salvatore, C.; Antonio, B.; Calvo-Henriquez, C.; et al. Single-nucleotide Polymorphism in Chronic Rhinosinusitis: A Systematic Review. Clin. Otolaryngol. 2022, 47, 14–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, F.; Zhang, L. Update on Pathomechanisms and Treatments in Allergic Rhinitis. Allergy 2022, 77, 3309–3319. [Google Scholar] [CrossRef]
- Ciprandi, G.; Cristofolini, M.; Mira, E. Comano Thermal Water Inhalations in the Treatment of Allergic Rhinitis: Preliminary Results. Eur. Ann. Allergy Clin. Immunol. 2016, 48, 220–223. [Google Scholar]
- Viegas, J.; Esteves, A.F.; Cardoso, E.M.; Arosa, F.A.; Vitale, M.; Taborda-Barata, L. Biological Effects of Thermal Water-Associated Hydrogen Sulfide on Human Airways and Associated Immune Cells: Implications for Respiratory Diseases. Front. Public Health 2019, 7, 128. [Google Scholar] [CrossRef]
- Keller, S.; König, V.; Mösges, R. Thermal Water Applications in the Treatment of Upper Respiratory Tract Diseases: A Systematic Review and Meta-Analysis. J. Allergy 2014, 2014, 943824. [Google Scholar] [CrossRef] [PubMed]
- Zajac, D. Inhalations with Thermal Waters in Respiratory Diseases. J. Ethnopharmacol. 2021, 281, 114505. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, P.; Gobbi, G.; Micheloni, C.; Vaccarezza, M.; Di Marcantonio, D.; Ruscitti, F.; de Panfilis, G.; Vitale, M. Hydrogen Sulfide Inhibits IL-8 Expression in Human Keratinocytes via MAP Kinase Signaling. Lab. Investig. 2011, 91, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Hayden, P.J.; Harbell, J.W. Special Review Series on 3D Organotypic Culture Models: Introduction and Historical Perspective. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Mercier, C.; Jacqueroux, E.; He, Z.; Hodin, S.; Constant, S.; Perek, N.; Boudard, D.; Delavenne, X. Pharmacological Characterization of the 3D MucilAirTM Nasal Model. Eur. J. Pharm. Biopharm. 2019, 139, 186–196. [Google Scholar] [CrossRef]
- Lacroix, G.; Koch, W.; Ritter, D.; Gutleb, A.C.; Larsen, S.T.; Loret, T.; Zanetti, F.; Constant, S.; Chortarea, S.; Rothen-Rutishauser, B.; et al. Air–Liquid Interface In Vitro Models for Respiratory Toxicology Research: Consensus Workshop and Recommendations. Appl. Vitr. Toxicol. 2018, 4, 91–106. [Google Scholar] [CrossRef]
- Zhang, N.; Van Crombruggen, K.; Gevaert, E.; Bachert, C. Barrier Function of the Nasal Mucosa in Health and Type-2 Biased Airway Diseases. Allergy 2016, 71, 295–307. [Google Scholar] [CrossRef]
- Ordovas-Montanes, J.; Dwyer, D.F.; Nyquist, S.K.; Buchheit, K.M.; Vukovic, M.; Deb, C.; Wadsworth, M.H.; Hughes, T.K.; Kazer, S.W.; Yoshimoto, E.; et al. Allergic Inflammatory Memory in Human Respiratory Epithelial Progenitor Cells. Nature 2018, 560, 649–654. [Google Scholar] [CrossRef]
- Gizurarson, S. Anatomical and Histological Factors Affecting Intranasal Drug and Vaccine Delivery. Curr. Drug Deliv. 2012, 9, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Laulajainen-Hongisto, A.; Toppila-Salmi, S.K.; Luukkainen, A.; Kern, R. Airway Epithelial Dynamics in Allergy and Related Chronic Inflammatory Airway Diseases. Front. Cell Dev. Biol. 2020, 8, 204. [Google Scholar] [CrossRef]
