The Intersection of Trauma and Immunity: Immune Dysfunction Following Hemorrhage
Abstract
:1. Introduction
2. Physiologic Response to Hemorrhage
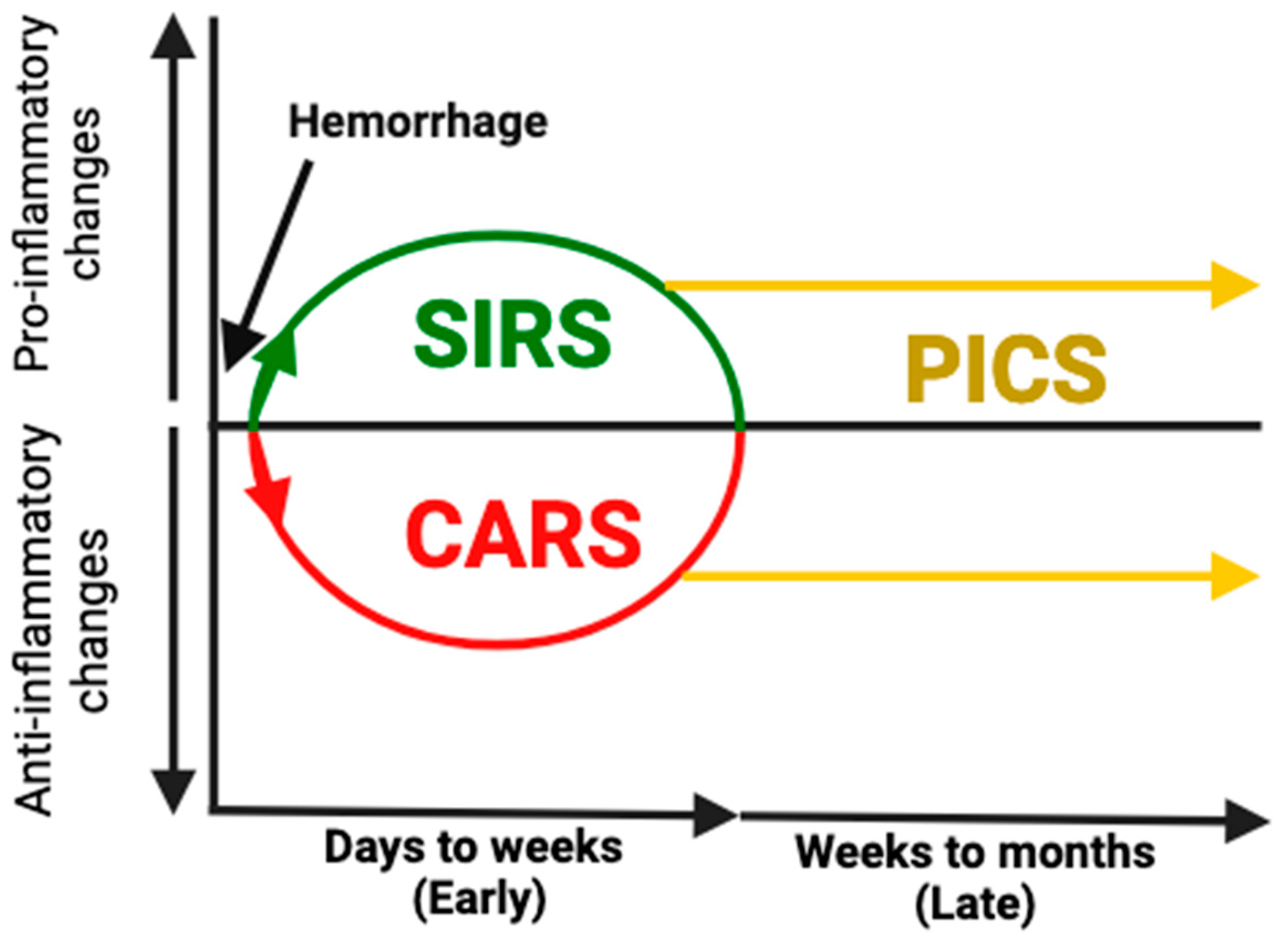
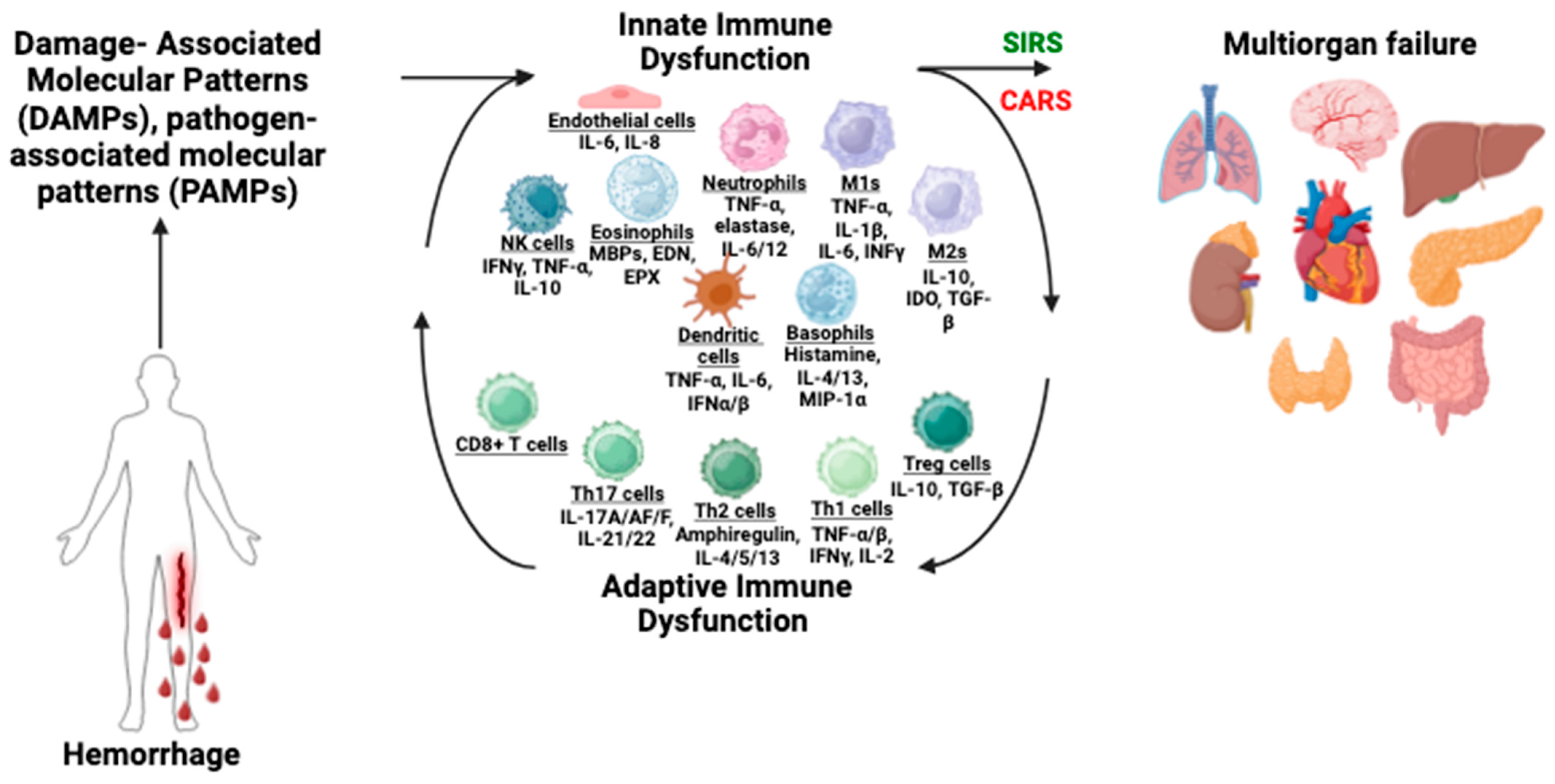
3. Immune Dysfunction Following Hemorrhage
3.1. Innate Immune Dysfunction
3.2. Adaptive Immune Dysfunction
3.3. MDSCs
3.4. Consequences of Immune Dysfunction
4. Metabolic Changes After Hemorrhage
5. Current Therapeutic Strategies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cannon, J.W. Hemorrhagic shock. N. Engl. J. Med. 2018, 378, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Vicente, D.A.; Bradley, M.J.; Bograd, B.; Leonhardt, C.; Elster, E.A.; Davis, T.A. The impact of septic stimuli on the systemic inflammatory response and physiologic insult in a preclinical non-human primate model of polytraumatic injury. J. Inflamm. 2018, 15, 11. [Google Scholar] [CrossRef]
- Duchesne, J.; Taghavi, S.; Houghton, A.; Khan, M.; Perreira, B.; Cotton, B.; Tatum, D. Prehospital mortality due to hemorrhagic shock remains high and unchanged: A summary of current civilian EMS practices and new military changes. Shock 2021, 56, 3–8. [Google Scholar] [CrossRef]
- Rossiter, N.D. Trauma—The forgotten pandemic? Int. Orthop. 2022, 46, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Eastridge, B.J.; Holcomb, J.B.; Shackelford, S. Outcomes of traumatic hemorrhagic shock and the epidemiology of preventable death from injury. Transfusion 2019, 59 (Suppl. S2), 1423–1428. [Google Scholar] [CrossRef]
- Maegele, M. The Diagnosis and Treatment of Acute Traumatic Bleeding and Coagulopathy. Dtsch. Arztebl. Int. 2019, 116, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Dobson, G.P.; Morris, J.L.; Letson, H.L. Immune dysfunction following severe trauma: A systems failure from the central nervous system to mitochondria. Front. Med. 2022, 9, 968453. [Google Scholar] [CrossRef]
- Debler, L.; Palmer, A.; Braumüller, S.; Klohs, B.; Mollnes, T.E.; Holzmann, K.; Huber-Lang, M.; Halbgebauer, R. Hemorrhagic shock induces a rapid transcriptomic shift of the immune balance in leukocytes after experimental multiple injury. Mediators Inflamm. 2021, 1, 6654318. [Google Scholar] [CrossRef]
