The Hazards of Probiotics on Gut-Derived Pseudomonas aeruginosa Sepsis in Mice Undergoing Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Strains
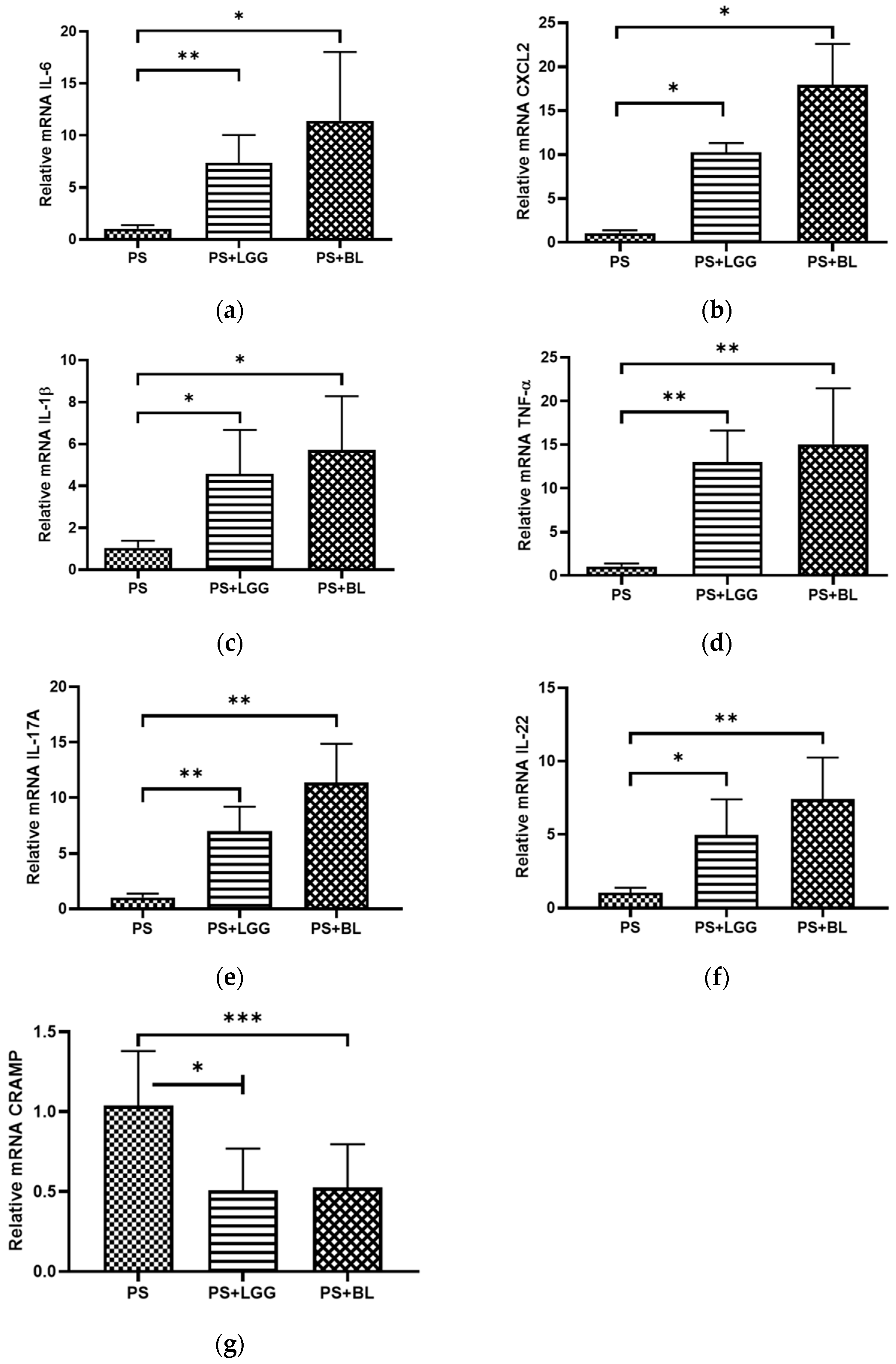
2.3. Quantitative Real-Time PCR Analysis of Cecum RNA
2.4. Animal Experiments
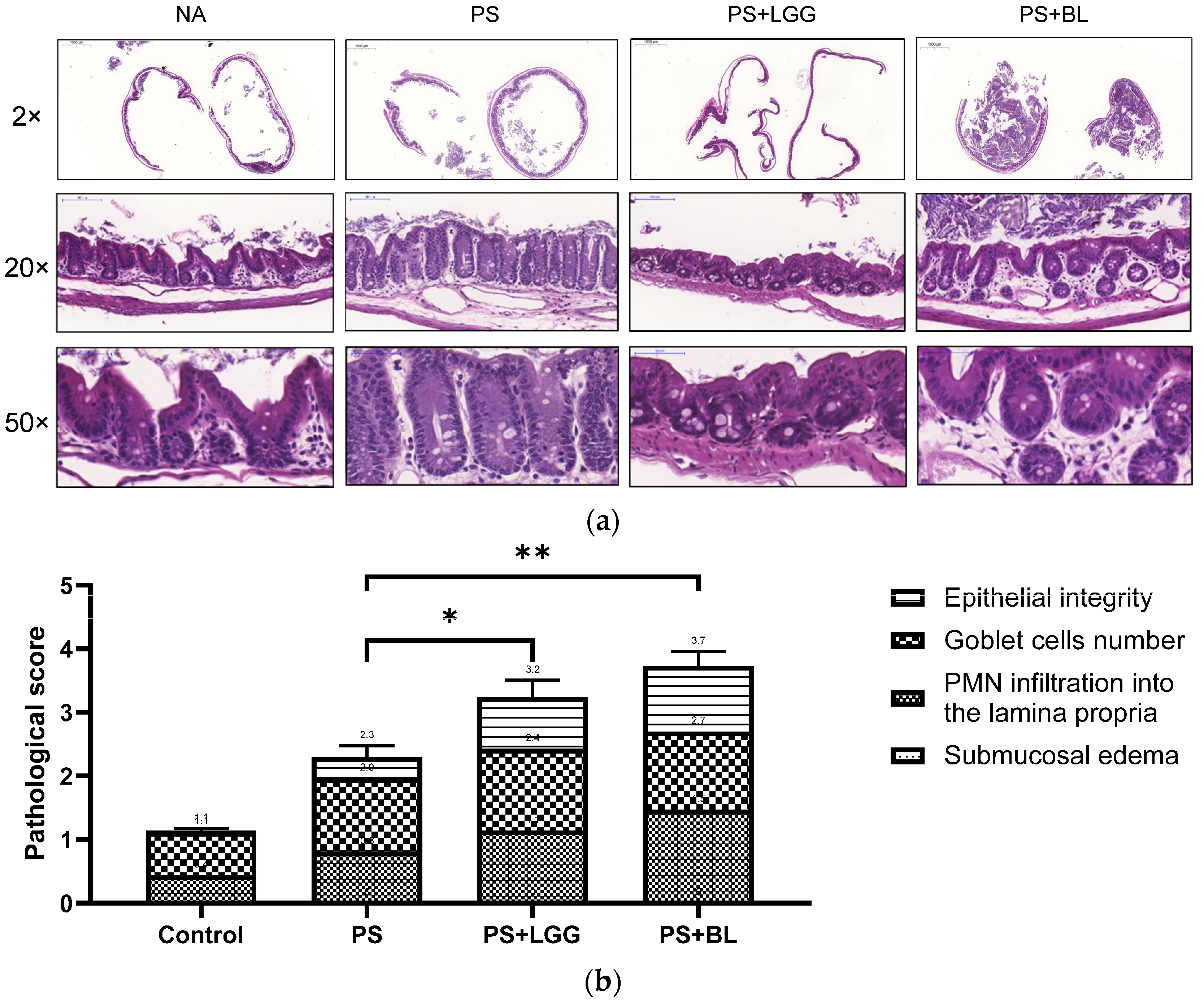
2.5. Histological Scores
2.6. Analysis of P. aeruginosa Loads in Liver, Spleen, and Blood
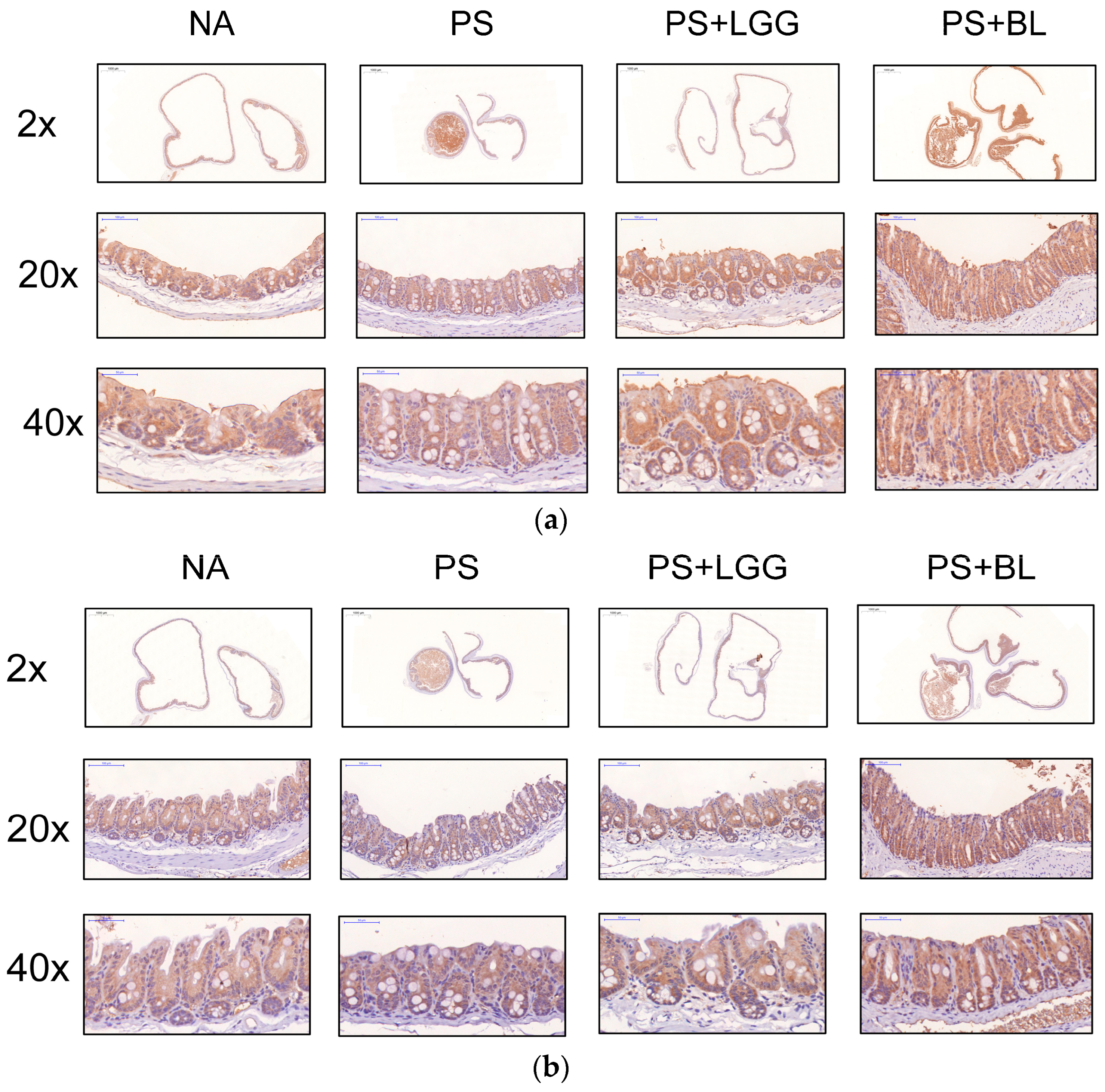
2.7. Immunohistochemistry (IHC) Staining Analysis for Tight Junction Proteins
2.8. Statistical Analysis
3. Results
3.1. Probiotics Exerted Exaggeration of Bacterial Translocation in Chemotherapy-Induced Gut-Derived P. aeruginosa Sepsis in Mice
3.2. Probiotics Exaggerated the Severity of Colitis in Mice Undergoing Chemotherapy and Suffering from Gut-Derived P. aeruginosa Sepsis
3.3. Probiotics LGG and BL Exaggerated Cecal Inflammatory Responses but Decreased Antimicrobial Peptide in Mice Undergoing Chemotherapy and Suffering from Gut-Derived P. aeruginosa Sepsis
3.4. Admixture of Probiotics Enhanced the Tight Junction Protein Expression in Cecal Mucosa of Mice Undergoing Chemotherapy and Suffering from Gut-Derived P. aeruginosa Sepsis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neuhauser, M.M.; Weinstein, R.A.; Rydman, R.; Danziger, L.H.; Karam, G.; Quinn, J.P. Antibiotic resistance among gram-negative bacilli in US intensive care units: Implications for fluoroquinolone use. JAMA 2003, 289, 885–888. [Google Scholar] [CrossRef]
- Vincent, J.L. Nosocomial infections in adult intensive-care units. Lancet 2003, 361, 2068–2077. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [CrossRef]
- King, S.; Tancredi, D.; Lenoir-Wijnkoop, I.; Gould, K.; Vann, H.; Connors, G.; Sanders, M.E.; Linder, J.A.; Shane, A.L.; Merenstein, D. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur. J. Public Health 2019, 29, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Armstrong, D. Infectious morbidity in critically ill patients with cancer. Crit. Care Clin. 2001, 17, 531–570, vii–viii. [Google Scholar] [CrossRef] [PubMed]
- Thuong, M.; Arvaniti, K.; Ruimy, R.; de la Salmoniere, P.; Scanvic-Hameg, A.; Lucet, J.C.; Regnier, B. Epidemiology of Pseudomonas aeruginosa and risk factors for carriage acquisition in an intensive care unit. J. Hosp. Infect. 2003, 53, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Okuda, J.; Hayashi, N.; Okamoto, M.; Sawada, S.; Minagawa, S.; Yano, Y.; Gotoh, N. Translocation of Pseudomonas aeruginosa from the intestinal tract is mediated by the binding of ExoS to an Na,K-ATPase regulator, FXYD3. Infect. Immun. 2010, 78, 4511–4522. [Google Scholar] [CrossRef]
- Driscoll, J.A.; Brody, S.L.; Kollef, M.H. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 2007, 67, 351–368. [Google Scholar] [CrossRef]
- van Vliet, M.J.; Tissing, W.J.; Dun, C.A.; Meessen, N.E.; Kamps, W.A.; de Bont, E.S.; Harmsen, H.J. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin. Infect. Dis. 2009, 49, 262–270. [Google Scholar] [CrossRef]
- Wada, M.; Nagata, S.; Saito, M.; Shimizu, T.; Yamashiro, Y.; Matsuki, T.; Asahara, T.; Nomoto, K. Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Support. Care Cancer 2010, 18, 751–759. [Google Scholar] [CrossRef]
- von Klitzing, E.; Ekmekciu, I.; Bereswill, S.; Heimesaat, M.M. Intestinal and Systemic Immune Responses upon Multi-drug Resistant Pseudomonas aeruginosa Colonization of Mice Harboring a Human Gut Microbiota. Front. Microbiol. 2017, 8, 2590. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, K.X.; Qu, J.M.; Wang, X.D. The changes induced by cyclophosphamide in intestinal barrier and microflora in mice. Eur. J. Pharmacol. 2013, 714, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Yan, J.; Liu, F.; Ding, P.; Chen, B.; Lu, Y.; Sun, Z. Probiotics in preventing and treating chemotherapy-induced diarrhea: A meta-analysis. Asia Pac. J. Clin. Nutr. 2019, 28, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Heus, P.; van de Wetering, F.T.; van Tienhoven, G.; Verleye, L.; Scholten, R.J. Probiotics for the prevention or treatment of chemotherapy- or radiotherapy-related diarrhoea in people with cancer. Cochrane Database Syst. Rev. 2018, 8, CD008831. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Holec, V.; Drgona, L.; Hainova, K.; Ciernikova, S.; Zajac, V. Probiotic bacteria in cancer patients undergoing chemotherapy and radiation therapy. Complement. Ther. Med. 2013, 21, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Machairas, N.; Pistiki, A.; Droggiti, D.I.; Georgitsi, M.; Pelekanos, N.; Damoraki, G.; Kouraklis, G.; Giamarellos-Bourboulis, E.J. Pre-treatment with probiotics prolongs survival after experimental infection by multidrug-resistant Pseudomonas aeruginosa in rodents: An effect on sepsis-induced immunosuppression. Int. J. Antimicrob. Agents 2015, 45, 376–384. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ishikawa, H.; Tateda, K.; Yaeshima, T.; Ishibashi, N.; Yamaguchi, K. Oral administration of Bifidobacterium longum prevents gut-derived Pseudomonas aeruginosa sepsis in mice. J. Appl. Microbiol. 2008, 104, 672–680. [Google Scholar] [CrossRef]
- Matsumoto, T.; Furuya, N.; Tateda, K.; Miyazaki, S.; Ohno, A.; Ishii, Y.; Hirakata, Y.; Yamaguchi, K. Effect of passive immunotherapy on murine gut-derived sepsis caused by Pseudomonas aeruginosa. J. Med. Microbiol. 1999, 48, 765–770. [Google Scholar] [CrossRef][Green Version]
- Huang, F.C.; Huang, S.C. The Pivotal Role of Aryl Hydrocarbon Receptor-Regulated Tight Junction Proteins and Innate Immunity on the Synergistic Effects of Postbiotic Butyrate and Active Vitamin D3 to Defense against Microbial Invasion in Salmonella Colitis. Nutrients 2023, 15, 305. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M.; Stiles, M.E.; Schleifer, K.H.; Holzapfel, W.H. Enterococci in foods—A conundrum for food safety. Int. J. Food Microbiol. 2003, 88, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tran, D.Q.; Fatheree, N.Y.; Marc Rhoads, J. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G177–G186. [Google Scholar] [CrossRef] [PubMed]
- Ammor, M.S.; Florez, A.B.; Mayo, B. Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol. 2007, 24, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. Gut microflora as a target for energy and metabolic homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef]
- Shin, W.; Kim, H.J. Intestinal barrier dysfunction orchestrates the onset of inflammatory host-microbiome cross-talk in a human gut inflammation-on-a-chip. Proc. Natl. Acad. Sci. USA 2018, 115, E10539–E10547. [Google Scholar] [CrossRef]
- Panigrahi, P.; Parida, S.; Nanda, N.C.; Satpathy, R.; Pradhan, L.; Chandel, D.S.; Baccaglini, L.; Mohapatra, A.; Mohapatra, S.S.; Misra, P.R.; et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017, 548, 407–412. [Google Scholar] [CrossRef]
- Costeloe, K.; Hardy, P.; Juszczak, E.; Wilks, M.; Millar, M.R.; Probiotics in Preterm Infants Study Collaborative, G. Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2016, 387, 649–660. [Google Scholar] [CrossRef]
- Kothari, D.; Patel, S.; Kim, S.K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, C.A.; Schulz, C.; Malfertheiner, P. Treating critically ill patients with probiotics: Beneficial or dangerous? Gut Pathog. 2011, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhang, R.; Tian, X.; Zhou, X.; Pan, X.; Wong, A. Assessing the Risk of Probiotic Dietary Supplements in the Context of Antibiotic Resistance. Front. Microbiol. 2017, 8, 908. [Google Scholar] [CrossRef] [PubMed]
- D’Agostin, M.; Squillaci, D.; Lazzerini, M.; Barbi, E.; Wijers, L.; Da Lozzo, P. Invasive Infections Associated with the Use of Probiotics in Children: A Systematic Review. Children 2021, 8, 924. [Google Scholar] [CrossRef]
- Liong, M.T. Safety of probiotics: Translocation and infection. Nutr. Rev. 2008, 66, 192–202. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Meng, M.; Klingensmith, N.J.; Coopersmith, C.M. New insights into the gut as the driver of critical illness and organ failure. Curr. Opin. Crit. Care 2017, 23, 143–148. [Google Scholar] [CrossRef]
- El Asmar, R.; Panigrahi, P.; Bamford, P.; Berti, I.; Not, T.; Coppa, G.V.; Catassi, C.; Fasano, A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 2002, 123, 1607–1615. [Google Scholar] [CrossRef]
- Li, C.; Gao, M.; Zhang, W.; Chen, C.; Zhou, F.; Hu, Z.; Zeng, C. Zonulin Regulates Intestinal Permeability and Facilitates Enteric Bacteria Permeation in Coronary Artery Disease. Sci. Rep. 2016, 6, 29142. [Google Scholar] [CrossRef]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef]
- Sturgeon, C.; Lan, J.; Fasano, A. Zonulin transgenic mice show altered gut permeability and increased morbidity/mortality in the DSS colitis model. Ann. N. Y. Acad. Sci. 2017, 1397, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Yoseph, B.P.; Klingensmith, N.J.; Liang, Z.; Breed, E.R.; Burd, E.M.; Mittal, R.; Dominguez, J.A.; Petrie, B.; Ford, M.L.; Coopersmith, C.M. Mechanisms of Intestinal Barrier Dysfunction in Sepsis. Shock 2016, 46, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Klaus, D.A.; Motal, M.C.; Burger-Klepp, U.; Marschalek, C.; Schmidt, E.M.; Lebherz-Eichinger, D.; Krenn, C.G.; Roth, G.A. Increased plasma zonulin in patients with sepsis. Biochem. Med. 2013, 23, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, C.; Huang, M.; Tong, C.; Zhang, X.; Wang, L.; Peng, H.; Lan, P.; Zhang, P.; Huang, N.; et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: A double-center and double-blind randomized clinical trial. BMC Gastroenterol. 2015, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Serek, P.; Oleksy-Wawrzyniak, M. The Effect of Bacterial Infections, Probiotics and Zonulin on Intestinal Barrier Integrity. Int. J. Mol. Sci. 2021, 22, 11359. [Google Scholar] [CrossRef] [PubMed]
- Ramezani Ahmadi, A.; Sadeghian, M.; Alipour, M.; Ahmadi Taheri, S.; Rahmani, S.; Abbasnezhad, A. The Effects of Probiotic/Synbiotic on Serum Level of Zonulin as a Biomarker of Intestinal Permeability: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2020, 49, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2018, 53, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Fouser, L.A.; Artis, D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011, 12, 383–390. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Nair, M.G.; Kirn, T.J.; Zaph, C.; Fouser, L.A.; Artis, D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 2010, 207, 1293–1305. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.-C.; Huang, S.-C. The Hazards of Probiotics on Gut-Derived Pseudomonas aeruginosa Sepsis in Mice Undergoing Chemotherapy. Biomedicines 2024, 12, 253. https://doi.org/10.3390/biomedicines12020253
Huang F-C, Huang S-C. The Hazards of Probiotics on Gut-Derived Pseudomonas aeruginosa Sepsis in Mice Undergoing Chemotherapy. Biomedicines. 2024; 12(2):253. https://doi.org/10.3390/biomedicines12020253
Chicago/Turabian StyleHuang, Fu-Chen, and Shun-Chen Huang. 2024. "The Hazards of Probiotics on Gut-Derived Pseudomonas aeruginosa Sepsis in Mice Undergoing Chemotherapy" Biomedicines 12, no. 2: 253. https://doi.org/10.3390/biomedicines12020253
APA StyleHuang, F.-C., & Huang, S.-C. (2024). The Hazards of Probiotics on Gut-Derived Pseudomonas aeruginosa Sepsis in Mice Undergoing Chemotherapy. Biomedicines, 12(2), 253. https://doi.org/10.3390/biomedicines12020253





