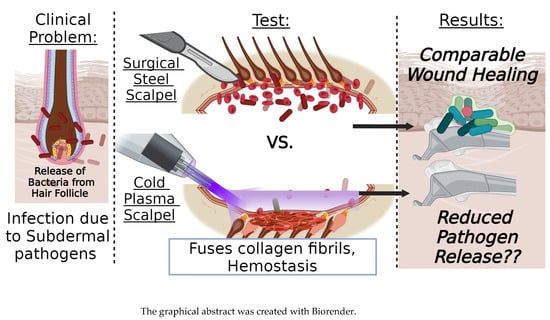
Evaluating the Healing Potential of J-Plasma Scalpel-Created Surgical Incisions in Porcine and Rat Models
Abstract
1. Introduction
2. Materials and Methods
3. Results
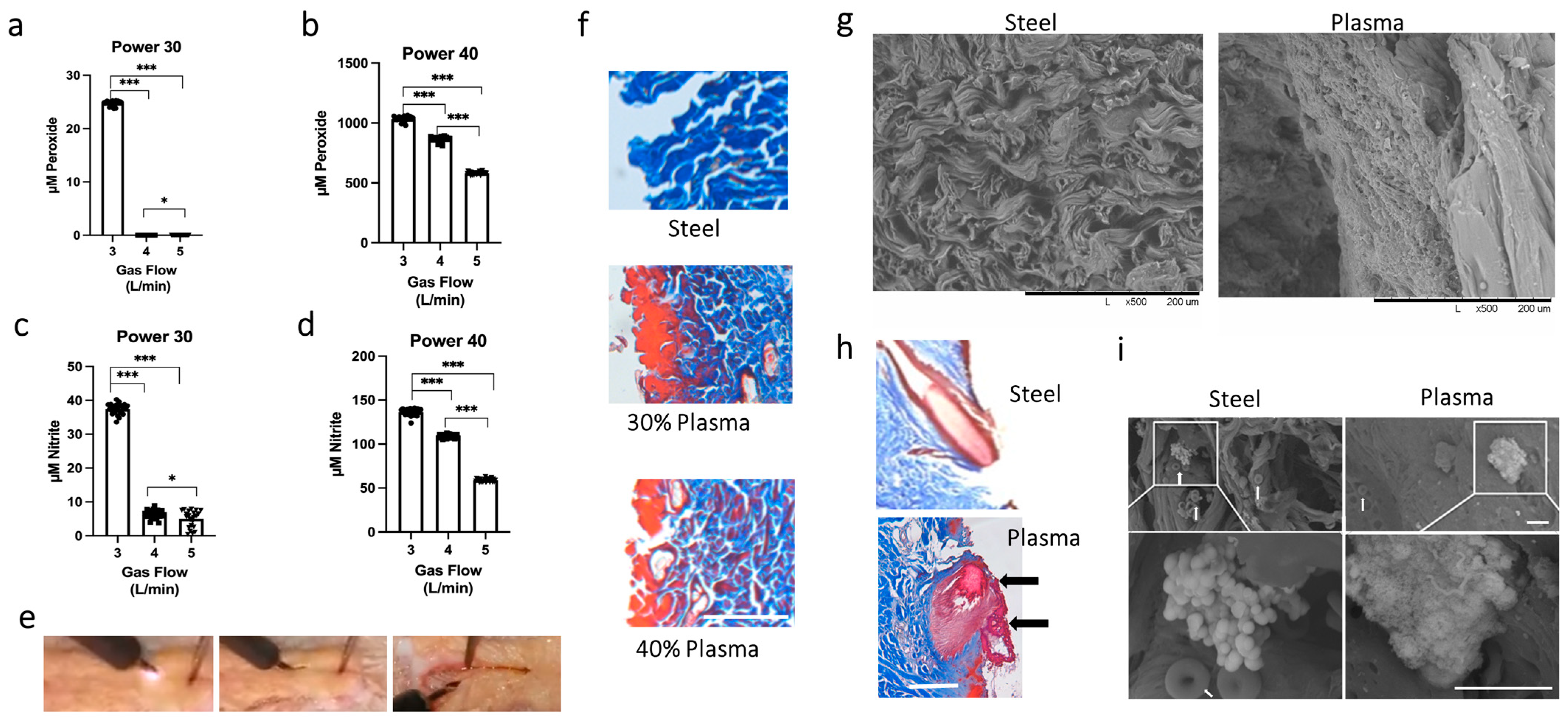
3.1. Determining the Optimal Settings for the J-Plasma Scalpel
3.2. Scanning Electron Microscope Results Support Plasma Fusion of Collagen Fibrils and Disruption of Bacterial Structure
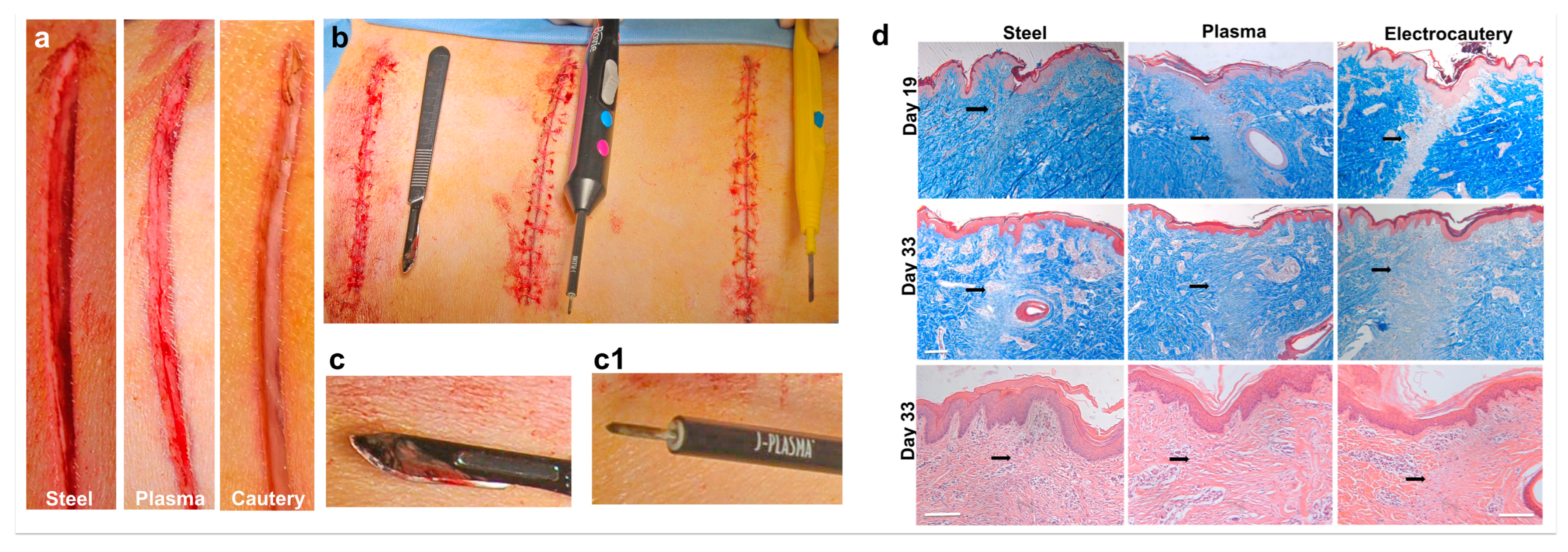
3.3. In Vivo Incisions in a Porcine Model Comparing Electrocautery and J-Plasma to a Steel Scalpel
3.4. J-Plasma Scalpel Showed Improved Tissue Healing Compared to Electrocautery in the Porcine Model
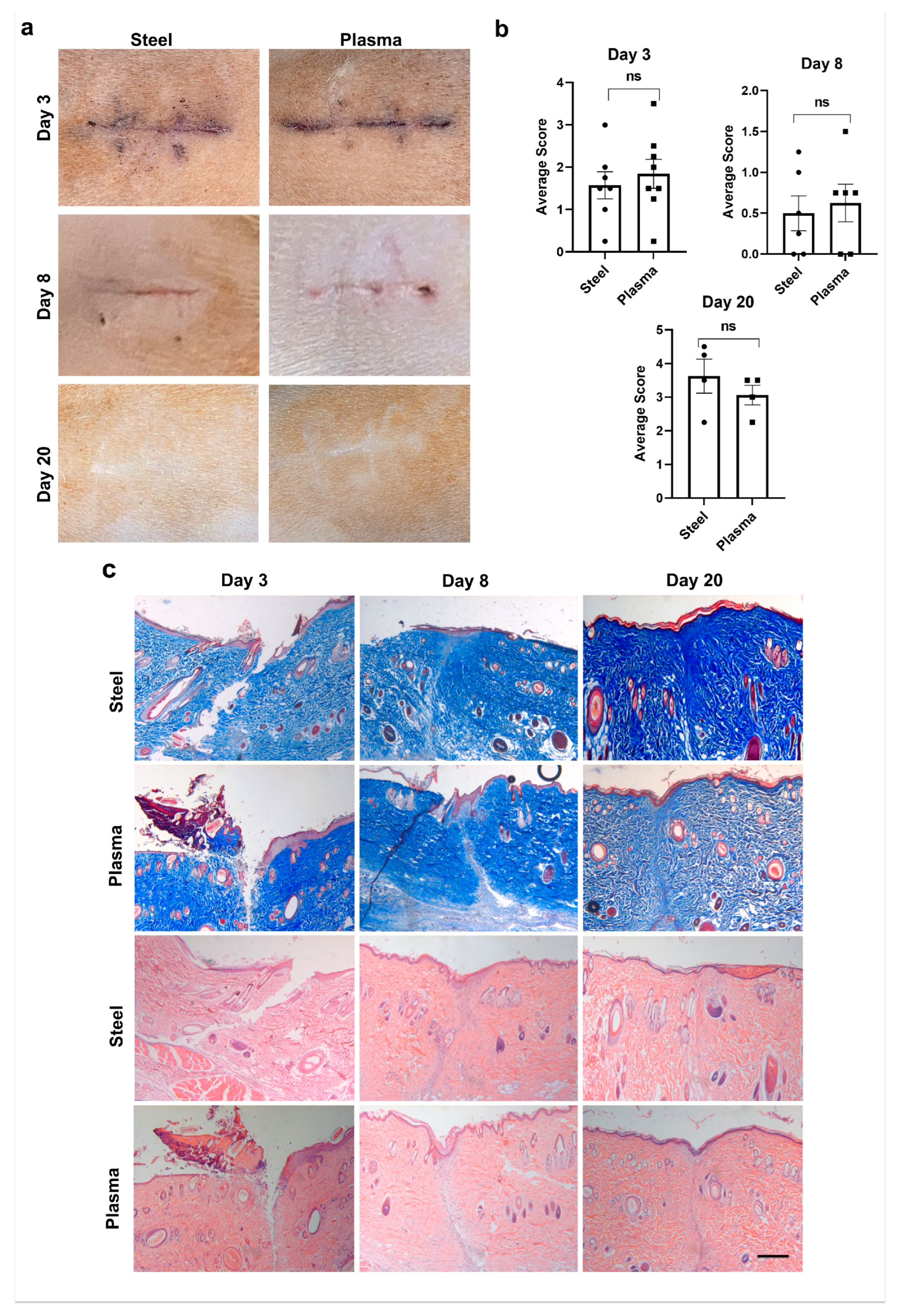
3.5. Plasma Scalpel Exhibits No Differences in External Wound Appearance When Compared to Steel Scalpel in the Rat Model
3.6. Quantitation of Histological Wound Healing Showed No Significant Differences between Steel and J-Plasma Scalpels in the Rat Model
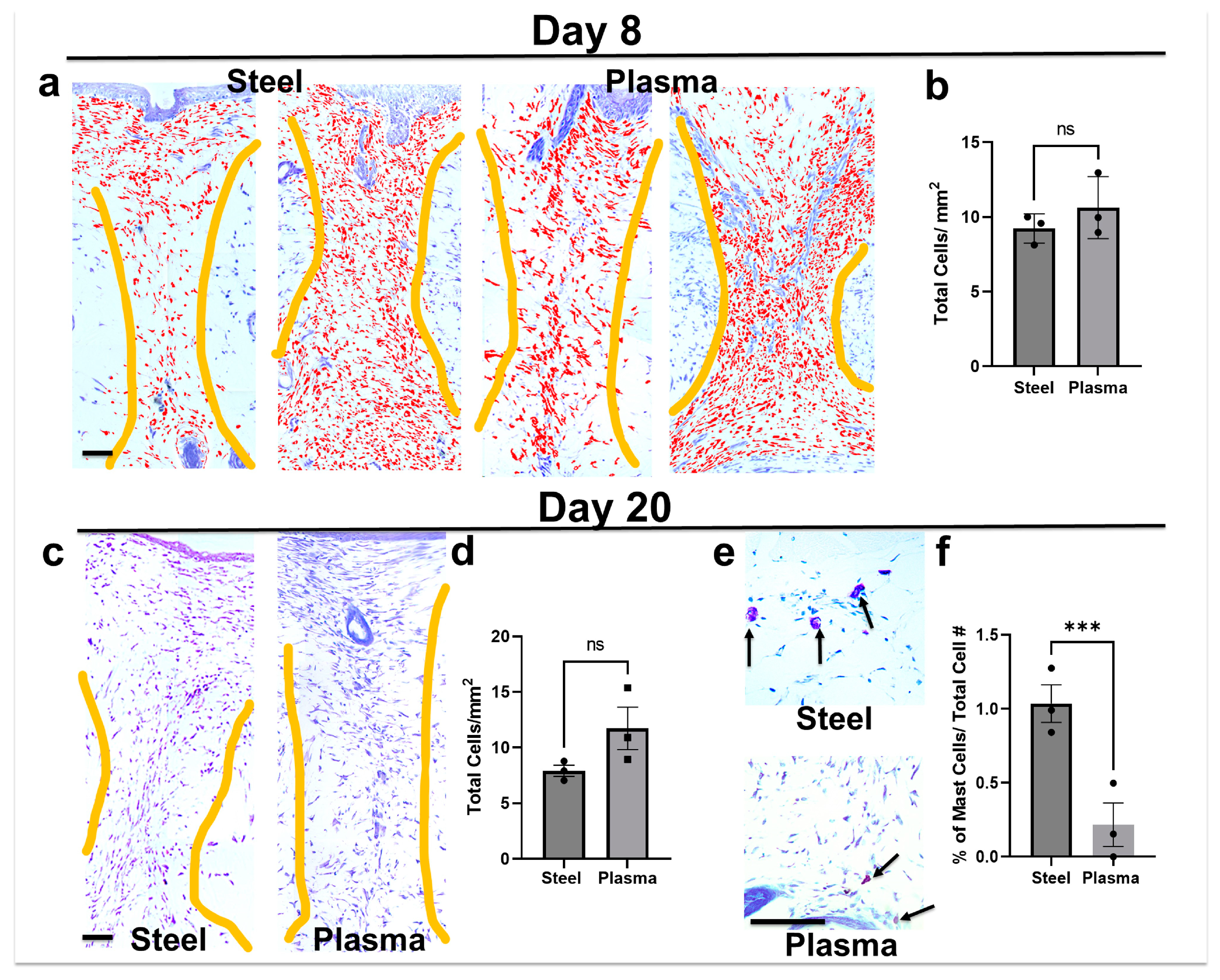
3.7. Total Cell Infiltrate in Granulation Tissue Is Similar in Both Steel and Plasma Wound Beds on Days 8 and 20 Post-Incision, but Steel Incisions Have a Significantly Greater Number of Mast Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dandu, N.; Nelson, B.B.; Easley, J.T.; Huddleston, H.P.; DeFroda, S.F.; Bisazza, K.T.; Garrigues, G.E.; Yanke, A.B. Quantifying the Magnitude of Local Tendon Injury from Electrosurgical Transection. J. Shoulder Elbow Surg. 2022, 31, 832–838. [Google Scholar] [CrossRef]