- Huang, S.; Wiszniewski, L.; Constant, S.; Roggen, E. Potential of in Vitro Reconstituted 3D Human Airway Epithelia (MucilAirTM) to Assess Respiratory Sensitizers. Toxicol. Vitr. 2013, 27, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.L.; Mann, D.A.; Wilson, J.A.; Fisher, A.J. The Role of the Fibroblast in Inflammatory Upper Airway Conditions. Am. J. Pathol. 2016, 186, 225–233. [Google Scholar] [CrossRef]
- Krausgruber, T.; Fortelny, N.; Fife-Gernedl, V.; Senekowitsch, M.; Schuster, L.C.; Lercher, A.; Nemc, A.; Schmidl, C.; Rendeiro, A.F.; Bergthaler, A.; et al. Structural Cells Are Key Regulators of Organ-Specific Immune Responses. Nature 2020, 583, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhu, X. Role of Chemokines and Inflammatory Cells in Respiratory Allergy. J. Asthma Allergy 2022, 15, 1805–1822. [Google Scholar] [CrossRef] [PubMed]
- Wagenmann, M.; Schumacher, L.; Bachert, C. The Time Course of the Bilateral Release of Cytokines and Mediators after Unilateral Nasal Allergen Challenge. Allergy 2005, 60, 1132–1138. [Google Scholar] [CrossRef]
- Gosset, P.; Malaquim, F.; Delnest, Y.; Wallaert, B.; Capron, A.; Joseph, M.; Tonnel, A. Interleukin-6 and Interleukin-1α Production Is Associated with Antigen-Induced Late Nasal Response. J. Allergy Clin. Immunol. 1993, 92, 878–890. [Google Scholar] [CrossRef]
- Jha, A.; Thwaites, R.S.; Tunstall, T.; Kon, O.M.; Shattock, R.J.; Hansel, T.T.; Openshaw, P.J.M. Increased Nasal Mucosal Interferon and CCL13 Response to a TLR7/8 Agonist in Asthma and Allergic Rhinitis. J. Allergy Clin. Immunol. 2021, 147, 694–703.e12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Yu, S.Q.; Jiang, J.Z.; Liu, G.J. Complementary DNA Microarray Analysis of Chemokines and Their Receptors in Allergic Rhinitis. J. Investig. Allergol. Clin. Immunol. 2007, 17, 329–336. [Google Scholar] [PubMed]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A Comprehensive Review on the Role of IL-1α in the Pathogenesis and Treatment of Autoimmune and Inflammatory Diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Girard, J.-P.; Turnquist, H.R. Interleukin-33 in Health and Disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef]
- Takatori, H.; Makita, S.; Ito, T.; Matsuki, A.; Nakajima, H. Regulatory Mechanisms of IL-33-ST2-Mediated Allergic Inflammation. Front. Immunol. 2018, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Baumann, R.; Rabaszowski, M.; Stenin, I.; Tilgner, L.; Scheckenbach, K.; Wiltfang, J.; Schipper, J.; Chaker, A.; Wagenmann, M. Comparison of the Nasal Release of IL-4, IL-10, IL-17, CCL13/MCP-4, and CCL26/Eotaxin-3 in Allergic Rhinitis during Season and after Allergen Challenge. Am. J. Rhinol. Allergy 2013, 27, 266–272. [Google Scholar] [CrossRef]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The Multifaceted Nature of IL-10: Regulation, Role in Immunological Homeostasis and Its Relevance to Cancer, COVID-19 and Post-COVID Conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Aljamaei, H.M.; Stadnyk, A.W. The Production and Function of Endogenous Interleukin-10 in Intestinal Epithelial Cells and Gut Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1343–1352. [Google Scholar] [CrossRef]
