Post-Operational Photodynamic Therapy of the Tumor Bed: Comparative Analysis for Cold Knife and Laser Scalpel Resection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Cohort
2.2. Resection Procedure
2.3. PDT Procedure
2.4. Follow-Up
2.5. Analysis of Photosensitizer Distribution
2.6. Monte Carlo Simulations
3. Results and Discussion
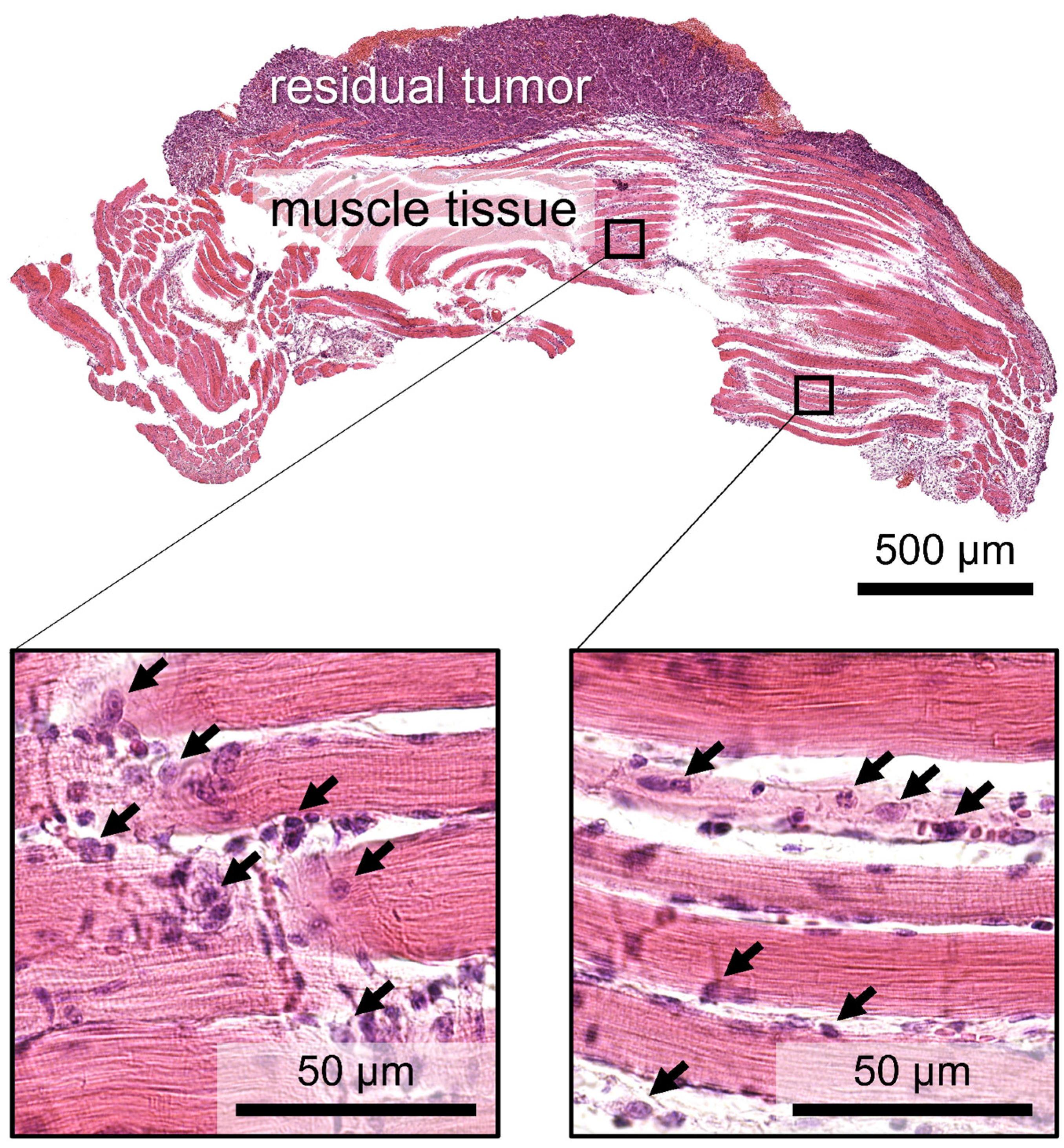
3.1. Histology Study
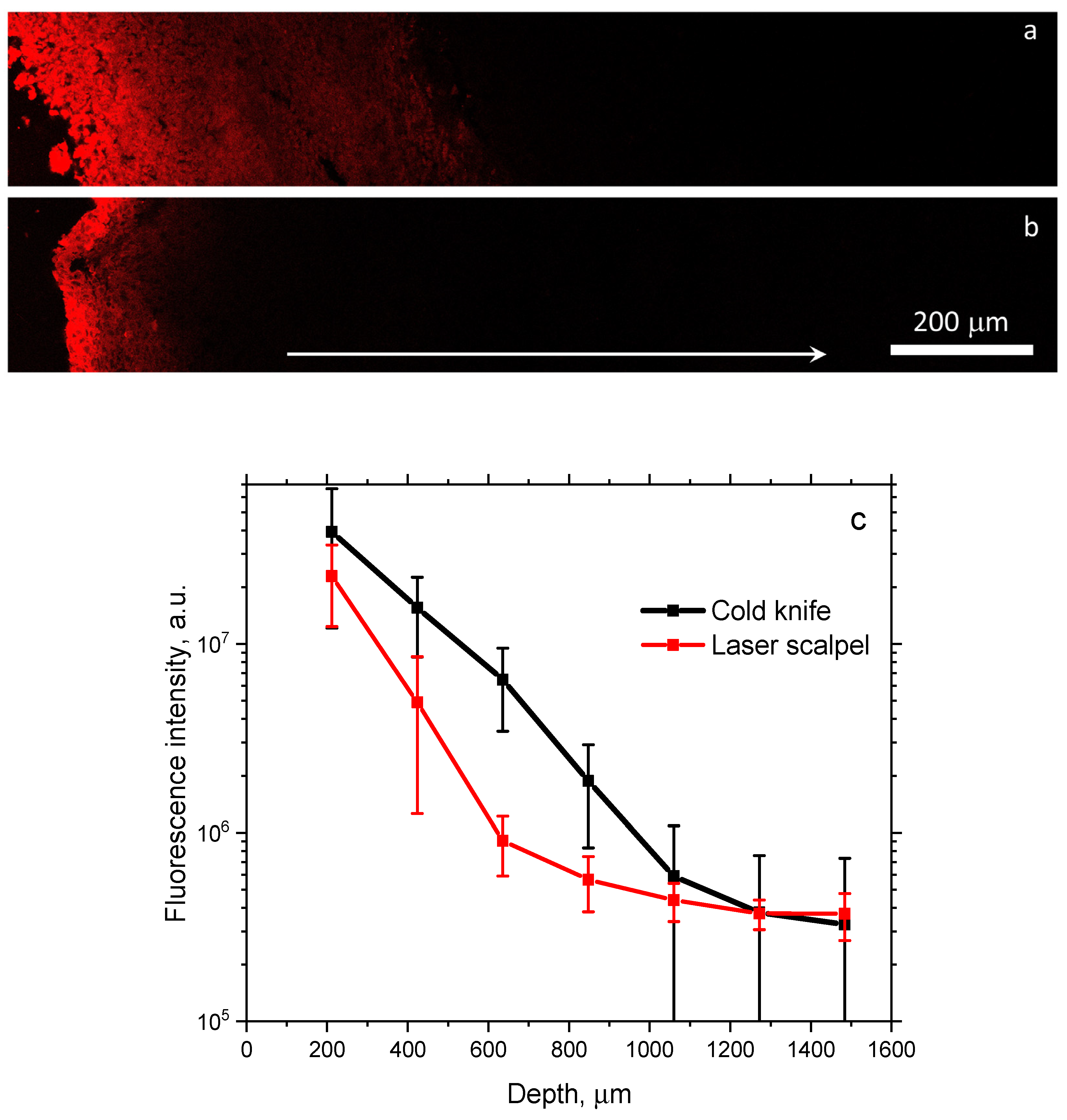
3.2. Fluorescence Microscopy
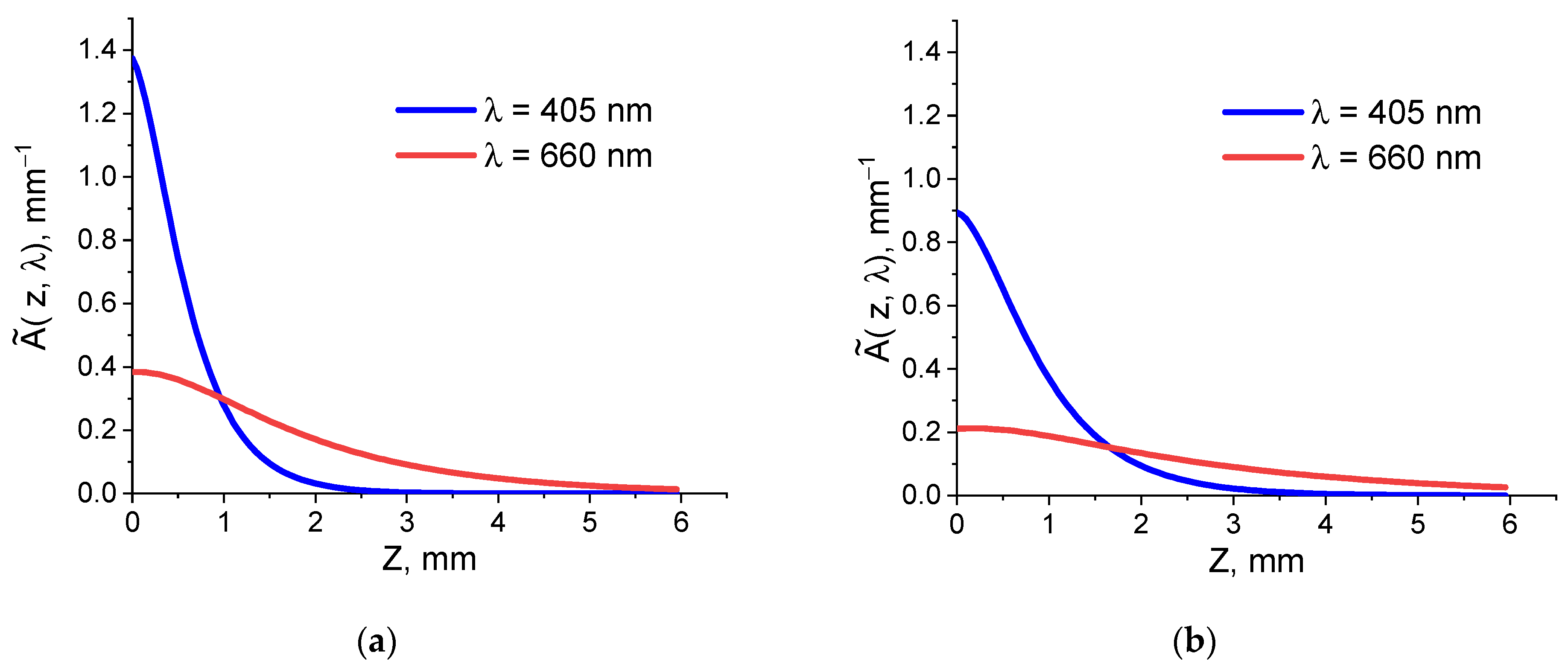
3.3. Monte Carlo Simulations
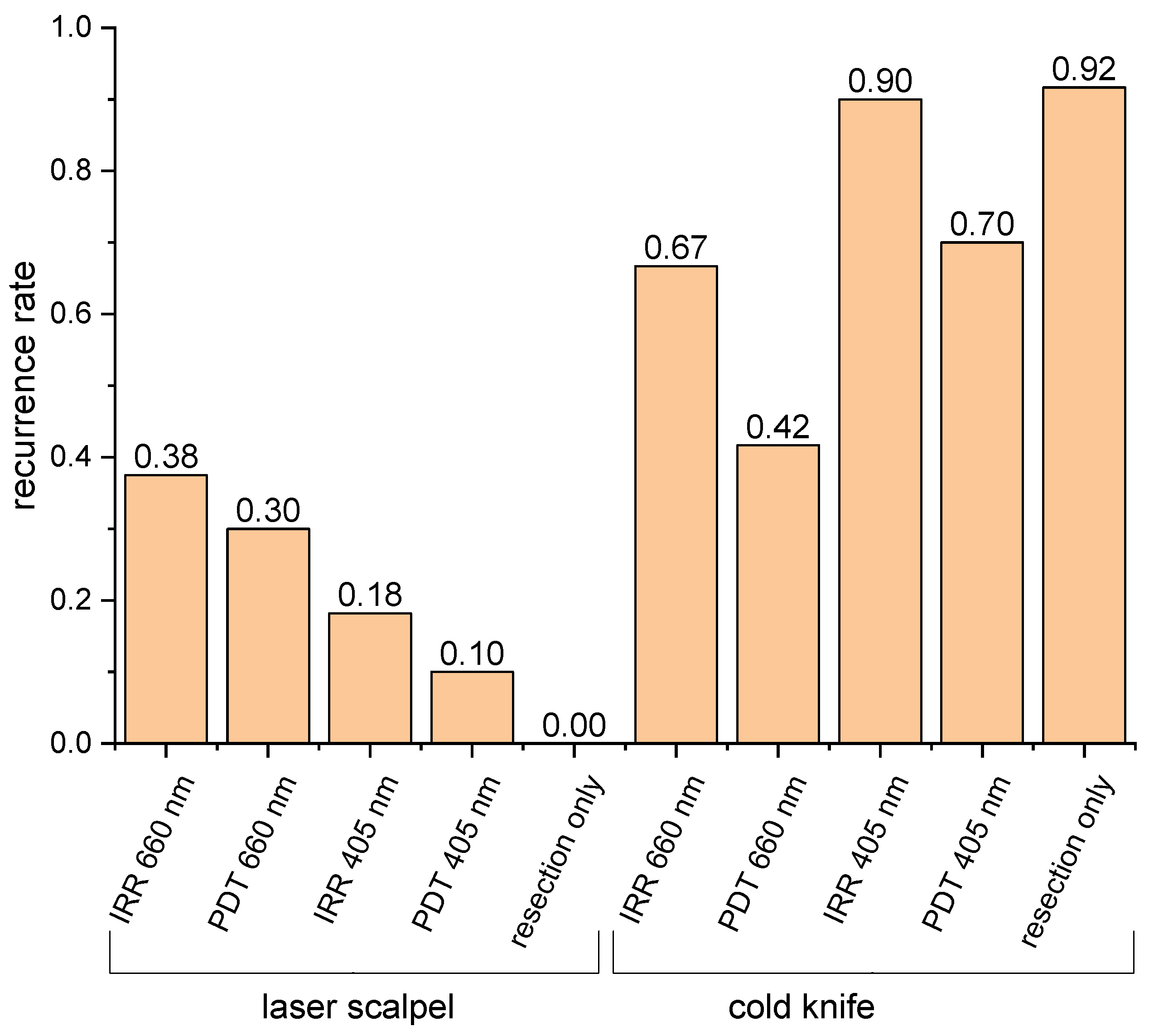
3.4. Animal Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, N.; Wei, X.; Zhang, Y.; Luo, H.; Su, Y.; Liang, L.; Chen, B.; Li, N.; Ren, X.; Yang, H. Effect of different surgical modalities on swallowing-related quality of life in patients with glottic laryngeal squamous cell carcinoma: How should we choose? Arch. Med. Sci. 2023, 19, 550–554. [Google Scholar] [CrossRef]
- Hans, S.; Baudouin, R.; Circiu, M.P.; Couineau, F.; Lisan, Q.; Crevier-Buchman, L.; Lechien, J.R. Laryngeal Cancer Surgery: History and Current Indications of Transoral Laser Microsurgery and Transoral Robotic Surgery. J. Clin. Med. 2022, 11, 5769. [Google Scholar] [CrossRef]
- Amaral, M.B.; de Avila, J.M.; Abreu, M.H.; Mesquita, R.A. Diode laser surgery versus scalpel surgery in the treatment of fibrous hyperplasia: A randomized clinical trial. Int. J. Oral. Maxillofac. Surg. 2015, 44, 1383–1389. [Google Scholar] [CrossRef]
- Jia, W.; King, E. The Role of Robotic Surgery in Laryngeal Cancer. Otolaryngol. Clin. N. Am. 2023, 56, 313–322. [Google Scholar] [CrossRef]
- Mahvi, D.A.; Liu, R.; Grinstaff, M.W.; Colson, Y.L.; Raut, C.P. Local Cancer Recurrence: The Realities, Challenges, and Opportunities for New Therapies. CA Cancer J. Clin. 2018, 68, 488–505. [Google Scholar] [CrossRef]
- Abulafi, A.M.; Dejode, M.; Allardice, J.T.; Ansell, J.; Rogers, J.; Williams, N.S. Adjuvant intraoperative photodynamic therapy in experimental colorectal cancer. Br. J. Surg. 1995, 82, 178–181. [Google Scholar] [CrossRef]
- Nanashima, A.; Yamaguchi, H.; Shibasaki, S.; Ide, N.; Sawai, T.; Tsuji, T.; Hidaka, S.; Sumida, Y.; Nakagoe, T.; Nagayasu, T. Adjuvant photodynamic therapy for bile duct carcinoma after surgery: A preliminary study. J. Gastroenterol. 2004, 39, 1095–1101. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, Z.; Wang, J.; Zhang, P.; Ding, Y.; Jiang, Y.; Wang, D.; Li, Y. Engineering autologous tumor cell vaccine to locally mobilize antitumor immunity in tumor surgical bed. Sci. Adv. 2020, 6, eaba4024. [Google Scholar] [CrossRef]
- Lamy, L.; Thomas, J.; Leroux, A.; Bisson, J.-F.; Myren, K.; Godal, A.; Stensrud, G.; Bezdetnaya, L. Antitumor effect and induced immune response following expo-sure of hexaminolevulinate and blue light in combination with checkpoint inhibitor in an orthotopic model of rat bladder cancer. Biomedicines 2022, 10, 548. [Google Scholar] [CrossRef]
- Giuliano, E.A.; Johnson, P.J.; Delgado, C.; Pearce, J.W.; Moore, C.P. Local photodynamic therapy delays recurrence of equine periocular squamous cell carcinoma compared to cryotherapy. Vet. Ophthalmol. 2014, 17 (Suppl. S1), 37–45. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.M.; Niu, L.Z.; Chen, J.B.; Xu, K.C. Advances in cryoablation for pancreatic cancer. World J. Gastroenterol. 2016, 22, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, K.; Kodresko, A.; Ghazal, H.; Mokbel, R.; Trembley, J.; Jouhara, H. The Evolving Role of Cryosurgery in Breast Cancer Management: A Comprehensive Review. Cancers 2023, 15, 4272. [Google Scholar] [CrossRef] [PubMed]
- Osintsev, A.M.; Vasilchenko, I.L.; Rodrigues, D.B.; Stauffer, P.R.; Braginsky, V.I.; Rynk, V.V.; Gromov, E.S.; Prosekov, A.Y.; Kaprin, A.D.; Kostin, A.A. Characterization of Ferromagnetic Composite Implants for Tumor Bed Hyperthermia. IEEE Trans. Magn. 2021, 57, 5400108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Weng, S.; Yu, L.; Zhu, N.; Yang, M.; Yuan, Y. The Role of Hyperthermia in the Multidisciplinary Treatment of Malignant Tumors. Integr. Cancer Ther. 2019, 18, 1534735419876345. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Hahner, S.; Polat, B.; Koschker, A.-C.; Kenn, W.; Flentje, M.; Allolio, B. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 4501–4504. [Google Scholar] [CrossRef]
- Haas, R.L.; Walraven, I.; Lecointe-Artzner, E.; van Houdt, W.J.; Strauss, D.; Schrage, Y.; Hayes, A.J.; Raut, C.P.; Fairweather, M.; Baldini, E.H.; et al. Extrameningeal solitary fibrous tumors—Surgery alone or surgery plus perioperative radiotherapy: A retrospective study from the global solitary fibrous tumor initiative in collaboration with the Sarcoma Patients EuroNet. Cancer 2020, 126, 3002–3012. [Google Scholar] [CrossRef]
- Guan, X.; Sun, L.; Shen, Y.; Jin, F.; Bo, X.; Zhu, C.; Han, X.; Li, X.; Chen, Y.; Xu, H.; et al. Nanoparticle-enhanced radiotherapy synergizes with PD-L1 blockade to limit post-surgical cancer recurrence and metastasis. Nat. Commun. 2022, 13, 2834. [Google Scholar] [CrossRef]
- Tong, C.W.; Wu, W.K.; Loong, H.H.; Cho, W.C.; To, K.K. Drug combination approach to overcome resistance to EGFR tyrosine kinase inhibitors in lung cancer. Cancer Lett. 2017, 405, 100–110. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.K.; Park, I.K.; Hwang, S.R. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines 2021, 9, 85. [Google Scholar] [CrossRef]