- Bekeschus, S.; Brüggemeier, J.; Hackbarth, C.; Von Woedtke, T.; Partecke, L.-I.; Van Der Linde, J. Platelets Are Key in Cold Physical Plasma-Facilitated Blood Coagulation in Mice. Clin. Plasma Med. 2017, 7–8, 58–65. [Google Scholar] [CrossRef]
- Bekeschus, S.; Clemen, R.; Metelmann, H.-R. Potentiating Anti-Tumor Immunity with Physical Plasma. Clin. Plasma Med. 2018, 12, 17–22. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef]
- Hertwig, C.; Meneses, N.; Mathys, A. Cold Atmospheric Pressure Plasma and Low Energy Electron Beam as Alternative Nonthermal Decontamination Technologies for Dry Food Surfaces: A Review. Trends Food Sci. Technol. 2018, 77, 131–142. [Google Scholar] [CrossRef]
- Taheri, A.; Mansoori, P.; Sandoval, L.F.; Feldman, S.R.; Pearce, D.; Williford, P.M. Electrosurgery. J. Am. Acad. Dermatol. 2014, 70, 591.e1–591.e14. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, M.; Starnoni, M.; De Santis, G. The Use of Cold Atmospheric Plasma Device in Flap Elevation. Plast. Reconstr. Surg.-Glob. Open 2020, 8, e2815. [Google Scholar] [CrossRef]
- Filis, K.; Galyfos, G.; Sigala, F.; Zografos, G. Utilization of Low-Temperature Helium Plasma (J-Plasma) for Dissection and Hemostasis during Carotid Endarterectomy. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 152–155. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.; Poorun, D.; Liu, D.; Lu, X.; He, M.; Duan, X.; Chen, H. Medical Applications of Nonthermal Atmospheric Pressure Plasma in Dermatology. JDDG J. Dtsch. Dermatol. Ges. 2018, 16, 7–13. [Google Scholar] [CrossRef]
- Musavi, E.S.; Khorashadizadeh, S.M.; Fallah, R.; Rahmanian Sharifabad, A. Effect of Nonthermal Atmospheric Pressure Plasma on Plasma Coagulation in Healthy Persons and Patients under Treatment with Warfarin. Contrib. Plasma Phys. 2019, 59, 354–357. [Google Scholar] [CrossRef]