- Hyun, J.; Romero, L.; Riveron, R.; Flores, C.; Kanagavelu, S.; Chung, K.D.; Alonso, A.; Sotolongo, J.; Ruiz, J.; Manukyan, A.; et al. Human Intestinal Epithelial Cells Express Interleukin-10 through Toll-Like Receptor 4-Mediated Epithelial-Macrophage Crosstalk. J. Innate Immun. 2015, 7, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, T.; Piao, C.H.; Fan, Y.J.; Shin, D.-U.; Kim, S.Y.; Song, H.-J.; Song, C.H.; Shin, H.S.; Chai, O.H. Anti-Allergic Rhinitis Activity of α-Lipoic Acid via Balancing Th17/Treg Expression and Enhancing Nrf2/HO-1 Pathway Signaling. Sci. Rep. 2020, 10, 12528. [Google Scholar] [CrossRef]
- Fang, Z.; Yi, F.; Peng, Y.; Zhang, J.; Zhang, L.; Deng, Z.; Chen, F.; Li, C.; He, Y.; Huang, C.; et al. Inhibition of TRPA1 Reduces Airway Inflammation and Hyperresponsiveness in Mice with Allergic Rhinitis. FASEB J. 2021, 35, e21428. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Zhao, Y.; Liu, R. Effects of Differentially Expressed MRNAs Screened Based on GEO Database on Inflammatory Infiltration of Nasal Mucosa in Mice with Allergic Rhinitis. Altern. Ther. Health Med. 2023, 29, 608–612. [Google Scholar]
- Elbrecht, D.H.; Long, C.J.; Hickman, J.J. Transepithelial/Endothelial Electrical Resistance (TEER) Theory and Applications for Microfluidic Body-on-a-Chip Devices. J. Rare Dis. Res. Treat. 2016, 1, 46–52. [Google Scholar] [CrossRef]
- Burgos, M.A.; Sanmiguel-Rojas, E.; del Pino, C.; Sevilla-García, M.A.; Esteban-Ortega, F. New CFD Tools to Evaluate Nasal Airflow. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 3121–3128. [Google Scholar] [CrossRef]
- Wen, J.; Inthavong, K.; Tu, J.; Wang, S. Numerical Simulations for Detailed Airflow Dynamics in a Human Nasal Cavity. Respir. Physiol. Neurobiol. 2008, 161, 125–135. [Google Scholar] [CrossRef]
- Martonen, T.B.; Quan, L.; Zhang, Z.; Musante, C.J. Flow Simulation in the Human Upper Respiratory Tract. Cell Biochem. Biophys. 2002, 37, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Doorly, D.J.; Taylor, D.J.; Schroter, R.C. Mechanics of Airflow in the Human Nasal Airways. Respir. Physiol. Neurobiol. 2008, 163, 100–110. [Google Scholar] [CrossRef]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology, 13th ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 2013206534. [Google Scholar]
- Kumar, H.; Jain, R.; Douglas, R.G.; Tawhai, M.H. Airflow in the Human Nasal Passage and Sinuses of Chronic Rhinosinusitis Subjects. PLoS ONE 2016, 11, e0156379. [Google Scholar] [CrossRef]
