Prediabetes and Cardiometabolic Risk: The Need for Improved Diagnostic Strategies and Treatment to Prevent Diabetes and Cardiovascular Disease
Abstract
1. Introduction
2. Cardiovascular Risk, Morbidity, and Mortality in Prediabetes
3. An Adequate or Insufficient Diagnostic Criterion?
3.1. Controversy in the Established Cut-Off Values
3.2. Proposed New Cut-Off Points in the OGTT for the Diagnosis of Prediabetes
3.3. Other Approaches to Define Patients at Risk
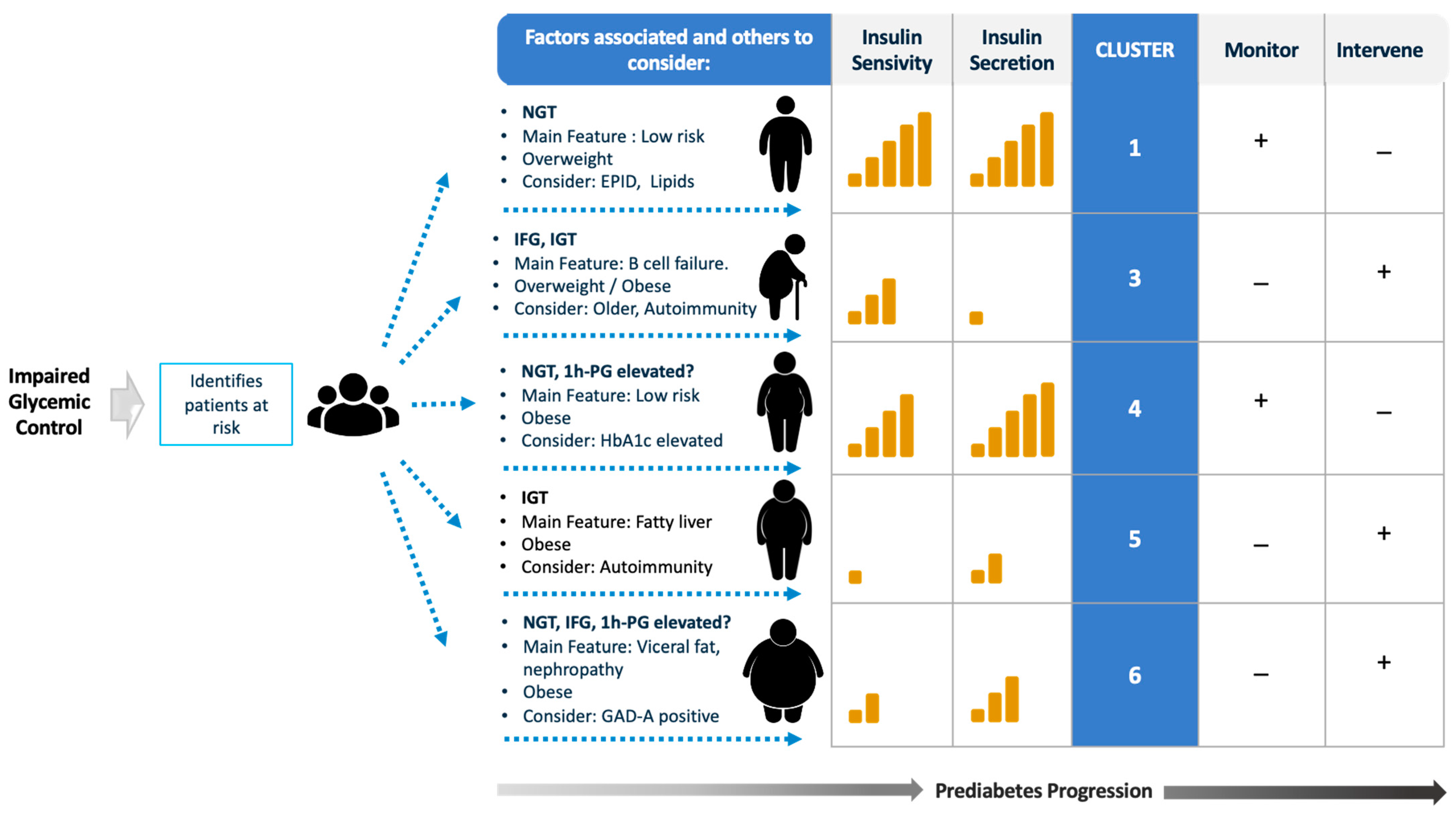
4. An Approach to Risk-Stratify Patients with Prediabetes
4.1. Diagnostic Criteria and Glycemic Cut-Point Thresholds for Diagnosis
4.2. Pathophysiology According to the Defect in Glycemic Control
4.3. Associated Risk Factors
5. Established Phenotypes Related to The Development of T2DM
6. What Is the Importance of Intervening in Patients with Prediabetes?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. S1), S19–S40. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Perreault, L.; Ji, L.; Dagogo-Jack, S. Diagnosis and Management of Prediabetes: A Review. JAMA 2023, 329, 1206. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- World Health Organization; International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation. 2006. Available online: https://apps.who.int/iris/handle/10665/43588 (accessed on 22 August 2023).
