Mucins as Precision Biomarkers in Glioma: Emerging Evidence for Their Potential in Biospecimen Analysis and Outcome Prediction
Abstract
:1. Introduction
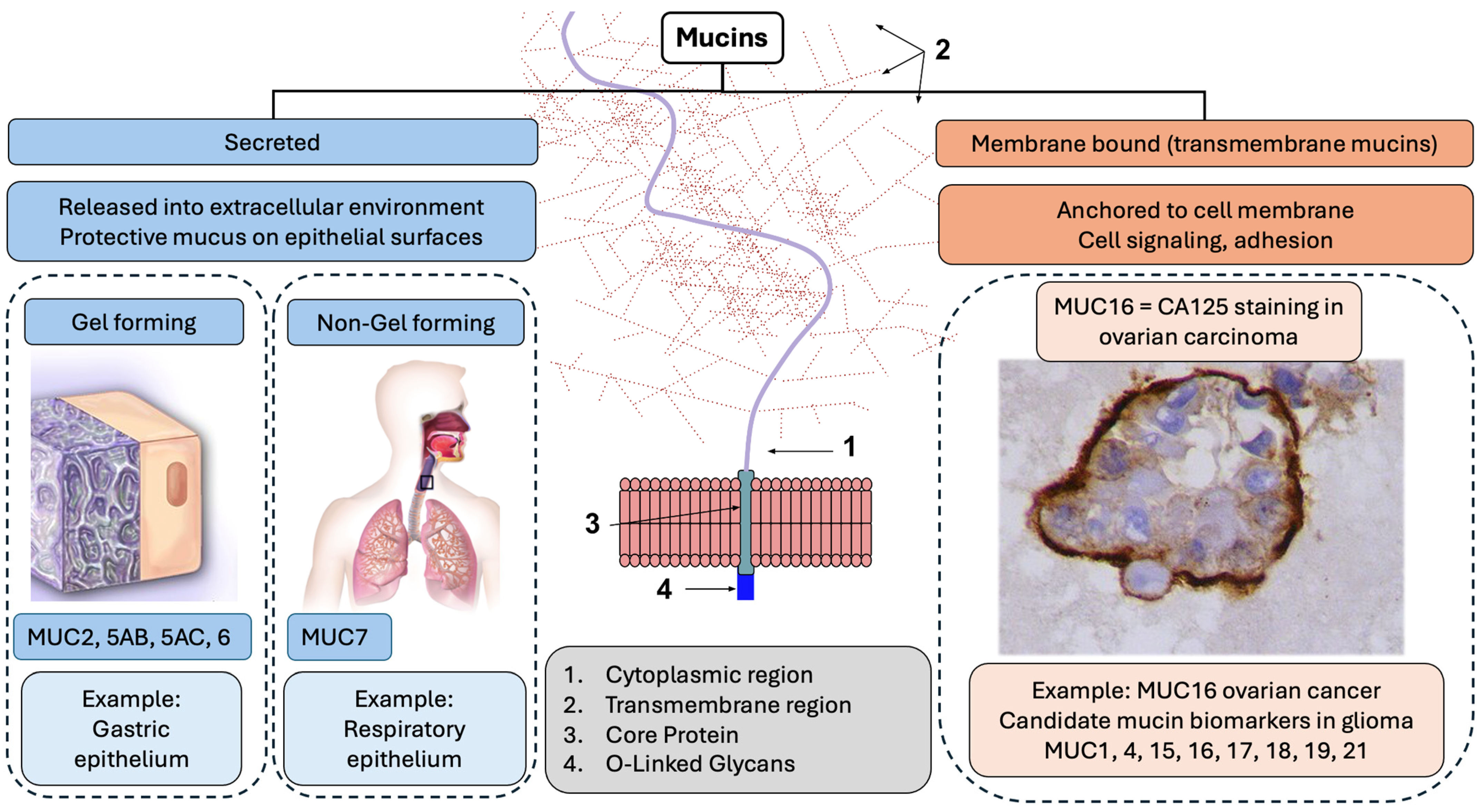
2. Biological Role of Mucins and Their Role in Glioma

3. Means of Mucin Measurement and Levels of Detection in Glioma
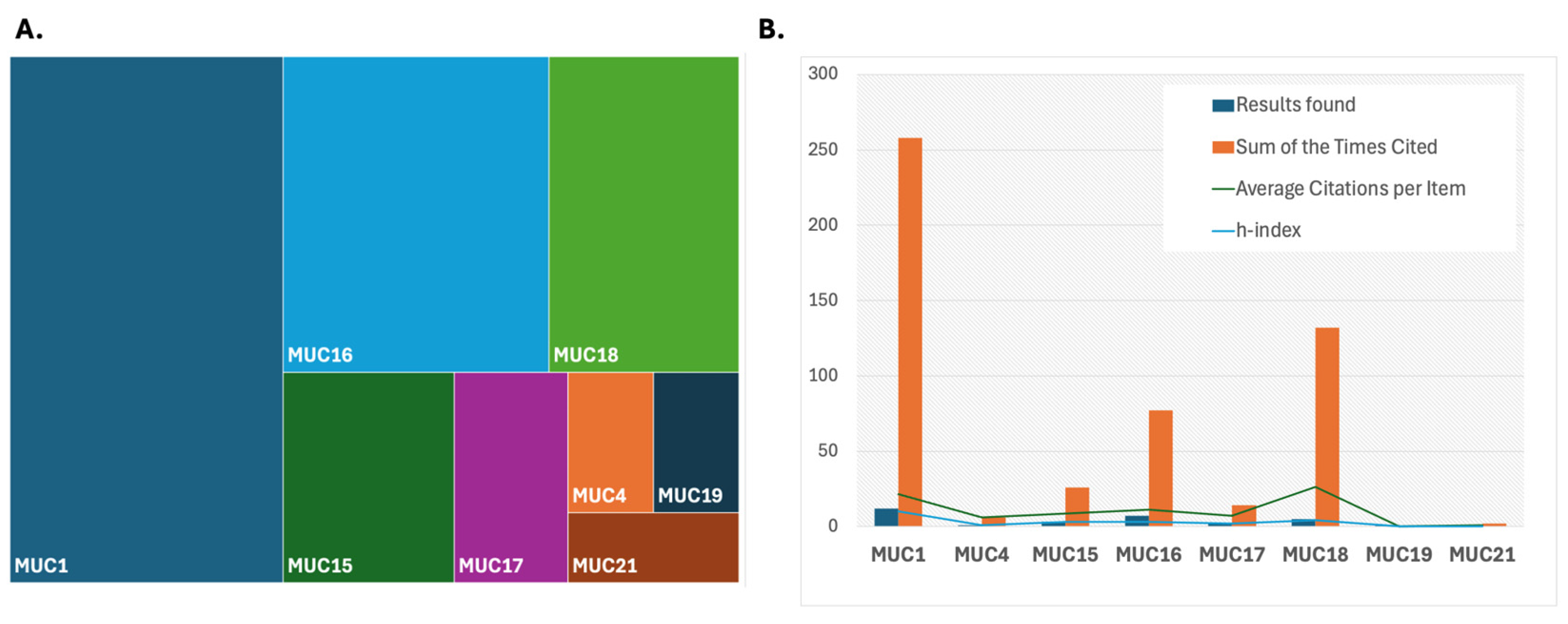
4. Mucins as Biomarker Candidates in Glioma—A Framework for Analysis and Evaluation
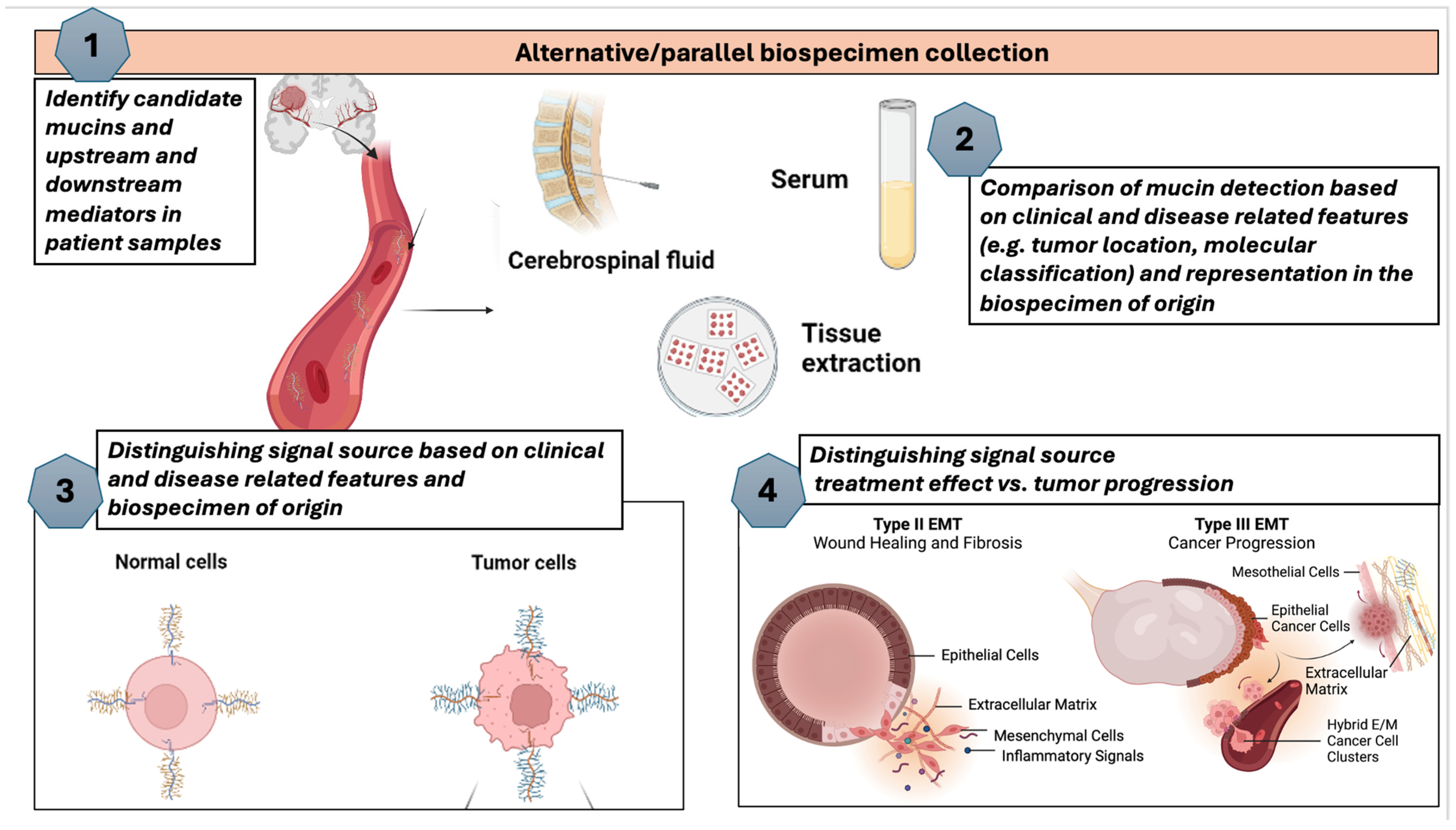
5. Measurement of Mucins in Metastatic Settings, Including the CNS
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma |
| MUC | Mucin |
| PTS | Proline-Threonine-Serine |
| VWD | Von Willebrand Domain |
| EGF | Epidermal growth factor |
| PIK/AKT | Phosphoinositide kinase (PIK)/Protein kinase B |
| MAPK/ERK | Mitogen-activated protein kinase/Extracellular signal-regulated kinase |
| Raf | Rapidly accelerated fibrosarcoma |
| ESCC | Esophageal squamous cell carcinoma |
| OVGP1 | Oviductal glycoprotein one |
| LGG | Low-grade glioma |
| CTC | Circulating tumor cell |