- Burrowes, K.S.; De Backer, J.; Kumar, H. Image-based Computational Fluid Dynamics in the Lung: Virtual Reality or New Clinical Practice? WIREs Syst. Biol. Med. 2017, 9, e1392. [Google Scholar] [CrossRef]
- Mygind, N.; Dahl, R. Anatomy, Physiology and Function of the Nasal Cavities in Health and Disease. Adv. Drug Deliv. Rev. 1998, 29, 3–12. [Google Scholar] [CrossRef]
- Buijs, E.F.M.; Covello, V.; Pipolo, C.; Saibene, A.M.; Felisati, G.; Quadrio, M. Thermal Water Delivery in the Nose: Experimental Results Describing Droplet Deposition through Computational Fluid Dynamics. Acta Otorhinolaryngol. Ital. 2019, 39, 396–403. [Google Scholar] [CrossRef]
- Pellegrini, M.; Fanin, D.; Nowicki, Y.; Guarnieri, G.; Bordin, A.; Faggian, D.; Plebani, M.; Saetta, M.; Maestrelli, P. Effect of Inhalation of Thermal Water on Airway Inflammation in Chronic Obstructive Pulmonary Disease. Respir. Med. 2005, 99, 748–754. [Google Scholar] [CrossRef]
- Salami, A. Sulphurous Thermal Water Inhalations in the Treatment of Chronic Rhinosinusitis. Rhinol. J. 2010, 48, 71. [Google Scholar] [CrossRef] [PubMed]
- Seite, S. Thermal Waters as Cosmeceuticals: La Roche-Posay Thermal Spring Water Example. Clin. Cosmet. Investig. Dermatol. 2013, 6, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Oliveira, A.S.; Vaz, C.V.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R.; Pereira, C.M.F.; Palmeira-de-Oliveira, A.; et al. Anti-Inflammatory Potential of Portuguese Thermal Waters. Sci. Rep. 2020, 10, 22313. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, G.; Gobbi, G.; Masselli, E.; Carubbi, C.; Presta, V.; Ambrosini, L.; Vitale, M.; Mirandola, P. Buffering Adaptive Immunity by Hydrogen Sulfide. Cells 2022, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, V.; Neubauer, A.; Gevaert, P.; Zidarn, M.; Worm, M.; Aberer, W.; Malling, H.J.; Pfaar, O.; Klimek, L.; Pfützner, W.; et al. Safety and Efficacy of Immunotherapy with the Recombinant B-Cell Epitope–Based Grass Pollen Vaccine BM32. J. Allergy Clin. Immunol. 2018, 142, 497–509.e9. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Rodrigues, M.; Mourelle, M.L.; Araujo, A.R.T.S. Thermal Spring Waters as an Active Ingredient in Cosmetic Formulations. Cosmetics 2023, 10, 27. [Google Scholar] [CrossRef]
- Joly, F.; Gardille, C.; Barbieux, E.; Lefeuvre, L. Beneficial Effect of a Thermal Spring Water on the Skin Barrier Recovery after Injury: Evidence for Claudin-6 Expression in Human Skin. J. Cosmet. Dermatol. Sci. Appl. 2012, 2, 273–276. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Vaz, C.V.; Silva, A.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R.; Pereira, C.; Cruz, M.T.; et al. In Vitro Evaluation of Potential Benefits of a Silica-Rich Thermal Water (Monfortinho Thermal Water) in Hyperkeratotic Skin Conditions. Int. J. Biometeorol. 2020, 64, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Rasmont, V.; Valois, A.; Gueniche, A.; Sore, G.; Kerob, D.; Nielsen, M.; Berardesca, E. Vichy Volcanic Mineralizing Water Has Unique Properties to Strengthen the Skin Barrier and Skin Defenses against Exposome Aggressions. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.; Ceci, C.; Marabini, L.; Nappi, G. The Antioxidant Activity of Sulphurous Thermal Water Protects against Oxidative DNA Damage: A Comet Assay Investigation. Drug Res. 2013, 63, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Dankers, A.C.A.; Kuper, C.F.; Boumeester, A.J.; Fabriek, B.O.; Kooter, I.M.; Gröllers-Mulderij, M.; Tromp, P.; Nelissen, I.; Zondervan-Van Den Beuken, E.K.; Vandebriel, R.J. A Practical Approach to Assess Inhalation Toxicity of Metal Oxide Nanoparticles in Vitro. J. Appl. Toxicol. 2018, 38, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Donkers, J.M.; Höppener, E.M.; Grigoriev, I.; Will, L.; Melgert, B.N.; van der Zaan, B.; van de Steeg, E.; Kooter, I.M. Advanced Epithelial Lung and Gut Barrier Models Demonstrate Passage of Microplastic Particles. Microplastics Nanoplastics 2022, 2, 6. [Google Scholar] [CrossRef]
- George, I.; Uboldi, C.; Bernard, E.; Sobrido, M.; Dine, S.; Hagège, A.; Vrel, D.; Herlin, N.; Rose, J.; Orsière, T.; et al. Toxicological Assessment of ITER-Like Tungsten Nanoparticles Using an In Vitro 3D Human Airway Epithelium Model. Nanomaterials 2019, 9, 1374. [Google Scholar] [CrossRef]
- Kooter, I.; Ilves, M.; Gröllers-Mulderij, M.; Duistermaat, E.; Tromp, P.C.; Kuper, F.; Kinaret, P.; Savolainen, K.; Greco, D.; Karisola, P.; et al. Molecular Signature of Asthma-Enhanced Sensitivity to CuO Nanoparticle Aerosols from 3D Cell Model. ACS Nano 2019, 13, 6932–6946. [Google Scholar] [CrossRef] [PubMed]
- Meindl, C.; Absenger-Novak, M.; Jeitler, R.; Roblegg, E.; Fröhlich, E. Assessment of Carbon Nanotubes on Barrier Function, Ciliary Beating Frequency and Cytokine Release in In Vitro Models of the Respiratory Tract. Nanomaterials 2023, 13, 682. [Google Scholar] [CrossRef]
- Movia, D.; Di Cristo, L.; Alnemari, R.; McCarthy, J.E.; Moustaoui, H.; Lamy de la Chapelle, M.; Spadavecchia, J.; Volkov, Y.; Prina-Mello, A. The Curious Case of How Mimicking Physiological Complexity in in Vitro Models of the Human Respiratory System Influences the Inflammatory Responses. A Preliminary Study Focused on Gold Nanoparticles. J. Interdiscip. Nanomed. 2017, 2, 110–130. [Google Scholar] [CrossRef]
- Metz, J.; Knoth, K.; Groß, H.; Lehr, C.-M.; Stäbler, C.; Bock, U.; Hittinger, M. Combining MucilAirTM and Vitrocell® Powder Chamber for the In Vitro Evaluation of Nasal Ointments in the Context of Aerosolized Pollen. Pharmaceutics 2018, 10, 56. [Google Scholar] [CrossRef]
- Czekala, L.; Wieczorek, R.; Simms, L.; Yu, F.; Budde, J.; Trelles Sticken, E.; Rudd, K.; Verron, T.; Brinster, O.; Stevenson, M.; et al. Multi-Endpoint Analysis of Human 3D Airway Epithelium Following Repeated Exposure to Whole Electronic Vapor Product Aerosol or Cigarette Smoke. Curr. Res. Toxicol. 2021, 2, 99–115. [Google Scholar] [CrossRef]
- McGee Hargrove, M.; Parr-Dobrzanski, B.; Li, L.; Constant, S.; Wallace, J.; Hinderliter, P.; Wolf, D.C.; Charlton, A. Use of the MucilAir Airway Assay, a New Approach Methodology, for Evaluating the Safety and Inhalation Risk of Agrochemicals. Appl. Vitr. Toxicol. 2021, 7, 50–60. [Google Scholar] [CrossRef]
- De Servi, B.; Ranzini, F.; Piqué, N. Protective Barrier Properties of Rhinosectan® Spray (Containing Xyloglucan) on an Organotypic 3D Airway Tissue Model (MucilAir): Results of an in Vitro Study. Allergy Asthma Clin. Immunol. 2017, 13, 37. [Google Scholar] [CrossRef]
- Sivars, K.B.; Sivars, U.; Hornberg, E.; Zhang, H.; Brändeń, L.; Bonfante, R.; Huang, S.; Constant, S.; Robinson, I.; Betts, C.J.; et al. A 3D Human Airway Model Enables Prediction of Respiratory Toxicity of Inhaled Drugs in Vitro. Toxicol. Sci. 2018, 162, 301–308. [Google Scholar] [CrossRef]
- Boda, B.; Benaoudia, S.; Huang, S.; Bonfante, R.; Wiszniewski, L.; Tseligka, E.D.; Tapparel, C.; Constant, S. Antiviral Drug Screening by Assessing Epithelial Functions and Innate Immune Responses in Human 3D Airway Epithelium Model. Antiviral Res. 2018, 156, 72–79. [Google Scholar] [CrossRef]
- Robinot, R.; Hubert, M.; de Melo, G.D.; Lazarini, F.; Bruel, T.; Smith, N.; Levallois, S.; Larrous, F.; Fernandes, J.; Gellenoncourt, S.; et al. SARS-CoV-2 Infection Induces the Dedifferentiation of Multiciliated Cells and Impairs Mucociliary Clearance. Nat. Commun. 2021, 12, 4354. [Google Scholar] [CrossRef]
- Haswell, L.E.; Smart, D.; Jaunky, T.; Baxter, A.; Santopietro, S.; Meredith, S.; Camacho, O.M.; Breheny, D.; Thorne, D.; Gaca, M.D. The Development of an in Vitro 3D Model of Goblet Cell Hyperplasia Using MUC5AC Expression and Repeated Whole Aerosol Exposures. Toxicol. Lett. 2021, 347, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Kooter, I.M.; Gröllers-Mulderij, M.; Duistermaat, E.; Kuper, F.; Schoen, E.D. Factors of Concern in a Human 3D Cellular Airway Model Exposed to Aerosols of Nanoparticles. Toxicol. Vitr. 2017, 44, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Haenuki, Y.; Matsushita, K.; Futatsugi-Yumikura, S.; Ishii, K.J.; Kawagoe, T.; Imoto, Y.; Fujieda, S.; Yasuda, M.; Hisa, Y.; Akira, S.; et al. A Critical Role of IL-33 in Experimental Allergic Rhinitis. J. Allergy Clin. Immunol. 2012, 130, 184–194.e11. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.; Wallace, J.; Lansley, A.B.; Roper, C. Evaluation of the Toxicity of Sodium Dodecyl Sulphate (SDS) in the MucilAirTM Human Airway Model in Vitro. Regul. Toxicol. Pharmacol. 2021, 125, 105022. [Google Scholar] [CrossRef]
- Bessa, M.J.; Brandão, F.; Fokkens, P.; Cassee, F.R.; Salmatonidis, A.; Viana, M.; Vulpoi, A.; Simon, S.; Monfort, E.; Teixeira, J.P.; et al. Toxicity Assessment of Industrial Engineered and Airborne Process-Generated Nanoparticles in a 3D Human Airway Epithelial in Vitro Model. Nanotoxicology 2021, 15, 542–557. [Google Scholar] [CrossRef]
- Hufnagel, M.; May, N.; Wall, J.; Wingert, N.; Garcia-Käufer, M.; Arif, A.; Hübner, C.; Berger, M.; Mülhopt, S.; Baumann, W.; et al. Impact of Nanocomposite Combustion Aerosols on A549 Cells and a 3D Airway Model. Nanomaterials 2021, 11, 1685. [Google Scholar] [CrossRef]
- Huang, S.; Constant, S.; De Servi, B.; Meloni, M.; Culig, J.; Bertini, M.; Saaid, A. In Vitro Safety and Performance Evaluation of a Seawater Solution Enriched with Copper, Hyaluronic Acid, and Eucalyptus for Nasal Lavage. Med. Devices Evid. Res. 2019, 12, 399–410. [Google Scholar] [CrossRef]
- Barreto-Duran, E.; Szczepański, A.; Gałuszka-Bulaga, A.; Surmiak, M.; Siedlar, M.; Sanak, M.; Rajfur, Z.; Milewska, A.; Lenart, M.; Pyrć, K. The Interplay between the Airway Epithelium and Tissue Macrophages during the SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 991991. [Google Scholar] [CrossRef] [PubMed]
- Chapman, F.; Pour, S.J.; Wieczorek, R.; Trelles Sticken, E.; Budde, J.; Röwer, K.; Otte, S.; Mason, E.; Czekala, L.; Nahde, T.; et al. Twenty-Eight Day Repeated Exposure of Human 3D Bronchial Epithelial Model to Heated Tobacco Aerosols Indicates Decreased Toxicological Responses Compared to Cigarette Smoke. Front. Toxicol. 2023, 5, 1076752. [Google Scholar] [CrossRef] [PubMed]
- Cervena, T.; Vrbova, K.; Rossnerova, A.; Topinka, J.; Rossner, P. Short-Term and Long-Term Exposure of the MucilAirTM Model to Polycyclic Aromatic Hydrocarbons. ATLA Altern. Lab. Anim. 2019, 47, 9–18. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Result | Method | |
|---|---|---|---|
| Physico-chemical | |||
| Temperature at source | 37.5 °C | ||
| pH (at 24 °C) | 8.43 | SMEWW 4500 H+ | |
| Conductivity (at 20 °C) | 303 µS·cm−1 | NP EN 27888:1996 | |
| Resistivity | 3.3 × 103 Ω·cm | LAE 4.3 A | |
| Total sulfur | 0.14 mmol·L−1 | M.M. (CI) | |
| Total sulfuring of sulfide | 13 mL (I2 0.01 N·L−1) | M.M. 3.11 (21 May 2013) | |
| Hydrogen sulfide | <0.5 mg (H2S/L) | M.M. 2.2.7 (7 February 2003) | |
| Total alkalinization | 75.5 mg (CaCO3)·L−1 | SMEWW 2320 | |
| Hardness | 10 mg (CaCO3)·L−1 | SMEWW 2340B | |
| Silica (SO2) | 55 mg (SiO2)·L−1 | SMEWW 4500 Si-C | |
| Total silicon | 57 mg (SiO2)·L−1 | SMEWW 4500 Si-C | |
| Dry residue | 226 mg·L−1 | SMEWW 1030 E | |
| Total mineralization | 268 mg·L−1 | M.M. 2.1.11 (3 April 2009) | |
| Anions | |||
| Bicarbonate (HCO3−) | 82.9 mg (HCO3)·L−1 | 1.36 mEq·L−1 | M.M. 2.2.7 (7 February 2003) |
| Carbonate | <2 mg (CO3)·L−1 | ___ | M.M. 2.2.7 (7 February 2003) |
| Chloride (Cl−) | 26 mg·L−1 | 0.73 mEq·L−1 | SMEWW 4110B |
| Fluoride (F−) | 16 mg·L−1 | 0.84 mEq·L−1 | SMEWW 4110B |
| Hydrosulfide | 2.2 mg (HS)·L−1 | 0.07 mEq·L−1 | M.M. 2.2.7 |
| Silicate | 3.4 mg (H3SiO4)·L−1 | M.M. 2.2.7 (7 February 2003) | |
| Nitrate | <0.3 mg (NO3)·L−1 | ___ | SMEWW 4110B |
| Nitrite | <0.010 mg (NO2)·L−1 | ___ | SMEWW 4500 NO2-B |
| Silicate | 3.4 mg (H3SiO4)·L−1 | 0.04 mEq·L−1 | M.M. 2.2.7 (7 February 2003) |
| Sulphate (SO42−) | 7.9 mg (SO42−)·L−1 | 0.16 mEq·L−1 | SMEWW 4110 B |
| Cations | |||