- The International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009, 32, 1327–1334. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.; Hansen, T.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Brannick, B.; Dagogo-Jack, S. Prediabetes and Cardiovascular Disease. Endocrinol. Metab. Clin. N. Am. 2018, 47, 33–50. [Google Scholar] [CrossRef]
- Haffner, S.M. Obesity and the metabolic syndrome: The San Antonio Heart Study. Br. J. Nutr. 2000, 83 (Suppl. S1), S67–S70. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Neuenschwander, M.; Barbaresko, J.; Lang, A.; Maalmi, H.; Rathmann, W.; Roden, M.; Herder, C. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: Umbrella review of meta-analyses of prospective studies. Diabetologia 2022, 65, 275–285. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, Y.; Li, M.; Wu, J.H.; Mai, L.; Li, J.; Yang, Y.; Hu, Y.; Huang, Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ 2020, 370, m2297. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Manco, M.; Sesti, G.; Dankner, R.; Pareek, M.; Jagannathan, R.; Chetrit, A.; Abdul-Ghani, M.; Buysschaert, M.; Olsen, M.H.; et al. Petition to replace current OGTT criteria for diagnosing prediabetes with the 1-hour post-load plasma glucose ≥155 mg/dL (8.6 mmol/L). Diabetes Res. Clin. Pract. 2018, 146, 18–33. [Google Scholar] [CrossRef]
- Cefalu, W.T. “Prediabetes”: Are There Problems with This Label? No, We Need Heightened Awareness of This Condition! Diabetes Care 2016, 39, 1472–1477. [Google Scholar] [CrossRef]
- Hare, M.J.; Magliano, D.J.; Zimmet, P.Z.; Söderberg, S.; Joonas, N.; Pauvaday, V.; Larhubarbe, J.; Tuomilehto, J.; Kowlessur, S.; Alberti, K.G.M.; et al. Glucose-Independent Ethnic Differences in HbA1c in People Without Known Diabetes. Diabetes Care 2013, 36, 1534–1540. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Abdul-Ghani, T.; Ali, N.; DeFronzo, R.A. One-Hour Plasma Glucose Concentration and the Metabolic Syndrome Identify Subjects at High Risk for Future Type 2 Diabetes. Diabetes Care 2008, 31, 1650–1655. [Google Scholar] [CrossRef]
- Bergman, M.; Abdul-Ghani, M.; DeFronzo, R.A.; Manco, M.; Sesti, G.; Fiorentino, T.V.; Ceriello, A.; Rhee, M.; Phillips, L.S.; Chung, S.; et al. Review of methods for detecting glycemic disorders. Diabetes Res. Clin. Pract. 2020, 165, 108233. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Marini, M.A.; Andreozzi, F.; Arturi, F.; Succurro, E.; Perticone, M.; Sciacqua, A.; Hribal, M.; Perticone, F.; Sesti, G. One-Hour Postload Hyperglycemia Is a Stronger Predictor of Type 2 Diabetes Than Impaired Fasting Glucose. J. Clin. Endocrinol. Metab. 2015, 100, 3744–3751. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Miccoli, R.; Trombetta, M.; Giorgino, F.; Frontoni, S.; Faloia, E.; Marchesini, G.; Dolci, M.; Cavalot, F.; Cavallo, G.; et al. Elevated 1-Hour Postload Plasma Glucose Levels Identify Subjects with Normal Glucose Tolerance but Impaired β-Cell Function, Insulin Resistance, and Worse Cardiovascular Risk Profile: The GENFIEV Study. J. Clin. Endocrinol. Metab. 2013, 98, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Tura, A.; Grespan, E.; Göbl, C.S.; Koivula, R.W.; Franks, P.W.; Pearson, E.R.; Walker, M.; Forgie, I.; Giordano, G.; Pavo, I.; et al. rofiles of Glucose Metabolism in Different Prediabetes Phenotypes, Classified by Fasting Glycemia, 2-Hour OGTT, Glycated Hemoglobin, and 1-Hour OGTT: An IMI DIRECT Study. Diabetes 2021, 70, 2092–2106. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Hata, J.; Yoshinari, M.; Higashioka, M.; Yoshida, D.; Shibata, M.; Honda, T.; Sakata, S.; Kato, H.; Teramoto, T. 30-minute postload plasma glucose levels during an oral glucose tolerance test predict the risk of future type 2 diabetes: The Hisayama Study. BMJ Open Diabetes Res Care 2020, 8, e001156. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, R.; Weber, M.B.; Anjana, R.M.; Ranjani, H.; Staimez, L.R.; Ali, M.K.; Mohan, V.; Narayan, K. Clinical utility of 30-min plasma glucose for prediction of type 2 diabetes among people with prediabetes: Ancillary analysis of the diabetes community lifestyle improvement program. Diabetes Res. Clin. Pract. 2020, 161, 108075. [Google Scholar] [CrossRef] [PubMed]
- Hulman, A.; Gujral, U.P.; Narayan, K.M.V.; Pradeepa, R.; Mohan, D.; Anjana, R.M.; Mohan, V.; Færch, K.; Witte, D. Glucose patterns during the OGTT and risk of future diabetes in an urban Indian population: The CARRS study. Diabetes Res. Clin. Pract. 2017, 126, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, R.; Sevick, M.A.; Fink, D.; Dankner, R.; Chetrit, A.; Roth, J.; Buysschaert, M.; Bergman, M. The 1-hour post-load glucose level is more effective than HbA1c for screening dysglycemia. Acta Diabetol. 2016, 53, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, D.; Colantonio, L.D.; Tanner, R.M.; Carson, A.P.; Sakhuja, S.; Jaeger, B.C.; Carey, R.; Cohen, L.; Shimbo, D.; Butler, M.; et al. Prediabetes and Risk for Cardiovascular Disease by Hypertension Status in Black Adults: The Jackson Heart Study. Diabetes Care 2019, 42, 2322–2329. [Google Scholar] [CrossRef] [PubMed]
- Tagi, V.M.; Mainieri, F.; Chiarelli, F. Hypertension in Patients with Insulin Resistance: Etiopathogenesis and Management in Children. Int. J. Mol. Sci. 2022, 23, 5814. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, A.; Brogi, G.; Di Legge, V.; Bernini, G.P. The Inter-Relationship between Insulin Resistance and Hypertension. Drugs 1993, 46 (Suppl. S2), 149–159. [Google Scholar] [CrossRef] [PubMed]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell. Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef]
- Laakso, M.; Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol. 2014, 10, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Laichuthai, N.A.; DeFronzo, R. Abnormal Glucose Tolerance in Prediabetes Patients with Acute Myocardial Infarction: Implications for Therapy. J. Endocrinol. Sci. 2021, 3, 16–21. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009. Diabetologia 2010, 53, 1270–1287. [Google Scholar] [CrossRef]
- Gong, Q.; Zhang, P.; Wang, J.; Ma, J.; An, Y.; Chen, Y.; Zhang, B.; Feng, X.; Li, H.; Chen, X.; et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019, 7, 452–461. [Google Scholar] [CrossRef]
- Goldberg, R.B.; Orchard, T.J.; Crandall, J.P.; Boyko, E.J.; Budoff, M.; Dabelea, D.; Gadde, K.; Knowler, W.; Lee, C.; Nathan, D.; et al. Effects of Long-term Metformin and Lifestyle Interventions on Cardiovascular Events in the Diabetes Prevention Program and Its Outcome Study. Circulation 2022, 145, 1632–1641. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Marini, M.A.; Succurro, E.; Andreozzi, F.; Perticone, M.; Hribal, M.L.; Sciacqua, A.; Perticone, F.; Sesti, G. One-Hour Postload Hyperglycemia: Implications for Prediction and Prevention of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3131–3143. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Jagannathan, R.; Buysschaert, M.; Pareek, M.; Olsen, M.H.; Nilsson, P.M.; Medina, J.; Roth, J.; Chetrit, A.; Groop, L.; et al. Lessons learned from the 1-hour post-load glucose level during OGTT: Current screening recommendations for dysglycaemia should be revised. Diabetes/Metab. Res. Rev. 2018, 34, e2992. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Selvin, E. Prediabetes and What It Means: The Epidemiological Evidence. Annu. Rev. Public Health 2021, 42, 59–77. [Google Scholar] [CrossRef] [PubMed]
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up Report on the Diagnosis of Diabetes Mellitus. Diabetes Care 2003, 26, 3160–3167. [Google Scholar] [CrossRef]
- Davidson, M.B.; Kahn, R.A. A Reappraisal of Prediabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Peddinti, G.; Bergman, M.; Tuomi, T.; Groop, L. 1-Hour Post-OGTT Glucose Improves the Early Prediction of Type 2 Diabetes by Clinical and Metabolic Markers. J. Clin. Endocrinol. Metab. 2019, 104, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Pareek, M.; Bhatt, D.L.; Nielsen, M.L.; Jagannathan, R.; Eriksson, K.F.; Nilsson, P.M.; Bergman, M.; Olsen, M. Enhanced Predictive Capability of a 1-Hour Oral Glucose Tolerance Test: A Prospective Population-Based Cohort Study. Diabetes Care 2018, 41, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gregg, E.W.; Williamson, D.F.; Barker, L.E.; Thomas, W.; Bullard, K.M.; Imperatore, G.; Williams, D.; Albright, A. A1C Level and Future Risk of Diabetes: A Systematic Review. Diabetes Care 2010, 33, 1665–1673. [Google Scholar] [CrossRef]
- Guo, F.; Moellering, D.R.; Garvey, W.T. Use of HbA1c for Diagnoses of Diabetes and Prediabetes: Comparison with Diagnoses Based on Fasting and 2-Hr Glucose Values and Effects of Gender, Race, and Age. Metab. Syndr. Relat. Disord. 2014, 12, 258–268. [Google Scholar] [CrossRef]
- Lizarzaburu-Robles, J.C.; Torres-Aparcana, L.; Mansilla, R.; Valera, J.; Vargas, G.; Vento, F.; Laca, J.; Cornetero, V.; Herman, W. A cross-Sectional Study of the Association between the 1-Hour Oral Glucose Tolerance Test and the Metabolic Syndrome in a High-Risk Sample with Impaired Fasting Glucose. Endocr. Pract. 2020, 26, 529–534. [Google Scholar] [CrossRef]
- Ferrannini, E.; Natali, A.; Camastra, S.; Nannipieri, M.; Mari, A.; Adam, K.P.; Milburn, M.; Kastenmüller, G.; Adamski, J.; Tuomi, T.; et al. Early Metabolic Markers of the Development of Dysglycemia and Type 2 Diabetes and Their Physiological Significance. Diabetes 2013, 62, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulos, E.; Andreadis, E.; Tsourous, G.; Ifanti, G.; Katsanou, P.; Georgiopoulos, D.; Vassilopoulos, C.; Dimitriadis, G.; Raptis, S. Metabolic Syndrome and Prediabetes Identify Overlapping but Not Identical Populations. Exp. Clin. Endocrinol. Diabetes 2006, 114, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, A.; Scicali, R.; Marchisello, S.; Zanoli, L.; Ferrara, V.; Urbano, F.; Filippello, A.; Di Mauro, S.; Scamporrino, A.; Piro, P.; et al. High glomerular filtration rate is associated with impaired arterial stiffness and subendocardial viability ratio in prediabetic subjects. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3393–3400. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Larco, R.M.; Aparcana-Granda, D.J.; Mejia, J.R.; Bernabé-Ortiz, A. FINDRISC in Latin America: A systematic review of diagnosis and prognosis models. BMJ Open Diabetes Res. Care 2020, 8, e001169. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.; Roberts, S.; Oke, J.; Vijayaraghavan, S.; Normansell, R.; Greenhalgh, T. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: Systematic review and meta-analysis of screening tests and interventions. BMJ 2017, 356, i6538. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Bullard, K.M.; Rolka, D.B.; Geiss, L.S.; Williams, D.E.; Cowie, C.C.; Albright, A.; Gregg, E. Implications of Alternative Definitions of Prediabetes for Prevalence in U.S. Adults. Diabetes Care 2011, 34, 387–391. [Google Scholar] [CrossRef]
- Greiner, G.G.; Emmert-Fees, K.M.F.; Becker, J.; Rathmann, W.; Thorand, B.; Peters, A.; Quante, A.; Schwettmann, L.; Laxy, M. Toward targeted prevention: Risk factors for prediabetes defined by impaired fasting glucose, impaired glucose tolerance and increased HbA1c in the population-based KORA study from Germany. Acta Diabetol. 2020, 57, 1481–1491. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of β-Cell Dysfunction and Insulin Resistance to the Pathogenesis of Impaired Glucose Tolerance and Impaired Fasting Glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef]
- Del Prato, S. Loss of early insulin secretion leads to postprandial hyperglycaemia. Diabetologia 2003, 46 (Suppl. S1), M2–M8. [Google Scholar] [CrossRef]
- Tura, A.; Göbl, C.; Moro, E.; Pacini, G. Insulin resistance and beta-cell dysfunction in people with prediabetes according to criteria based on glycemia and glycosylated hemoglobin. Endocr. J. 2017, 64, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jokelainen, J.; Auvinen, J.; Puukka, K.; Keinänen-Kiukaanniemi, S.; Järvelin, M.R.; Kettunen, J.; Mäkinen, V.-P.; Ala-Korpela, M. Insulin resistance and systemic metabolic changes in oral glucose tolerance test in 5340 individuals: An interventional study. BMC Med. 2019, 17, 217. [Google Scholar] [CrossRef] [PubMed]
- Calanna, S.; Scicali, R.; Di Pino, A.; Knop, F.K.; Piro, S.; Rabuazzo, A.M.; Purrello, F. Alpha- and beta-cell abnormalities in haemoglobin A1c-defined prediabetes and type 2 diabetes. Acta Diabetol. 2014, 51, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.; Bergman, B.C.; Playdon, M.C.; Dalla Man, C.; Cobelli, C.; Eckel, R.H. Impaired fasting glucose with or without impaired glucose tolerance: Progressive or parallel states of prediabetes? Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E428–E435. [Google Scholar] [CrossRef] [PubMed]
- Rocca-Nación, J.; Calderon, M. Cardiovascular risk, fatty liver disease, glucose and insulin curve among prediabetes phenotypes in Peruvian population. Am. J. Med. Open 2022, 7, 100007. [Google Scholar] [CrossRef]