- Vanzant, E.L.; Lopez, C.M.; Ozrazgat-Baslanti, T.; Ungaro, R.; Davis, R.; Cuenca, A.G.; Gentile, L.F.; Nacionales, D.C.; Cuenca, A.L.; Bihorac, A.; et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J. Trauma Acute Care Surg. 2014, 76, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, P.; Faure, E.; Kipnis, E. Inflammasomes in tissue damages and immune disorders after trauma. Front. Immunol. 2018, 9, 1900. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Gulati, A. Controls of central and peripheral blood pressure and hemorrhagic/hypovolemic shock. J. Clin. Med. 2023, 12, 1108. [Google Scholar] [CrossRef]
- Szopinski, J.; Kusza, K.; Semionow, M. Microcirculatory responses to hypovolemic shock. J. Trauma Acute Care Surg. 2011, 71, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G. Regulation of breathing and autonomic outflows by chemoreceptors. Compr. Physiol. 2014, 4, 1511. [Google Scholar] [PubMed]
- Liu, H.; Xiao, X.; Sun, C.; Sun, D.; Li, Y.; Yang, M. Systemic inflammation and multiple organ injury in traumatic hemorrhagic shock. Front. Biosci. 2015, 20, 927–933. [Google Scholar]
- Fage, N.; Asfar, P.; Radermacher, P.; Demiselle, J. Norepinephrine and vasopressin in hemorrhagic shock: A focus on renal hemodynamics. Int. J. Mol. Sci. 2023, 24, 4103. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Lambris, J.D.; Ward, P.A. Innate immune responses to trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Angele, M.K.; Schneider, C.P.; Chaudry, I.H. Bench-to-bedside review: Latest results in hemorrhagic shock. Crit. Care 2008, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Biller, J.; Andalib, S.; Hinduja, A.; Rodrigues, R.; Rodriguez, M.; Sabbagh, S.Y.; Selim, M.; et al. Neuroinflammation in acute ischemic and hemorrhagic stroke. Curr. Neurol. Neurosci. Rep. 2023, 23, 407–431. [Google Scholar] [CrossRef]
- van Meurs, M.; Wulfert, F.M.; Knol, A.J.; De Haes, A.; Houwertjes, M.; Aarts, L.P.; Molema, G. Early organ-specific endothelial activation during hemorrhagic shock and resuscitation. Shock 2008, 29, 91–299. [Google Scholar] [CrossRef]
- Huang, Q.; Gao, S.; Yao, Y.; Wang, Y.; Li, J.; Chen, J.; Guo, C.; Zhao, D.; Li, X. Innate immunity and immunotherapy for hemorrhagic shock. Front. Immunol. 2022, 13, 918380. [Google Scholar] [CrossRef] [PubMed]
- Esmann, L.; Idel, C.; Sarkar, A.; Hellberg, L.; Behnen, M.; Möller, S.; van Zandbergen, G.; Klinger, M.; Köhl, J.; Bussmeyer, U.; et al. Phagocytosis of apoptotic cells by neutrophil granulocytes: Diminished proinflammatory neutrophil functions in the presence of apoptotic cells. J. Imunol. 2010, 184, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, D.; Bergamini, C.; Martelucci, J.; Alemanno, G.; Bruscinot, A.; Maltinti, G.; Sheiterle, M.; Viligiardi, R.; Panconesi, R.; Guagni, T.; et al. The role of damps in burns and hemorrhagic shock immune response: Pathophysiology and clinical issues. Int. J. Mol. Sci. 2021, 2213, 7020. [Google Scholar] [CrossRef] [PubMed]
- Dufour-Gaume, F.; Frescaline, N.; Cardona, V.; Prat, N.J. Danger signals in traumatic hemorrhagic shock and new lines for clinical applications. Front. Physiol. 2023, 13, 999011. [Google Scholar] [CrossRef] [PubMed]
- Akkari, L.; Amit, I.; Bronte, V.; Fridlender, Z.G.; Gabrilovich, D.I.; Ginhoux, F.; Hedrick, C.C.; Ostrand-Rosenberg, S. Defining myeloid-derived suppressor cells. Nat. Rev. Immunol. 2024, 24, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008, 181, 5791–5802. [Google Scholar] [CrossRef]
- Mira, J.C.; Brakenridge, S.C.; Moldawer, L.L.; Moore, F.A. Persistent inflammation, immunosuppression, and catabolism syndrome. Crit. Care Clin. 2017, 33, 245–258. [Google Scholar] [CrossRef]
- Kelly, L.S.; Apple, C.G.; Darden, D.B.; Kannan, K.B.; Pons, E.E.; Fenner, B.P.; Parvataneni, H.K.; Hagen, J.E.; Brakenridge, S.C.; Efron, P.A.; et al. Transcriptomic changes within human bone marrow after severe trauma. Shock 2022, 57, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Farooq, M.; Hwang, M.J.; Haseeb, M.; Choi, S. Autoimmune neuroinflammatory diseases: Role of interleukins. Int. J. Mol. Sci. 2023, 24, 7960. [Google Scholar] [CrossRef]
- Miller, E.S.; Loftus, T.J.; Kannan, K.B.; Plazas, J.M.; Efron, P.A.; Mohr, A.M. Systemic regulation of bone marrow stromal cytokines after severe trauma. J. Surg. Res. 2019, 243, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; van der Poll, T. Endothelial barrier dysfunction in septic shock. J. Intern. Med. 2015, 277.3, 277–293. [Google Scholar] [CrossRef]
- Tao, Y.L.; Wang, J.R.; Liu, M.; Liu, Y.N.; Zhang, J.Q.; Zhou, Y.J.; Li, S.W.; Zhu, S.F. Progress in the study of the correlation between sepsis and intestinal microecology. Front. Cell. Infect. Microbiol. 2024, 14, 1357178. [Google Scholar] [CrossRef]
- Schoenmann, N.; Tannenbaum, N.; Hodgeman, R.M.; Raju, R.P. Regulating mitochondrial metabolism by targeting pyruvate dehydrogenase with dichloroacetate, a metabolic messenger. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166769. [Google Scholar] [CrossRef]
- Zhang, L.; Du, W.Q.; Zong, Z.W.; Zhong, X.; Jia, Y.J.; Jiang, R.Q.; Ye, Z. Modified Glucose-insulin-potassium Therapy for Hemorrhage-induced Traumatic Cardiac Arrest in Rabbits. Curr. Med. Sci. 2023, 43, 1238–1246. [Google Scholar] [CrossRef]
- Şimşek, T.; Şimşek, H.U.; Cantürk, N.Z. Response to trauma and metabolic changes: Posttraumatic metabolism. Turk. J. Surg. 2014, 30, 153. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Yates, J.; Lau, A.; Lampron, J.; Matar, M. Permissive hypotension versus conventional resuscitation strategies in adult trauma patients with hemorrhagic shock: A systematic review and meta-analysis of randomized controlled trials. J. Trauma Acute Care Surg. 2018, 84, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Ketchum, L.; Hess, J.R.; Hiippala, S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J. Trauma Acute Care Surg. 2006, 60, S51–S58. [Google Scholar] [CrossRef] [PubMed]
- Mishima, H.; Nakagawa, K.; Takeuchi, H.; Takahashi, H.; Saito, S.; Sakanashi, S.; Saitoh, D.; Takyu, H.; Tanaka, H. Impact of Pre-Hospital Intravenous Infusion on Physiological Parameters in Severe Trauma Patients. Cureus 2024, 16, 71770. [Google Scholar] [CrossRef] [PubMed]
- Bjerkvig, C.K.; Strandenes, G.; Hervig, T.; Sunde, G.A.; Apelseth, T.O. Prehospital whole blood transfusion programs in Norway. Transfus. Med. Hemother. 2021, 48, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Valade, G.; Libert, N.; Martinaud, C.; Vicaut, E.; Banzet, S.; Peltzer, J. Therapeutic potential of mesenchymal stromal cell-derived extracellular vesicles in the prevention of organ injuries induced by traumatic hemorrhagic shock. Front. Immunol. 2021, 12, 749659. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Möbius, W.; Simon, P.; Krämer-Albers, E.M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef]
- Willaims, A.M.; Wu, Z.; Bhatti, U.F.; Biesterveld, B.E.; Kemp, M.T.; Wakam, G.K.; Vercruysse, C.A.; Chtraklin, K.; Siddiqui, A.Z.; Pickell, Z.; et al. Early single dose of exosome treatment improves neurologic outcomes in a 7-day swine model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 2020, 892, 388–396. [Google Scholar] [CrossRef]
- Alsaadi, N.; Srinivasan, A.J.; Seshadri, A.; Shiel, M.; Neal, M.D.; Scott, M.J. The emerging therapeutic potential of extracellular vesicles in trauma. J. Leukoc. Biol. 2022, 111, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Matijevic, N.; Wang, Y.W.; Kostousov, V.; Wade, C.E.; Vijayan, K.V.; Holcomb, J.B. Decline in platelet microparticles contributes to reduced hemostatic potential of stored plasma. Thromb. Res. 2011, 128, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dyer, M.R.; Alexander, W.; Hassoune, A.; Chen, Q.; Brzoska, T.; Alvikas, J.; Liu, Y.; Haldeman, S.; Plautz, W.; Loughran, P.; et al. Platelet-derived extracellular vesicles released after trauma promote hemostasis and contribute to DVT in mice. J. Thromb. Haemost. 2019, 17, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Sligl, W.I.; Milner, D.A.; Sundar, S.; Mphatswe, W.; Majumdar, S.R. Safety and efficacy of corticosteroids for the treatment of septic shock: A systematic review and meta-analysis. Clin. Infect. Dis. 2009, 49, 93–101. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef] [PubMed]
- Vanden, B.T.; Demon, D.; Bogaert, P.; Vandendriessche, B.; Goethals, A.; Depuydt, B.; Vuylsteke, M.; Roelandt, R.; Van Wonterghem, E.; Vandenbroecke, J.; et al. Simultaneous targeting of IL-1 and IL-18 is required for protection against inflammatory and septic shock. Am. J. Respir. Crit. Care Med. 2014, 189, 282–291. [Google Scholar]
- Beckmann, N.; Salyer, C.E.; Crisologo, P.A.; Nomellini, V.; Caldwell, C.C. Staging and personalized intervention for infection and sepsis. Surg. Infect. 2020, 21, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets—An updated view. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef] [PubMed]
- Parrish, W.R.; Gallowitsch-Puerta, M.; Czura, C.J.; Tracey, K.J. Experimental therapeutic strategies for severe sepsis: Mediators and mechanisms. Ann. N. Y. Acad. Sci. 2008, 1144, 210–236. [Google Scholar] [CrossRef]
- Perl, M.; Chung, C.S.; Garber, M.; Huang, X.; Ayala, A. Contribution of anti-inflammatory/immune suppressive processes to the pathology of sepsis. Front. Biosci. 2006, 11, 272–299. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, C.H.; Wang, F.S. Thymosin alpha 1: Biological activities, applications, and genetic engineering production. Peptides 2010, 31, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Dominari, A.; Hathaway Iii, D.; Pandav, K.; Matos, W.; Biswas, S.; Reddy, G.; Thevuthasan, S.; Khan, M.A.; Mathew, A.; Makkar, S.S.; et al. Thymosin alpha 1: A comprehensive review of the literature. World J. Virol. 2020, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, L.; Liu, J.; Ma, G.; Kou, Q.; He, Z.; Chen, J.; Ou-Yang, B.; Chen, M.; Li, Y.; et al. The efficacy of thymosin alpha 1 for severe sepsis (ETASS): A multicenter, single-blind, randomized, and controlled trial. Crit. Care 2013, 17, R8. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Yang, Y.; Liu, J.; Yu, J.; Guo, Q.; Guan, W.; Fan, L. Down-regulation of mir-18b-5p protects against splenic hemorrhagic shock by directly targeting HIF-1α/iNOS pathway. Immunobiology 2022, 227, 152188. [Google Scholar] [CrossRef]
- Kokubo, K.; Onodera, A.; Kiuchi, M.; Tsuji, K.; Hirahara, K.; Nakayama, T. Conventional and pathogenic Th2 cells in inflammation, tissue repair, and fibrosis. Front. Immunol. 2022, 13, 945063. [Google Scholar] [CrossRef] [PubMed]
- Luís, A.; Hackl, M.; Jafarmadar, M.; Keibl, C.; Jilge, J.M.; Grillari, J.; Bahrami, S.; Kozlov, A.V. Circulating miRNAs Associated With ER Stress and Organ Damage in a Preclinical Model of Trauma Hemorrhagic Shock. Front. Med. 2020, 7, 568096. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvo, N.; Charles, A.M.; Mohr, A.M. The Intersection of Trauma and Immunity: Immune Dysfunction Following Hemorrhage. Biomedicines 2024, 12, 2889. https://doi.org/10.3390/biomedicines12122889
Salvo N, Charles AM, Mohr AM. The Intersection of Trauma and Immunity: Immune Dysfunction Following Hemorrhage. Biomedicines. 2024; 12(12):2889. https://doi.org/10.3390/biomedicines12122889
Chicago/Turabian StyleSalvo, Nicholas, Angel M. Charles, and Alicia M. Mohr. 2024. "The Intersection of Trauma and Immunity: Immune Dysfunction Following Hemorrhage" Biomedicines 12, no. 12: 2889. https://doi.org/10.3390/biomedicines12122889
APA StyleSalvo, N., Charles, A. M., & Mohr, A. M. (2024). The Intersection of Trauma and Immunity: Immune Dysfunction Following Hemorrhage. Biomedicines, 12(12), 2889. https://doi.org/10.3390/biomedicines12122889