- Momma, T.; Hamblin, M.R.; Wu, H.C.; Hasan, T. Photodynamic therapy of orthotopic prostate cancer with benzoporphyrin derivative: Local control and distant metastasis. Cancer Res. 1998, 58, 5425–5431. [Google Scholar]
- Grandi, V.; Sessa, M.; Pisano, L.; Rossi, R.; Galvan, A.; Gattai, R.; Mori, M.; Tiradritti, L.; Bacci, S.; Zuccati, G.; et al. Photodynamic therapy with topical photosensitizers in mucosal and semimucosal areas: Review from a dermatologic perspective. Photodiagnosis Photodyn. Ther. 2018, 23, 119–131. [Google Scholar] [CrossRef]
- Kirillin, M.; Khilov, A.; Kurakina, D.; Orlova, A.; Perekatova, V.; Shishkova, V.; Malygina, A.; Mironycheva, A.; Shlivko, I.; Gamayunov, S.; et al. Dual-Wavelength Fluorescence Monitoring of Photodynamic Therapy: From Analytical Models to Clinical Studies. Cancers 2021, 13, 5807. [Google Scholar] [CrossRef]
- Shakhova, M.; Loginova, D.; Meller, A.; Sapunov, D.; Orlinskaya, N.; Shakhov, A.; Khilov, A.; Kirillin, M. Photodynamic therapy with chlorin-based photosensitizer at 405 nm: Numerical, morphological, and clinical study. J. Biomed. Opt. 2018, 23, 091412. [Google Scholar] [CrossRef]
- Kirillin, M.; Kurakina, D.; Khilov, A.; Orlova, A.; Shakhova, M.; Orlinskaya, N.; Sergeeva, E. Red and blue light in antitumor photodynamic therapy with chlorin-based photosensitizers: A comparative animal study assisted by optical imaging modalities. Biomed. Opt. Express. 2021, 12, 872–892. [Google Scholar] [CrossRef]
- Zeitouni, N.C.; Bhatia, N.; Ceilley, R.I.; Cohen, J.L.; Del Rosso, J.Q.; Moore, A.Y.; Munavalli, G.; Pariser, D.M.; Schlesinger, T.; Siegel, D.M.; et al. Photodynamic Therapy with 5-aminolevulinic Acid 10% Gel and Red Light for the Treatment of Actinic Keratosis, Nonmelanoma Skin Cancers, and Acne: Current Evidence and Best Practices. J. Clin. Aesthet. Dermatol. 2021, 14, E53–E65. [Google Scholar]
- Yang, H.; Zhang, Z.; Zhao, K.; Zhang, Y.; Yin, X.; Zhu, G.; Wang, Z.; Sui, Y.; Li, X.; Li, C.; et al. Initial Experience with Extraperitoneal Laparoscopic Radical Cystectomy With Pelvic Organ-Preserving and Orthotopic Neobladder Techniques for Bladder Cancer in Female Patients. Urology 2023, 171, 77–82. [Google Scholar] [CrossRef]
- Hanke, A.; Fimmers, R.; Frentzen, M.; Meister, J. Quantitative determination of cut efficiency during soft tissue surgery using diode lasers in the wavelength range between 400 and 1500 nm. Lasers Med. Sci. 2021, 36, 1633–1647. [Google Scholar] [CrossRef]
- Sapogova, N.; Bredikhin, V.; Bityurin, N.; Kamensky, V.; Zhigarcov, V.; Yusupov, V. Model for indirect laser surgery. Biomed. Opt. Express 2016, 8, 104–111. [Google Scholar] [CrossRef]
- Kuznetsova, D.S.; Karabut, M.M.; Elagin, V.V.; Shakhova, M.A.; Bredikhin, V.I.; Baskina, O.S.; Snopova, L.B.; Shakhov, A.V.; Kamensky, V.A. Comparative Analysis of Biotissue Laser Resection Using Strongly Absorbing Optical Fiber Tips. Opt. Photonics J. 2015, 5, 1–5. [Google Scholar] [CrossRef][Green Version]
- Khilov, A.; Kirillin, M.; Loginova, D.; Turchin, I. Estimation of chlorin-based photosensitizer penetration depth prior to photodynamic therapy procedure with dual-wavelength fluorescence imaging. Laser Phys. Lett. 2018, 15, 126202. [Google Scholar] [CrossRef]
- Greening, G.; Mundo, A.; Rajaram, N.; Muldoon, T.J. Sampling depth of a diffuse reflectance spectroscopy probe for in-vivo physiological quantification of murine subcutaneous tumor allografts. J. Biomed. Opt. 2018, 23, 085006. [Google Scholar] [CrossRef]
- Honda, N.; Ishii, K.; Terada, T.; Nanjo, T.; Awazu, K. Determination of the tumor tissue optical properties during and after photodynamic therapy using inverse Monte Carlo method and double integrating sphere between 350 and 1000 nm. J. Biomed. Opt. 2011, 16, 058003. [Google Scholar] [CrossRef]
- Loginova, D.A.; Sergeeva, E.A.; Krainov, A.D.; Agrba, P.D.; Kirillin, M.Y. Liquid optical phantoms mimicking spectral characteristics of laboratory mouse biotissues. Quantum Electron. 2016, 46, 528. [Google Scholar] [CrossRef]
- Zabotnov, S.V.; Skobelkina, A.V.; Sergeeva, E.A.; Kurakina, D.A.; Khilov, A.V.; Kashaev, F.V.; Kaminskaya, T.P.; Presnov, D.E.; Agrba, P.D.; Shuleiko, D.V.; et al. Nanoparticles Produced via Laser Ablation of Porous Silicon and Silicon Nanowires for Optical Bioimaging. Sensors 2020, 20, 4874. [Google Scholar] [CrossRef]
- Plekhanov, A.A.; Sirotkina, M.A.; Sovetsky, A.A.; Gubarkova, E.V.; Kuznetsov, S.S.; Matveyev, A.L.; Matveev, L.A.; Zagaynova, E.V.; Gladkova, N.D.; Zaitsev, V.Y. Histological validation of in vivo assessment of cancer tissue inhomogeneity and automated morphological segmentation enabled by Optical Coherence Elastography. Sci. Rep. 2020, 10, 11781. [Google Scholar] [CrossRef]
- Elagin, V.V.; Shakhova, M.A.; Sirotkina, M.A.; Shakhov, A.V.; Pavlova, N.P.; Snopova, L.B.; Bredikhin, V.I.; Kamensky, V.A. Can “Indirect” Contact Laser Surgery be Used for Fluorescence-Image Guided Tumor Resections? Preliminary Results. Technol. Cancer Res. Treat. 2018, 17, 1533033818805715. [Google Scholar] [CrossRef]
- Berger, N.A.; Eeg, P.H. Veterinary Laser Surgery: A Practical Guide; Wiley-Blackwell: Hoboken, NJ, USA, 2006; 256p. [Google Scholar]
- Svaasand, L.O.; Wyss, P.; Wyss, M.-T.; Tadir, Y.; Tromberg, B.J.; Berns, M.W. Dosimetry model for photodynamic therapy with topically administered photosensitizers. Lasers Surg. Med. 1996, 18, 139–149. [Google Scholar] [CrossRef]
- Li, L.-B.; Xie, J.-M.; Zhang, X.-N.; Chen, J.-Z.; Luo, Y.-L.; Zhang, L.-Y.; Luo, R.-C. Retrospective study of photodynamic therapy vs photodynamic therapy combined with chemotherapy and chemotherapy alone on advanced esophageal cancer. Photodiagnosis Photodyn. Ther. 2010, 7, 139–143. [Google Scholar] [CrossRef]
- Pech, O.; Behrens, A.; May, A.; Nachbar, L.; Gossner, L.; Rabenstein, T.; Manner, H.; Guenter, E.; Huijsmans, J.; Vieth, M.; et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008, 57, 1200–1206. [Google Scholar] [CrossRef]
- Yujie, S.; Mingming, L.; Fang, S.; Zhang, Y.; Qu, C.; Zhou, M.; Shen, F.; Xu, L. Low-dose photodynamic therapy-induced increase in the metastatic potential of pancreatic tumor cells and its blockade by simvastatin. J. Photochem. Photobiol. B Biol. 2020, 207, 111889. [Google Scholar]
- Kara, C.; Selamet, H.; Gökmenoğlu, C.; Kara, N. Low level laser therapy induces increased viability and proliferation in isolated cancer cells. Cell Prolif. 2018, 51, e12417. [Google Scholar] [CrossRef]
- De Castro, J.L.; Pinheiro, A.L.; Werneck, C.E.; Soares, C.P. The effect of laser therapy on the proliferation of oral KB carcinoma cells: An in vitro study. Photomed. Laser Surg. 2005, 23, 586–589. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Chen, S.-Y.; Hsieh, Y.-L.; Teng, Y.-H.; Cheng, Y.-J. Low-level laser therapy prevents endothelial cells from TNF-α/cycloheximide-induced apoptosis. Lasers Med. Sci. 2018, 33, 279–286. [Google Scholar] [CrossRef]
| Group No. | Resection Method | Treatment of the Tumor Bed | Number of Animals |
|---|---|---|---|
| 1 | laser scalpel | Irradiation only @660 nm | 11 (3) |
| 2 | laser scalpel | PDT: Radagel® @660 nm | 11 (1) |
| 3 | laser scalpel | Irradiation only @405 nm | 11 (-) |
| 4 | laser scalpel | PDT: Radagel® @405 nm | 11 (1) |
| 5 | laser scalpel | No additional treatment | 11 (-) |
| 6 | cold knife | Irradiation only @660 nm | 11 (2) |
| 7 | cold knife | PDT: Radagel® @660 nm | 11 (-) |
| 8 | cold knife | Irradiation only @405 nm | 10 (-) |
| 9 | cold knife | PDT: Radagel® @405 nm | 11 (1) |
| 10 | cold knife | No additional treatment | 13 (1) |
| Total | 113 (9) |
| Wavelength, nm | n | ||
|---|---|---|---|
| Tumor tissue | |||
| 405 nm | 0.92 | 1.29 | 1.4 |
| 660 nm | 0.18 | 0.67 | 1.4 |
| Muscle tissue | |||
| 405 nm | 0.57 | 1.17 | 1.4 |
| 660 nm | 0.07 | 0.58 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakhova, M.; Elagin, V.; Plekhanov, A.; Khilov, A.; Kurakina, D.; Kamensky, V.; Kirillin, M. Post-Operational Photodynamic Therapy of the Tumor Bed: Comparative Analysis for Cold Knife and Laser Scalpel Resection. Biomedicines 2024, 12, 291. https://doi.org/10.3390/biomedicines12020291
Shakhova M, Elagin V, Plekhanov A, Khilov A, Kurakina D, Kamensky V, Kirillin M. Post-Operational Photodynamic Therapy of the Tumor Bed: Comparative Analysis for Cold Knife and Laser Scalpel Resection. Biomedicines. 2024; 12(2):291. https://doi.org/10.3390/biomedicines12020291
Chicago/Turabian StyleShakhova, Maria, Vadim Elagin, Anton Plekhanov, Aleksandr Khilov, Daria Kurakina, Vladislav Kamensky, and Mikhail Kirillin. 2024. "Post-Operational Photodynamic Therapy of the Tumor Bed: Comparative Analysis for Cold Knife and Laser Scalpel Resection" Biomedicines 12, no. 2: 291. https://doi.org/10.3390/biomedicines12020291
APA StyleShakhova, M., Elagin, V., Plekhanov, A., Khilov, A., Kurakina, D., Kamensky, V., & Kirillin, M. (2024). Post-Operational Photodynamic Therapy of the Tumor Bed: Comparative Analysis for Cold Knife and Laser Scalpel Resection. Biomedicines, 12(2), 291. https://doi.org/10.3390/biomedicines12020291