- Nguyen, L.; Lu, P.; Boehm, D.; Bourke, P.; Gilmore, B.F.; Hickok, N.J.; Freeman, T.A. Cold Atmospheric Plasma Is a Viable Solution for Treating Orthopedic Infection: A Review. Biol. Chem. 2018, 400, 77–86. [Google Scholar] [CrossRef]
- Weltmann, K.-D.; Von Woedtke, T. Plasma Medicine—Current State of Research and Medical Application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological Interactions with Cold Plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef]
- Chernets, N.; Kurpad, D.S.; Alexeev, V.; Rodrigues, D.B.; Freeman, T.A. Reaction Chemistry Generated by Nanosecond Pulsed Dielectric Barrier Discharge Treatment Is Responsible for the Tumor Eradication in the B16 Melanoma Mouse Model: DBD Generated ROS Eliminates Melanoma. Plasma Process. Polym. 2015, 12, 1400–1409. [Google Scholar] [CrossRef]
- Gilmore, B.F.; Flynn, P.B.; O’Brien, S.; Hickok, N.; Freeman, T.; Bourke, P. Cold Plasmas for Biofilm Control: Opportunities and Challenges. Trends Biotechnol. 2018, 36, 627–638. [Google Scholar] [CrossRef]
- Eisenhauer, P.; Chernets, N.; Song, Y.; Dobrynin, D.; Pleshko, N.; Steinbeck, M.J.; Freeman, T.A. Chemical Modification of Extracellular Matrix by Cold Atmospheric Plasma-Generated Reactive Species Affects Chondrogenesis and Bone Formation. J. Tissue Eng. Regen. Med. 2016, 10, 772–782. [Google Scholar] [CrossRef]
- Graves, D.B. Mechanisms of Plasma Medicine: Coupling Plasma Physics, Biochemistry, and Biology. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 1, 281–292. [Google Scholar] [CrossRef]
- Steinbeck, M.J.; Chernets, N.; Zhang, J.; Kurpad, D.S.; Fridman, G.; Fridman, A.; Freeman, T.A. Skeletal Cell Differentiation Is Enhanced by Atmospheric Dielectric Barrier Discharge Plasma Treatment. PLoS ONE 2013, 8, e82143. [Google Scholar] [CrossRef] [PubMed]
- Von Woedtke, T.; Weltmann, K.-D. Grundlagen der Plasmamedizin. MKG-Chirurg 2016, 9, 246–254. [Google Scholar] [CrossRef]
- Chernets, N.; Zhang, J.; Steinbeck, M.J.; Kurpad, D.S.; Koyama, E.; Friedman, G.; Freeman, T.A. Nonthermal Atmospheric Pressure Plasma Enhances Mouse Limb Bud Survival, Growth, and Elongation. Tissue Eng. Part A 2015, 21, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Leutner, S.; Eckert, A.; Müller, W.E. ROS Generation, Lipid Peroxidation and Antioxidant Enzyme Activities in the Aging Brain. J. Neural Transm. 2001, 108, 955–967. [Google Scholar] [CrossRef]
- Bekeschus, S.; Brüggemeier, J.; Hackbarth, C.; Weltmann, K.-D.; Von Woedtke, T.; Partecke, L.-I.; Van Der Linde, J. The Feed Gas Composition Determines the Degree of Physical Plasma-Induced Platelet Activation for Blood Coagulation. Plasma Sources Sci. Technol. 2018, 27, 034001. [Google Scholar] [CrossRef]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.D.; Masur, K.; von Woedtke, T. Risk Assessment of a Cold Argon Plasma Jet in Respect to Its Mutagenicity. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 798–799, 48–54. [Google Scholar] [CrossRef]
- Elston, M.J.; Dupaix, J.P.; Opanova, M.I.; Atkinson, R.E. Cutibacterium Acnes (Formerly Proprionibacterium Acnes) and Shoulder Surgery. Hawaii J. Health Soc. Welf. 2019, 78, 3–5. [Google Scholar] [PubMed]
- Saltzman, M.D.; Nuber, G.W.; Gryzlo, S.M.; Marecek, G.S.; Koh, J.L. Efficacy of Surgical Preparation Solutions in Shoulder Surgery. J. Bone Jt. Surg. 2009, 91, 1949–1953. [Google Scholar] [CrossRef]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine Models of Cutaneous Wound Healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Campwala, I.; Unsell, K.; Gupta, S. A Comparative Analysis of Surgical Wound Infection Methods: Predictive Values of the CDC, ASEPSIS, and Southampton Scoring Systems in Evaluating Breast Reconstruction Surgical Site Infections. Plast. Surg. 2019, 27, 93–99. [Google Scholar] [CrossRef]
- Fearmonti, R.; Bond, J.; Erdmann, D.; Levinson, H. A Review of Scar Scales and Scar Measuring Devices. Eplasty 2010, 10, e43. [Google Scholar]
- Sultana, J.; Molla, M.R.; Kamal, M.; Shahidullah, M.; Begum, F.; Bashar, M.A. Histological Differences in Wound Healing in Maxillofacial Region in Patients with or without Risk Factors. Bangladesh J. Pathol. 1970, 24, 3–8. [Google Scholar] [CrossRef]
- van de Vyver, M.; Boodhoo, K.; Frazier, T.; Hamel, K.; Kopcewicz, M.; Levi, B.; Maartens, M.; Machcinska, S.; Nunez, J.; Pagani, C.; et al. Histology Scoring System for Murine Cutaneous Wounds. Stem Cells Dev. 2021, 30, 1141–1152. [Google Scholar] [CrossRef]
- Heslin, C.; Boehm, D.; Milosavljevic, V.; Laycock, M.; Cullen, P.J.; Bourke, P. Quantitative Assessment of Blood Coagulation by Cold Atmospheric Plasma. Plasma Med. 2014, 4, 153–163. [Google Scholar] [CrossRef]
- Kramer, A.; Lindequist, U.; Weltmann, K.-D.; Wilke, C.; von Woedtke, T. Plasma Medicine—Its Perspective for Wound Therapy. GMS Krankenhaushygiene Interdiszip. 2008, 3, Dec16. [Google Scholar]
- Falconer, T.M.; Baba, M.; Kruse, L.M.; Dorrestijn, O.; Donaldson, M.J.; Smith, M.M.; Figtree, M.C.; Hudson, B.J.; Cass, B.; Young, A.A. Contamination of the Surgical Field with Propionibacterium Acnes in Primary Shoulder Arthroplasty. J. Bone Jt. Surg. 2016, 98, 1722–1728. [Google Scholar] [CrossRef]
- Bekeschus, S.; Seebauer, C.; Wende, K.; Schmidt, A. Physical Plasma and Leukocytes—Immune or Reactive? Biol. Chem. 2018, 400, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Von Woedtke, T.; Emmert, S.; Schmidt, A. Medical Gas Plasma-Stimulated Wound Healing: Evidence and Mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi Rad, Z.; Abbasi Davani, F.; Etaati, G. Determination of Proper Treatment Time for in Vivo Blood Coagulation and Wound Healing Application by Non-Thermal Helium Plasma Jet. Australas. Phys. Eng. Sci. Med. 2018, 41, 905–917. [Google Scholar] [CrossRef]
- Darmawati, S.; Nasruddin, N.; Putri, G.S.A.; Iswara, A.; Kurniasiwi, P.; Wahyuningtyas, E.S.; Nurani, L.H.; Hayati, D.N.; Ishijima, T.; Nakatani, T.; et al. Accelerated Healing of Chronic Wounds under a Combinatorial Therapeutic Regimen Based on Cold Atmospheric Plasma Jet Using Contact and Noncontact Styles. Plasma Med. 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Klebes, M.; Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Richter, H.; Bob, A.; Von Hutten, J.; Krediet, J.T.; Kramer, A.; et al. Combined Antibacterial Effects of Tissue-tolerable Plasma and a Modern Conventional Liquid Antiseptic on Chronic Wound Treatment. J. Biophotonics 2015, 8, 382–391. [Google Scholar] [CrossRef]
- Bailey, I.S.; Karran, S.E.; Toyn, K.; Brough, P.; Ranaboldo, C.; Karran, S.J. Community Surveillance of Complications after Hernia Surgery. BMJ 1992, 304, 469–471. [Google Scholar] [CrossRef]
- Wang, X.-F.; Fang, Q.-Q.; Jia, B.; Hu, Y.-Y.; Wang, Z.-C.; Yan, K.; Yin, S.-Y.; Liu, Z.; Tan, W.-Q. Potential Effect of Non-Thermal Plasma for the Inhibition of Scar Formation: A Preliminary Report. Sci. Rep. 2020, 10, 1064. [Google Scholar] [CrossRef]
- Lee, M.-H.; Lee, Y.S.; Kim, H.J.; Han, C.H.; Kang, S.U.; Kim, C.-H. Non-Thermal Plasma Inhibits Mast Cell Activation and Ameliorates Allergic Skin Inflammatory Diseases in NC/Nga Mice. Sci. Rep. 2019, 9, 13510. [Google Scholar] [CrossRef] [PubMed]
- Noli, C.; Miolo, A. The Mast Cell in Wound Healing. Vet. Dermatol. 2001, 12, 303–313. [Google Scholar] [CrossRef]
- Urb, M.; Sheppard, D.C. The Role of Mast Cells in the Defence against Pathogens. PLoS Pathog. 2012, 8, e1002619. [Google Scholar] [CrossRef] [PubMed]
- Iba, Y.; Shibata, A.; Kato, M.; Masukawa, T. Possible Involvement of Mast Cells in Collagen Remodeling in the Late Phase of Cutaneous Wound Healing in Mice. Int. Immunopharmacol. 2004, 4, 1873–1880. [Google Scholar] [CrossRef] [PubMed]

| Southampton Scoring System | |
|---|---|
| Grade | Appearance |
| 0 Normal Healing | |
| (1) Normal Healing with mild bruising or erythema | 0.25 some bruising |
| 0.50 considerable bruising | |
| 0.75 mild erythema | |
| (2) Erythema plus other signs of inflammation | 0.25 at one point |
| 0.50 around sutures | |
| 0.75 along wound | |
| 0.95 around wound | |
| (3) Clear or hemoserous discharge | 0.25 at one point only (<2 cm) |
| 0.50 along wound (>2 cm) | |
| 0.75 large volume | |
| 0.95 prolonged (>3 days) | |
| (4) Pus/purulent discharge | 0.25 at one point only (<2 cm) |
| 0.50 along wound (>2 cm) | |
| (5) Deep or severe wound infection with or without tissue breakdown | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmore, L.; Minissale, N.J.; Israel, L.; Katz, Z.; Safran, J.; Barba, A.; Austin, L.; Schaer, T.P.; Freeman, T.A. Evaluating the Healing Potential of J-Plasma Scalpel-Created Surgical Incisions in Porcine and Rat Models. Biomedicines 2024, 12, 277. https://doi.org/10.3390/biomedicines12020277
Elmore L, Minissale NJ, Israel L, Katz Z, Safran J, Barba A, Austin L, Schaer TP, Freeman TA. Evaluating the Healing Potential of J-Plasma Scalpel-Created Surgical Incisions in Porcine and Rat Models. Biomedicines. 2024; 12(2):277. https://doi.org/10.3390/biomedicines12020277
Chicago/Turabian StyleElmore, Lilith, Nicholas J. Minissale, Lauren Israel, Zoe Katz, Jordan Safran, Adriana Barba, Luke Austin, Thomas P. Schaer, and Theresa A. Freeman. 2024. "Evaluating the Healing Potential of J-Plasma Scalpel-Created Surgical Incisions in Porcine and Rat Models" Biomedicines 12, no. 2: 277. https://doi.org/10.3390/biomedicines12020277
APA StyleElmore, L., Minissale, N. J., Israel, L., Katz, Z., Safran, J., Barba, A., Austin, L., Schaer, T. P., & Freeman, T. A. (2024). Evaluating the Healing Potential of J-Plasma Scalpel-Created Surgical Incisions in Porcine and Rat Models. Biomedicines, 12(2), 277. https://doi.org/10.3390/biomedicines12020277