| Ammonia nitrogen | 0.08 mg (NH4)·L−1 | ___ | M.M. 4.1 (22 November 1997) |
| Calcium (Ca2+) | 3.9 mg·L−1 | 0.19 mEq·L−1 | EPA 300.7:1986 |
| Lithium | 0.3 mg·L−1 | 0.04 mEq·L−1 | EPA 300.7:1986 |
| Magnesium | 0.15 mg·L−1 | 0.01 mEq·L−1 | EPA 300.7:1986 |
| Sodium (Na+) | 67 mg·L−1 | 2.91 mEq·L−1 | EPA 300.7:1986 |
| Potassium (K+) | 2.0 mg·L−1 | 0.05 mEq·L−1 | EPA 300.7:1986 |
| Iron | <0.006 mg·L−1 | ___ | M.M. 5.4 (EAA-CG) (6 May 2013) |
| Identification Code | Batch Number | Age (Years Old) | Sex | Smoker | Origin | Pathology | Viral Status * |
|---|---|---|---|---|---|---|---|
| EP29 | HF-MD006201 | 36 | Male | No | Caucasian | Allergic rhinitis | Negative |
| EP14 | HF-MD041901 | 52 | Female | No | Caucasian | Allergic rhinitis | Negative |
| Set | ID Code | Number of Nebulizations | Duration of Nebulization (Minutes) | Condition (n) | |||
|---|---|---|---|---|---|---|---|
| STW | ISCS | CA | None (Incubator) | ||||
| Pilot | EP29 | 10 | 15 | 6 | 5 | - | NA |
| 1 | EP14 | 7 | 1 | 3 | 3 | - | NA |
| 2 | 3 | 3 | - | NA | |||
| 4 | 3 | 3 | - | NA | |||
| 8 | 3 | 3 | - | NA | |||
| 2 | EP14 | 7 | 1 | 2 | 2 | - | NA |
| 2 | 2 | 2 | - | NA | |||
| 4 | 2 | 2 | - | NA | |||
| 3 | EP14 | 0 | NA | NA | NA | NA | 3 |
| 5 | 1 | 3 | 3 | - | NA | ||
| 2 | 3 | 3 | 2 | NA | |||
| 4 | 3 | 3 | - | NA | |||
| 4 | EP14 | 0 | NA | NA | NA | NA | 2 |
| 3 | 1 | 11 | 11 | - | NA | ||
| 2 | 11 | 11 | 3 | NA | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, J.; Cardoso, E.M.; Bonneau, L.; Esteves, A.F.; Ferreira, C.L.; Alves, G.; Santos-Silva, A.J.; Vitale, M.; Arosa, F.A.; Taborda-Barata, L. A Novel Bionebulizer Approach to Study the Effects of Natural Mineral Water on a 3D In Vitro Nasal Model from Allergic Rhinitis Patients. Biomedicines 2024, 12, 408. https://doi.org/10.3390/biomedicines12020408
Viegas J, Cardoso EM, Bonneau L, Esteves AF, Ferreira CL, Alves G, Santos-Silva AJ, Vitale M, Arosa FA, Taborda-Barata L. A Novel Bionebulizer Approach to Study the Effects of Natural Mineral Water on a 3D In Vitro Nasal Model from Allergic Rhinitis Patients. Biomedicines. 2024; 12(2):408. https://doi.org/10.3390/biomedicines12020408
Chicago/Turabian StyleViegas, Joana, Elsa M. Cardoso, Lucile Bonneau, Ana Filipa Esteves, Catarina L. Ferreira, Gilberto Alves, António Jorge Santos-Silva, Marco Vitale, Fernando A. Arosa, and Luís Taborda-Barata. 2024. "A Novel Bionebulizer Approach to Study the Effects of Natural Mineral Water on a 3D In Vitro Nasal Model from Allergic Rhinitis Patients" Biomedicines 12, no. 2: 408. https://doi.org/10.3390/biomedicines12020408
APA StyleViegas, J., Cardoso, E. M., Bonneau, L., Esteves, A. F., Ferreira, C. L., Alves, G., Santos-Silva, A. J., Vitale, M., Arosa, F. A., & Taborda-Barata, L. (2024). A Novel Bionebulizer Approach to Study the Effects of Natural Mineral Water on a 3D In Vitro Nasal Model from Allergic Rhinitis Patients. Biomedicines, 12(2), 408. https://doi.org/10.3390/biomedicines12020408