| ctDNA | Circulating tumor DNA |
| UV | Ultraviolet |
| CSF | Cerebrospinal fluid |
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022, 24 (Suppl. S5), v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.R.; Wen, P.Y.; Lang-Orsini, M.; Chukwueke, U.N. World Health Organization 2021 Classification of Central Nervous System Tumors and Implications for Therapy for Adult-Type Gliomas: A Review. JAMA Oncol. 2022, 8, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.H.; Park, C.K. Classification and Diagnosis of Adult Glioma: A Scoping Review. Brain Neurorehabil. 2022, 15, e23. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, K.; Sokołowska, J.; Szmidt, M.; Sysa, P. Glioblastoma multiforme-an overview. Contemp. Oncol. 2014, 18, 307–312. [Google Scholar]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro-Oncol. 2016, 18 (Suppl. S5), v1–v75. [Google Scholar] [CrossRef]
- Ferrer, V.P.; Moura Neto, V.; Mentlein, R. Glioma infiltration and extracellular matrix: Key players and modulators. Glia 2018, 66, 1542–1565. [Google Scholar] [CrossRef]
- Mohiuddin, E.; Waikimoto, H. Extracellular matrix in glioblastoma: Opportunities for emerging therapeutic approaches. Am. J. Cancer Res. 2021, 11, 3742. [Google Scholar]
- Obrador, E.; Moreno-Murciano, P.; Oriol-Caballo, M.; López-Blanch, R.; Pineda, B.; Gutiérrez-Arroyo, J.L.; Loras, A.; Gonzalez-Bonet, L.G.; Martinez-Cadenas, C.; Estrela, J.M.; et al. Glioblastoma Therapy: Past, Present and Future. Int. J. Mol. Sci. 2024, 25, 2529. [Google Scholar] [CrossRef]
- Karami Fath, M.; Babakhaniyan, K.; Anjomrooz, M.; Jalalifar, M.; Alizadeh, S.D.; Pourghasem, Z.; Abbasi Oshagh, P.; Azargoonjahromi, A.; Almasi, F.; Manzoor, H.Z.; et al. Recent Advances in Glioma Cancer Treatment: Conventional and Epigenetic Realms. Vaccines 2022, 10, 1448. [Google Scholar] [CrossRef]
- Nicholson, J.G.; Fine, H.A. Diffuse glioma heterogeneity and its therapeutic implications. Cancer Discov. 2021, 11, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.K.; Khan, P.; Natarajan, G.; Atri, P.; Aithal, A.; Ganti, A.K.; