A Combined Computational and Experimental Analysis of PLA and PCL Hybrid Nanocomposites 3D Printed Scaffolds for Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Work
2.1.1. Materials
2.1.2. Fabrication of the PLA/PCL Hybrid Scaffolds
2.1.3. Mechanical Testing
2.2. Computational Work
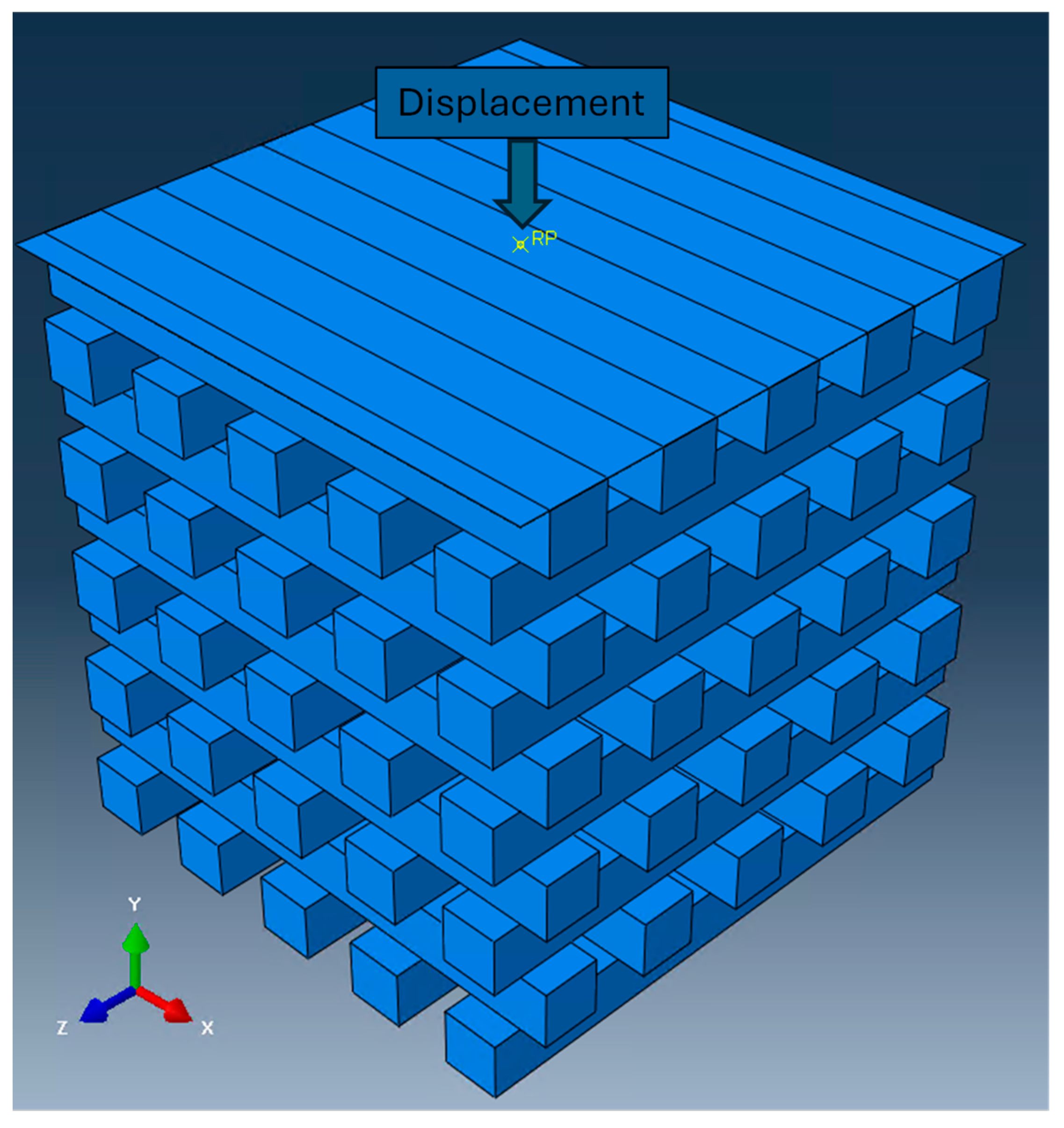
2.2.1. Mechanical Properties
2.2.2. Governing Equations and Boundary Conditions
2.2.3. Sensitivity Analysis
3. Results
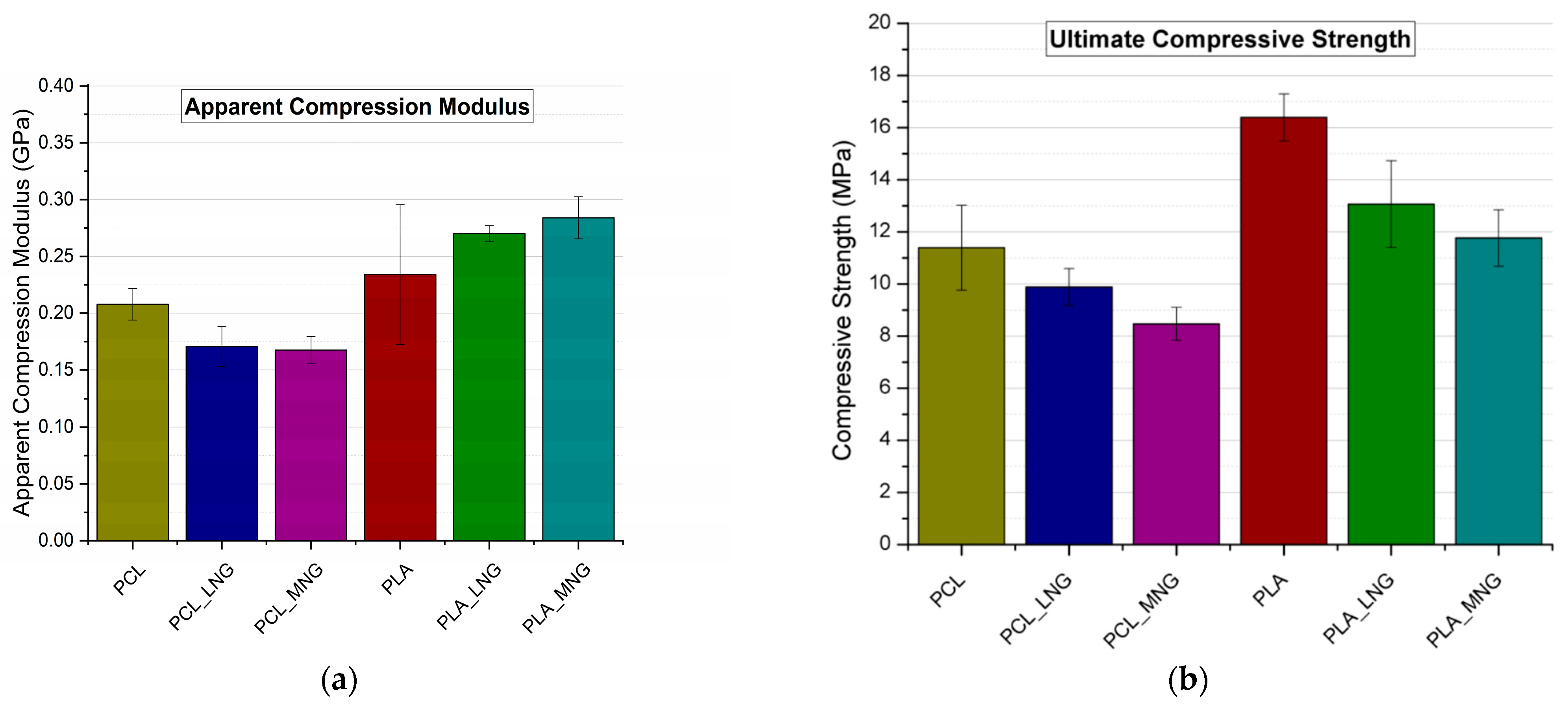
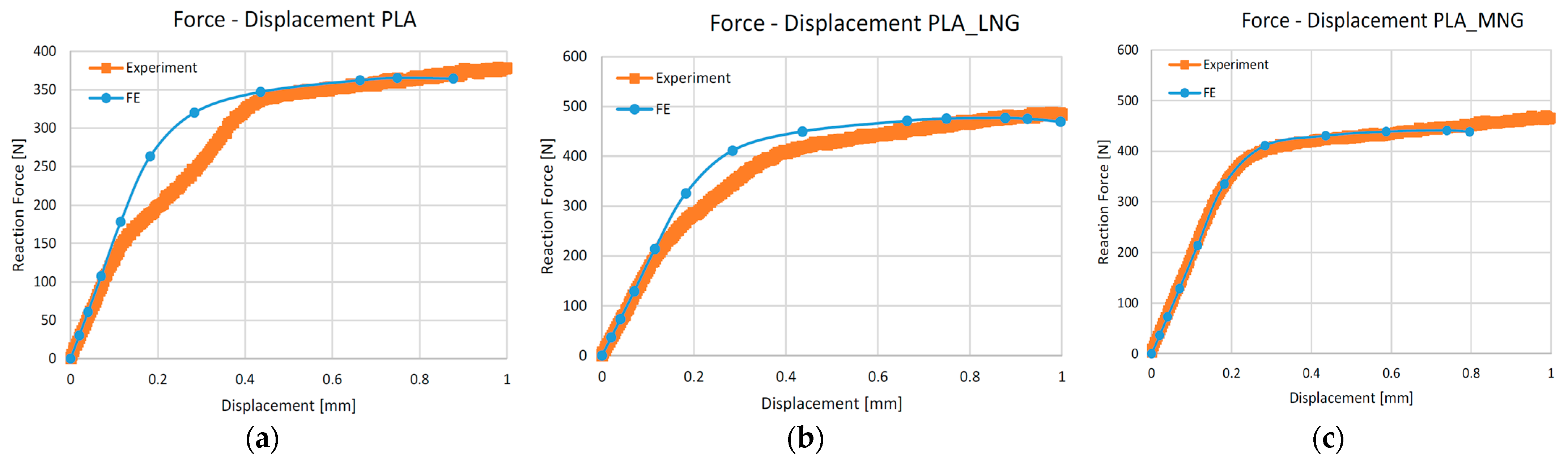
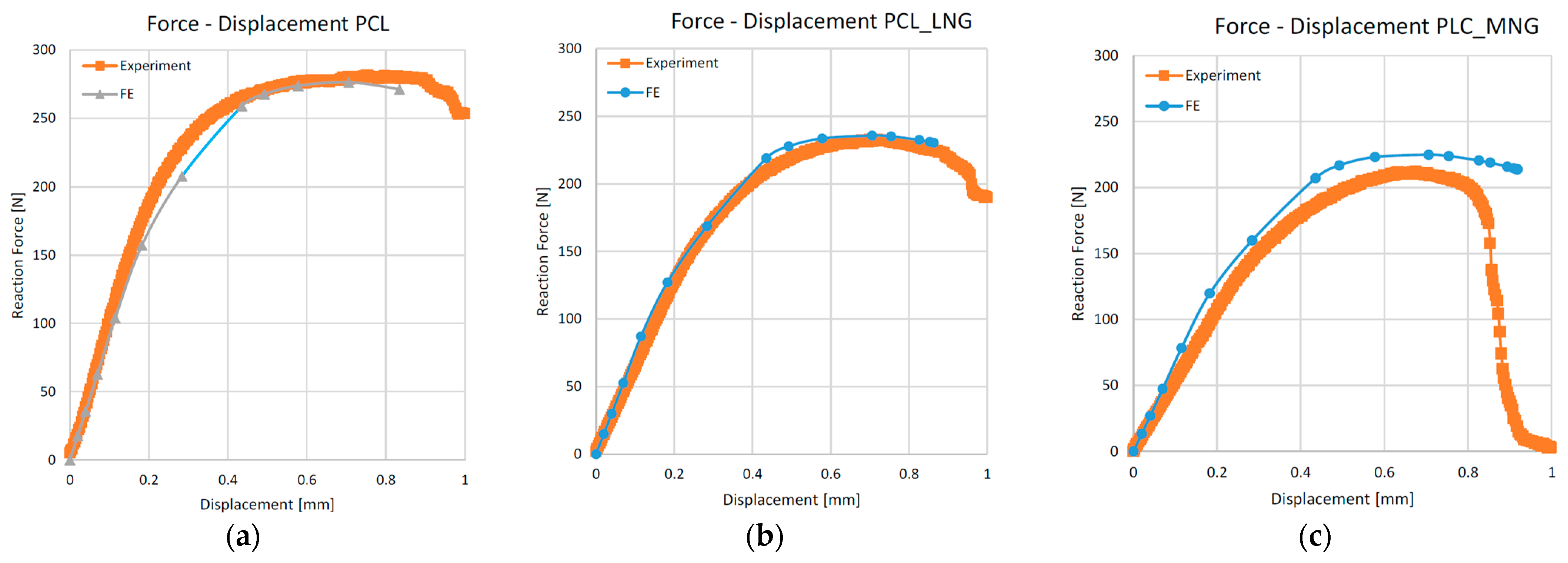
3.1. Experimental Results
3.2. Computational Results
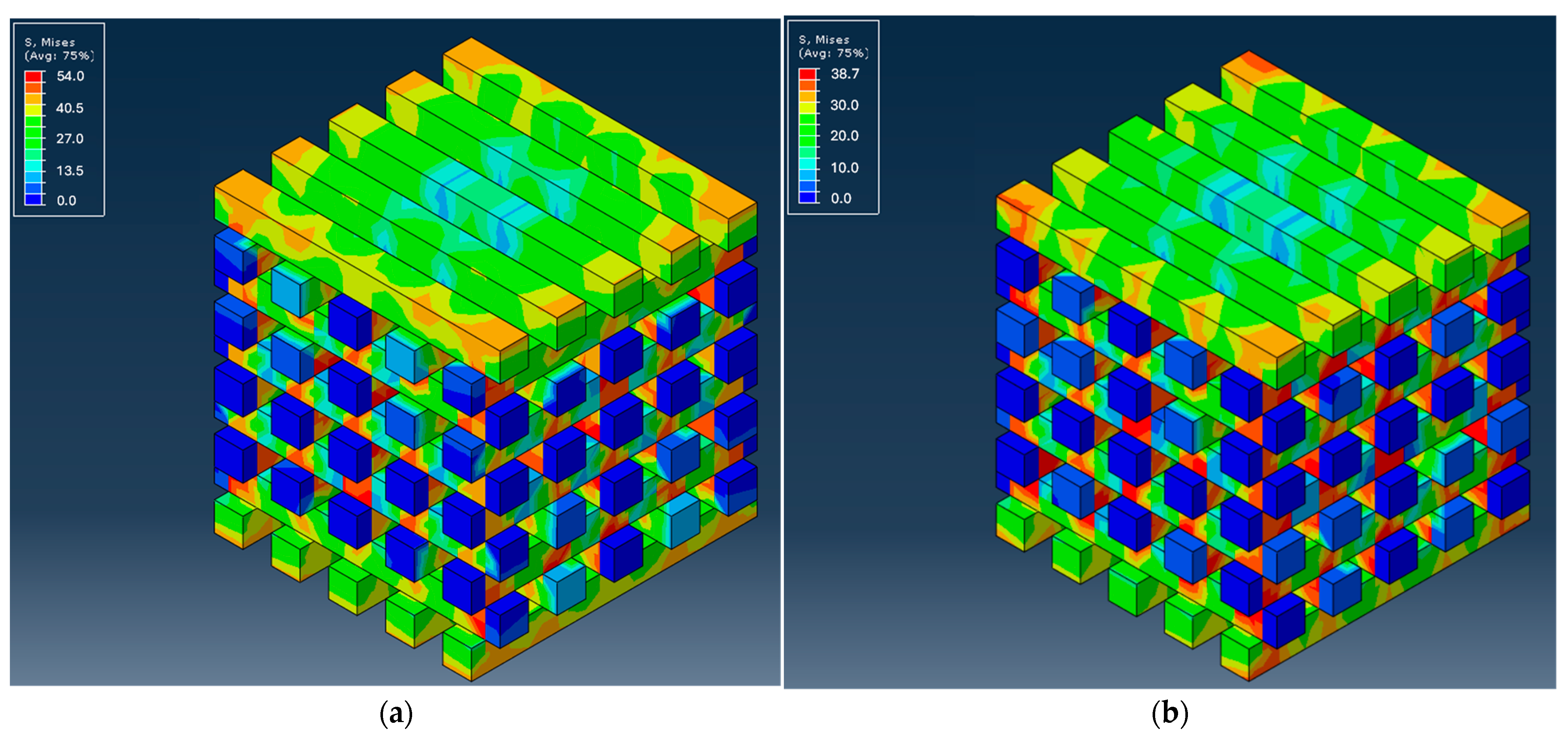
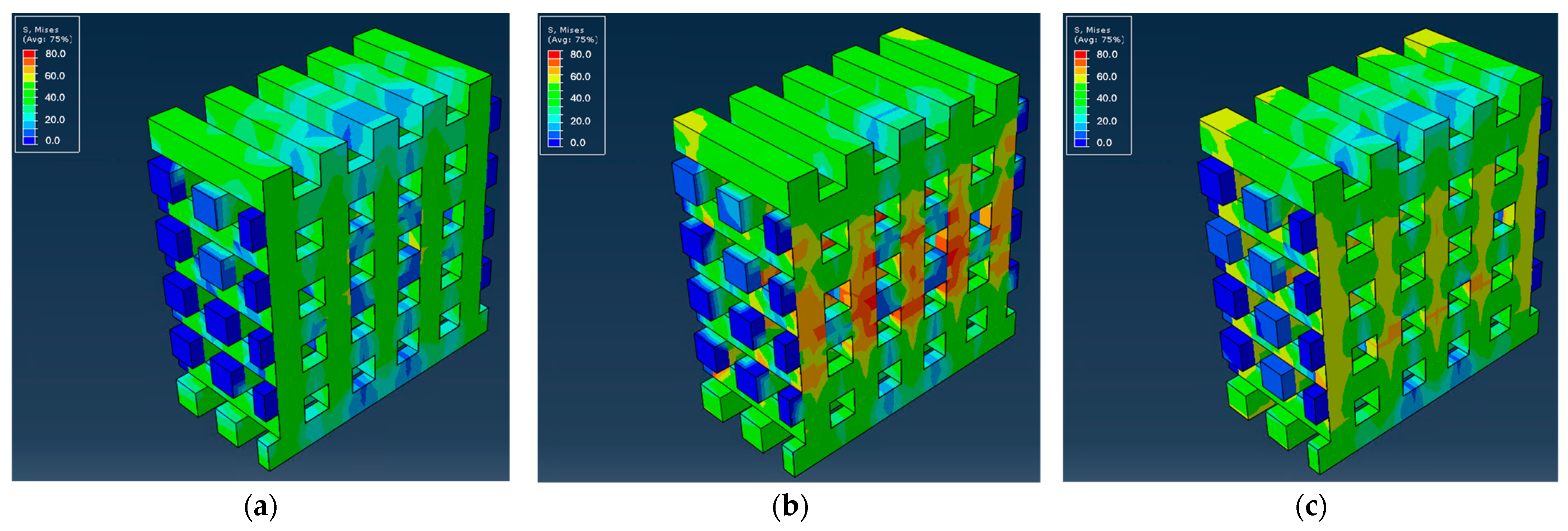
3.2.1. Mechanical Simulation
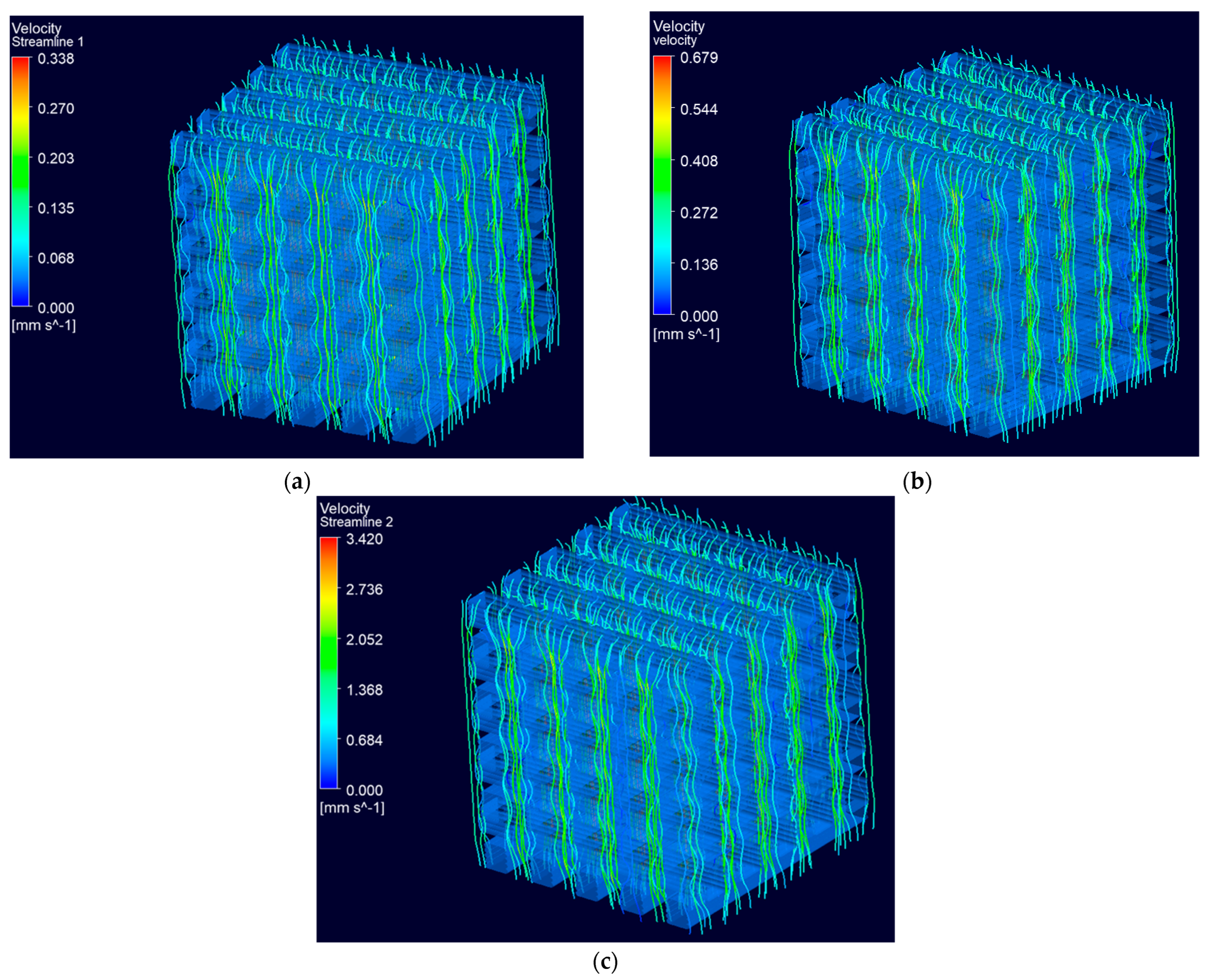
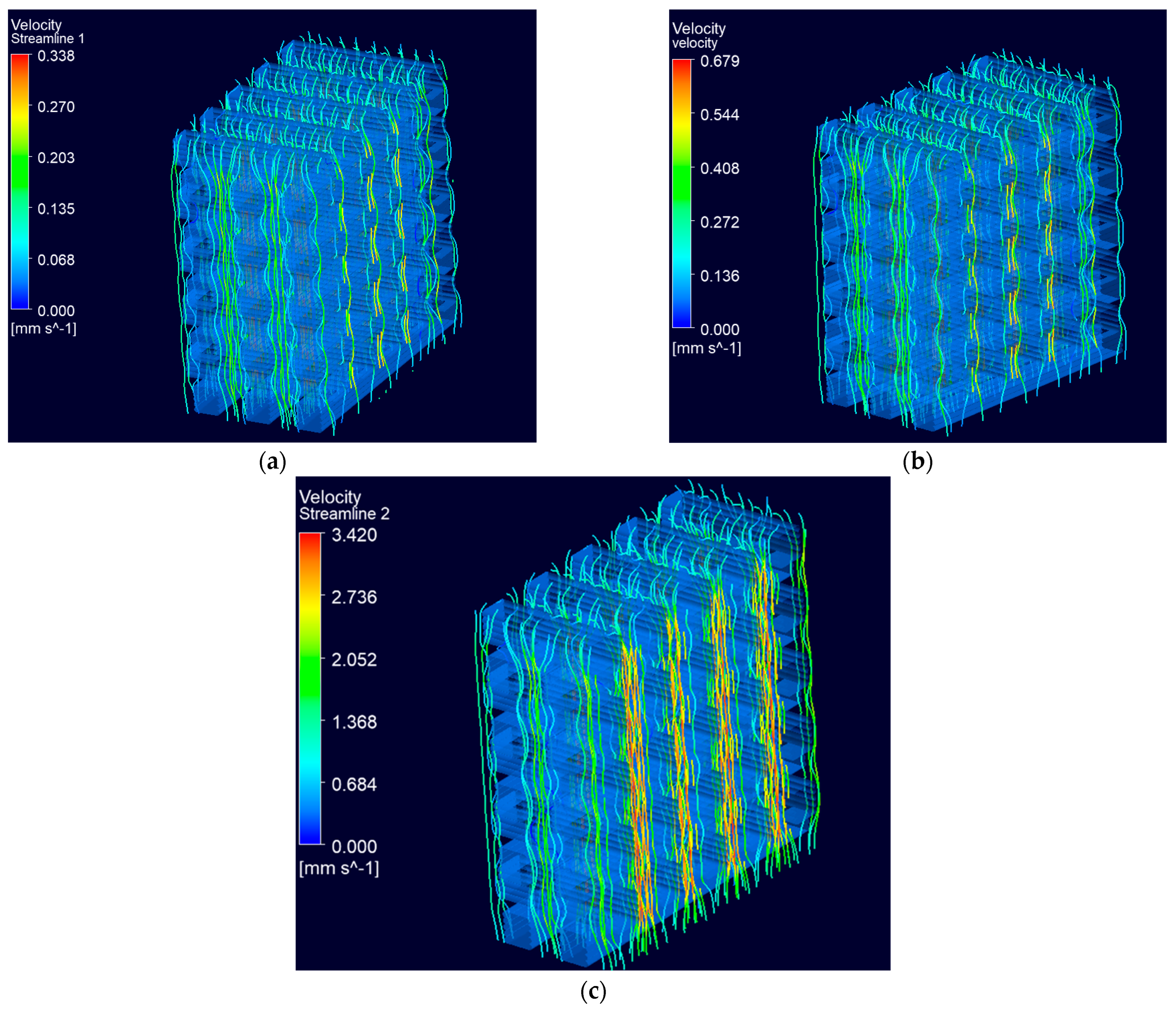
3.2.2. Fluid Flow Dynamics within Scaffolds
Permeability
3.2.3. Wall Shear Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahani, B.; Wang, X.; Brooks, A. Additive Manufacturing Techniques for Fabrication of Bone Scaffolds for Tissue Engineering Applications. Recent Prog. Mater. 2020, 2, 021. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Cao, L.; Zhang, X.; Wang, J.; Liu, C. Facilitated Vascularization and Enhanced Bone Regeneration by Manipulation Hierarchical Pore Structure of Scaffolds. Mater. Sci. Eng. C 2020, 110, 110622. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Accoto, D.; Trombetta, M.; Rainer, A. Current Trends in the Design of Scaffolds for Computer-Aided Tissue Engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef]
- Rezgui, F.; Swistek, M.; Hiver, J.M.; G’Sell, C.; Sadoun, T. Deformation and Damage upon Stretching of Degradable Polymers (PLA and PCL). Polymer 2005, 46, 7370–7385. [Google Scholar] [CrossRef]
- Zhao, F.; Lacroix, D.; Ito, K.; van Rietbergen, B.; Hofmann, S. Changes in Scaffold Porosity during Bone Tissue Engineering in Perfusion Bioreactors Considerably Affect Cellular Mechanical Stimulation for Mineralization. Bone Rep. 2020, 12, 100265. [Google Scholar] [CrossRef]
- Chao, L.; Jiao, C.; Liang, H.; Xie, D.; Shen, L.; Liu, Z. Analysis of Mechanical Properties and Permeability of Trabecular-Like Porous Scaffold by Additive Manufacturing. Front. Bioeng. Biotechnol. 2021, 9, 779854. [Google Scholar] [CrossRef]
- Olivares, A.L.; Marsal, È.; Planell, J.A.; Lacroix, D. Finite Element Study of Scaffold Architecture Design and Culture Conditions for Tissue Engineering. Biomaterials 2009, 30, 6142–6149. [Google Scholar] [CrossRef]
- Kakarla, A.B.; Kong, I.; Nukala, S.G.; Kong, W. Mechanical Behaviour Evaluation of Porous Scaffold for Tissue-Engineering Applications Using Finite Element Analysis. J. Compos. Sci. 2022, 6, 46. [Google Scholar] [CrossRef]
- Suffo, M.; López-Marín, C.J. A Comparative Study of Turbulence Methods Applied to the Design of a 3d-Printed Scaffold and the Selection of the Appropriate Numerical Scheme to Simulate the Scaffold for Tissue Engineering. Appl. Sci. 2022, 12, 191. [Google Scholar] [CrossRef]
- Ouyang, P.; Dong, H.; He, X.; Cai, X.; Wang, Y.; Li, J.; Li, H.; Jin, Z. Hydromechanical Mechanism behind the Effect of Pore Size of Porous Titanium Scaffolds on Osteoblast Response and Bone Ingrowth. Mater. Des. 2019, 183, 108151. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Deng, F.; Liu, L.; Li, Z.; Liu, J. 3D Printed Ti6Al4V Bone Scaffolds with Different Pore Structure Effects on Bone Ingrowth. J. Biol. Eng. 2021, 15, 4. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, F.; Zhai, W.; Cheng, S.; Li, J.; Wang, Y. Unraveling of Advances in 3D-Printed Polymer-Based Bone Scaffolds. Polymers 2022, 14, 566. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer-Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Feier, A.M.; Portan, D.; Manu, D.R.; Kostopoulos, V.; Kotrotsos, A.; Strnad, G.; Dobreanu, M.; Salcudean, A.; Bataga, T. Primary MSCs for Personalized Medicine: Ethical Challenges, Isolation and Biocompatibility Evaluation of 3D Electrospun and Printed Scaffolds. Biomedicines 2022, 10, 1563. [Google Scholar] [CrossRef]
- Pok, S.; Vitale, F.; Eichmann, S.L.; Benavides, O.M.; Pasquali, M.; Jacot, J.G. Biocompatible Carbon Nanotube-Chitosan Scaffold Matching the Electrical Conductivity of the Heart. ACS Nano 2014, 8, 9822–9832. [Google Scholar] [CrossRef]
- Zieliński, A.; Majkowska-Marzec, B. Whether Carbon Nanotubes Are Capable, Promising, and Safe for Their Application in Nervous System Regeneration. Some Critical Remarks and Research Strategies. Coatings 2022, 12, 1643. [Google Scholar] [CrossRef]
- Hendrikson, W.J.; Deegan, A.J.; Yang, Y.; van Blitterswijk, C.A.; Verdonschot, N.; Moroni, L.; Rouwkema, J. Influence of Additive Manufactured Scaffold Architecture on the Distribution of Surface Strains and Fluid Flow Shear Stresses and Expected Osteochondral Cell Differentiation. Front. Bioeng. Biotechnol. 2017, 5, 6. [Google Scholar] [CrossRef]
- Liu, M.; Wu, C.; Jiao, Y.; Xiong, S.; Zhou, C. Chitosan-Halloysite Nanotubes Nanocomposite Scaffolds for Tissue Engineering. J. Mater. Chem. B 2013, 1, 2078–2089. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Z.; Liu, L.; Huang, Z.; Li, Z.; Liu, J.; Hao, Y. Honeycomb Structure Is Promising for the Repair of Human Bone Defects. Mater. Des. 2021, 207, 109832. [Google Scholar] [CrossRef]
- Mohol, S.S.; Kumar, M.; Sharma, V. PLA-Based Nature-Inspired Architecture for Bone Scaffolds: A Finite Element Analysis. Comput. Biol. Med. 2023, 163, 107163. [Google Scholar] [CrossRef]
- Wu, Z.; Feng, W.; Feng, Y.; Liu, Q.; Xu, X.; Sekino, T.; Fujii, A.; Ozaki, M. Preparation and Characterization of Chitosan-Grafted Multiwalled Carbon Nanotubes and Their Electrochemical Properties. Carbon 2007, 45, 1212–1218. [Google Scholar] [CrossRef]
- Kallivokas, S.V.; Kontaxis, L.; Kakkos, I.; Deligianni, D.; Kostopoulos, V.; Matsopoulos, G.K. A Computational and Experimental Mechanical Study of Nanocomposites for 3D Printed Scaffolds with a New Geometry. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, NSW, Australia, 24–27 July 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Sadrehaghighi, I. Mesh Sensitivity & Mesh Independence Study; CFD Open Series: Annapolis, MD, USA, 2021; Volume 56. [Google Scholar] [CrossRef]
- Fallah, A.; Altunbek, M.; Bartolo, P.; Cooper, G.; Weightman, A.; Blunn, G.; Koc, B. 3D Printed Scaffold Design for Bone Defects with Improved Mechanical and Biological Properties. J. Mech. Behav. Biomed. Mater. 2022, 134, 105418. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, N.; Azri, A.; Mohd, N.A.N.; Khair, A.H.; Naja, A.A.; Amir, H.M.H.; Abd, S.M.S.H.; Muhammad, A.T.; Abdul, S.A. CFD Simulation on Permeability of Porous Scaffolds for Human Skeletal System. J. Hum. Centered Technol. 2022, 1, 39–47. [Google Scholar] [CrossRef]
- Ali, D.; Ozalp, M.; Blanquer, S.B.G.; Onel, S. Permeability and Fluid Flow-Induced Wall Shear Stress in Bone Scaffolds with TPMS and Lattice Architectures: A CFD Analysis. Eur. J. Mech. B/Fluids 2020, 79, 376–385. [Google Scholar] [CrossRef]
- Foroughi, A.H.; Razavi, M.J. Multi-Objective Shape Optimization of Bone Scaffolds: Enhancement of Mechanical Properties and Permeability. Acta Biomater. 2022, 146, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Lughmani, W.A.; Obeidi, M.A.; Brabazon, D.; Ahad, I.U. Additive Manufacturing of Bone Scaffolds Using Polyjet and Stereolithography Techniques. Appl. Sci. 2021, 11, 7336. [Google Scholar] [CrossRef]
- Omar, A.M.; Hassan, M.H.; Daskalakis, E.; Ates, G.; Bright, C.J.; Xu, Z.; Powell, E.J.; Mirihanage, W.; Bartolo, P.J.D.S. Geometry-Based Computational Fluid Dynamic Model for Predicting the Biological Behavior of Bone Tissue Engineering Scaffolds. J. Funct. Biomater. 2022, 13, 104. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Kourinou, M.; Velidakis, E.; Mountakis, N.; Fischer-Griffiths, P.E.; Grammatikos, S.; Tzounis, L. Additive Manufacturing of Multifunctional Polylactic Acid (PLA)—Multiwalled Carbon Nanotubes (MWCNTs) Nanocomposites. Nanocomposites 2021, 7, 184–199. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Ramy-Ratiarison, R.; Paint, Y.; Raquez, J.M.; Dubois, P. Recent Advances in Production of Poly(Lactic Acid) (PLA) Nanocomposites: A Versatile Method to Tune Crystallization Properties of PLA. Nanocomposites 2015, 1, 71–82. [Google Scholar] [CrossRef]
- Kontaxis, L.C.; Kozaniti, F.K.; Papanicolaou, G.C. Mechanical Behavior Modelling and Filler Geometry Effect of Glass Filler Reinforced Starch-epoxy Hybrid Matrix Composites. Materials 2021, 14, 6587. [Google Scholar] [CrossRef] [PubMed]
- Sezer, H.K.; Eren, O. FDM 3D Printing of MWCNT Re-Inforced ABS Nano-Composite Parts with Enhanced Mechanical and Electrical Properties. J. Manuf. Process. 2019, 37, 339–347. [Google Scholar] [CrossRef]
- Kallivokas, S.V.; Sgouros, A.P.; Theodorou, D.N. Kinetic Concepts and Local Failure in the Interfacial Shear Strength of Epoxy-Graphene Nanocomposites. Phys. Rev. E 2020, 102, 030501. [Google Scholar] [CrossRef]
- Mahammod, B.P.; Barua, E.; Deb, P.; Deoghare, A.B.; Pandey, K.M. Investigation of Physico-Mechanical Behavior, Permeability and Wall Shear Stress of Porous HA/PMMA Composite Bone Scaffold. Arab. J. Sci. Eng. 2020, 45, 5505–5515. [Google Scholar] [CrossRef]
- Hassan, M.N.; Yassin, M.A.; Suliman, S.; Lie, S.A.; Gjengedal, H.; Mustafa, K. The Bone Regeneration Capacity of 3D-Printed Templates in Calvarial Defect Models: A Systematic Review and Meta-Analysis. Acta Biomater. 2019, 91, 1–23. [Google Scholar] [CrossRef]




| Materials | Compression Modulus (GPa) | Standard Deviation (GPa) |
|---|---|---|
| PCL | 0.353 | 0.01061 |
| PCL_LNG | 0.361 | 0.00919 |
| PCL_MNG | 0.376 | 0.03764 |
| PLA | 3.122 | 0.12976 |
| PLA_LNG | 3.071 | 0.22762 |
| PLA_MNG | 3.305 | 0.18144 |
| Fluid Inlet Velocity [mm/s] | Permeability [m2] |
|---|---|
| 0.5 | 4.315 × 10−9 |
| 0.1 | 4.412 × 10−9 |
| 0.05 | 4.413 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallivokas, S.V.; Kontaxis, L.C.; Psarras, S.; Roumpi, M.; Ntousi, O.; Kakkos, I.; Deligianni, D.; Matsopoulos, G.K.; Fotiadis, D.I.; Kostopoulos, V. A Combined Computational and Experimental Analysis of PLA and PCL Hybrid Nanocomposites 3D Printed Scaffolds for Bone Regeneration. Biomedicines 2024, 12, 261. https://doi.org/10.3390/biomedicines12020261
Kallivokas SV, Kontaxis LC, Psarras S, Roumpi M, Ntousi O, Kakkos I, Deligianni D, Matsopoulos GK, Fotiadis DI, Kostopoulos V. A Combined Computational and Experimental Analysis of PLA and PCL Hybrid Nanocomposites 3D Printed Scaffolds for Bone Regeneration. Biomedicines. 2024; 12(2):261. https://doi.org/10.3390/biomedicines12020261
Chicago/Turabian StyleKallivokas, Spyros V., Lykourgos C. Kontaxis, Spyridon Psarras, Maria Roumpi, Ourania Ntousi, Iοannis Kakkos, Despina Deligianni, George K. Matsopoulos, Dimitrios I. Fotiadis, and Vassilis Kostopoulos. 2024. "A Combined Computational and Experimental Analysis of PLA and PCL Hybrid Nanocomposites 3D Printed Scaffolds for Bone Regeneration" Biomedicines 12, no. 2: 261. https://doi.org/10.3390/biomedicines12020261
APA StyleKallivokas, S. V., Kontaxis, L. C., Psarras, S., Roumpi, M., Ntousi, O., Kakkos, I., Deligianni, D., Matsopoulos, G. K., Fotiadis, D. I., & Kostopoulos, V. (2024). A Combined Computational and Experimental Analysis of PLA and PCL Hybrid Nanocomposites 3D Printed Scaffolds for Bone Regeneration. Biomedicines, 12(2), 261. https://doi.org/10.3390/biomedicines12020261