- Lazo-Porras, M.; Ruiz-Alejos, A.; Miranda, J.J.; Carrillo-Larco, R.M.; Gilman, R.H.; Smeeth, L.; Bernabé-Ortiz, A. Intermediate hyperglycaemia and 10-year mortality in resource-constrained settings: The PERU MIGRANT Study. Diabet. Med. 2020, 37, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell Deficit and Increased β-Cell Apoptosis in Humans with Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Vellanki, P.; Blonde, L.; Christofides, E.A.; Galindo, R.J.; Hirsch, I.B.; Isaacs, S.; Izuora, K.; Low Wang, C.; Twining, C.; et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm—2023 Update. Endocr. Pract. 2023, 29, 305–340. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The metabolic syndrome—What is it and how should it be managed? Eur. J. Prev. Cardiol. 2019, 26 (Suppl. S2), 33–46. [Google Scholar] [CrossRef]
- Bracco, P.A.; Schmidt, M.I.; Vigo, A.; Mill, J.G.; Vidigal, P.G.; Barreto, S.M.; Sander, M.; Mendes da Fonseca, M.; Duncan, B. Optimizing strategies to identify high risk of developing type 2 diabetes. Front. Endocrinol. 2023, 14, 1166147. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.; Aly, D.; Almgren, P. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Popovic, D.S.; Rizzo, M.; Stokic, E.; Papanas, N. New Sub-Phenotyping of Subjects at High Risk of Type 2 Diabetes: What Are the Potential Clinical Implications? Diabetes Ther. 2021, 12, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Heni, M.; Tabák, A.G.; Machann, J.; Schick, F.; Randrianarisoa, E.; Hrabě de Angelis, M.; Birkenfeld, A.; Stefan, N.; Peter, A.; et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat Med. 2021, 27, 49–57. [Google Scholar] [CrossRef]
- Danquah, I.; Mank, I.; Hampe, C.S.; Meeks, K.A.C.; Agyemang, C.; Owusu-Dabo, E.; Smeeth, L.; Klipstein-Grobusch, K.; Bahendeka, S.; Spranger, J.; et al. Subgroups of adult-onset diabetes: A data-driven cluster analysis in a Ghanaian population. Sci Rep. 2023, 13, 10756. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.; Lin, J.; Xiao, J.; Cao, H.; et al. Effects of Diet and Exercise in Preventing NIDDM in People with Impaired Glucose Tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Lindström, J.; Louheranta, A.; Mannelin, M.; Rastas, M.; Salminen, V.; Eriksson, J.; Uusitupa, M.; Tuomilehto, J. The Finnish Diabetes Prevention Study (DPS). Diabetes Care 2003, 26, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- De Fronzo, R.A.; Banerji, M.; Bray, G.A.; Buchanan, T.A.; Clement, S.; Henry, R.R.; Kitabchi, A.; Mudaliar, S.; Musi, N.; Ratner, R.; et al. Actos Now for the prevention of diabetes (ACT NOW) study. BMC Endocr. Disord. 2009, 9, 17. [Google Scholar]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef]
- The ORIGIN Trial Investigators. Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia. N. Engl. J. Med. 2012, 367, 319–328. [Google Scholar] [CrossRef]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjöström, L. XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study. Diabetes Care 2004, 27, 155–161. [Google Scholar] [CrossRef]
- Aronne, L.; Shanahan, W.; Fain, R.; Glicklich, A.; Soliman, W.; Li, Y.; Smith, S. Safety and Efficacy of Lorcaserin: A Combined Analysis of the BLOOM and BLOSSOM Trials. Postgrad. Med. 2014, 126, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Ryan, D.H.; Look, M.; Gadde, K.M.; Allison, D.B.; Peterson, C.A.; Schwiers, M.; Day, W.; Bowden, C. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): A randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 2012, 95, 297–308. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.; Le Roux, C.; Violante, R.; Bjørn Jensen, C.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Perreault, L.; Davies, M.; Frias, J.P.; Laursen, P.N.; Lingvay, I.; Machineni, S.; Varbo, A.; Wilding, J.; Rytter Wallenstein, S.; Le Roux, C. Changes in Glucose Metabolism and Glycemic Status with Once-Weekly Subcutaneous Semaglutide 2.4 mg Among Participants With Prediabetes in the STEP Program. Diabetes Care 2022, 45, 2396–2405. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Uusitupa, M.; Gregg, E.W.; Lindström, J. Type 2 Diabetes Prevention Programs—From Proof-of-Concept Trials to National Intervention and Beyond. J. Clin. Med. 2023, 12, 1876. [Google Scholar] [CrossRef]
- Li, G.; Zhang, P.; Wang, J.; An, Y.; Gong, Q.; Gregg, E.W.; Yang, W.; Zhang, B.; Shuai, Y.; Hong, J.; et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: A 23-year follow-up study. Lancet Diabetes Endocrinol. 2014, 2, 474–480. [Google Scholar] [CrossRef]
- Perreault, L.; Pan, Q.; Mather, K.J.; Watson, K.E.; Hamman, R.F.; Kahn, S.E. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: Results from the Diabetes Prevention Program Outcomes Study. Lancet 2012, 379, 2243–2251. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef]
- Xu, D.; Nair, A.; Sigston, C.; Ho, C.; Li, J.; Yang, D.; Liao, X.; Chen, W.; Kuang, M.; Li, Y.; et al. Potential Roles of Glucagon-Like Peptide 1 Receptor Agonists (GLP-1 RAs) in Nondiabetic Populations. Norgard NB, editor. Cardiovasc. Ther. 2022, 2022, 6820377. [Google Scholar] [CrossRef]
- Farr, O.M.; Mantzoros, C.S. Treating prediabetes in the obese: Are GLP-1 analogues the answer? Lancet 2017, 389, 1371–1372. [Google Scholar] [CrossRef]
| Time of Measurement | Name of the Hyperglycemic Condition | Organization | |
|---|---|---|---|
| Current Diagnostic Criteria | |||
| Glucose | |||
| Fasting | IFG | ADA |
| Fasting | IFG | WHO |
| 2 h-PG (75 g OGTT) | IGT | ADA/WHO |
| HbA1C | |||
| Any time | prediabetes | ADA |
| Any time | prediabetes | IEC |
| Proposed Criteria | |||
| Glucose | |||
| 30 min-PG (75 g OGTT) | - | - |
| 1 h-PG (75 g OGTT) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizarzaburu-Robles, J.C.; Herman, W.H.; Garro-Mendiola, A.; Galdón Sanz-Pastor, A.; Lorenzo, O. Prediabetes and Cardiometabolic Risk: The Need for Improved Diagnostic Strategies and Treatment to Prevent Diabetes and Cardiovascular Disease. Biomedicines 2024, 12, 363. https://doi.org/10.3390/biomedicines12020363
Lizarzaburu-Robles JC, Herman WH, Garro-Mendiola A, Galdón Sanz-Pastor A, Lorenzo O. Prediabetes and Cardiometabolic Risk: The Need for Improved Diagnostic Strategies and Treatment to Prevent Diabetes and Cardiovascular Disease. Biomedicines. 2024; 12(2):363. https://doi.org/10.3390/biomedicines12020363
Chicago/Turabian StyleLizarzaburu-Robles, Juan Carlos, William H. Herman, Alonso Garro-Mendiola, Alba Galdón Sanz-Pastor, and Oscar Lorenzo. 2024. "Prediabetes and Cardiometabolic Risk: The Need for Improved Diagnostic Strategies and Treatment to Prevent Diabetes and Cardiovascular Disease" Biomedicines 12, no. 2: 363. https://doi.org/10.3390/biomedicines12020363
APA StyleLizarzaburu-Robles, J. C., Herman, W. H., Garro-Mendiola, A., Galdón Sanz-Pastor, A., & Lorenzo, O. (2024). Prediabetes and Cardiometabolic Risk: The Need for Improved Diagnostic Strategies and Treatment to Prevent Diabetes and Cardiovascular Disease. Biomedicines, 12(2), 363. https://doi.org/10.3390/biomedicines12020363






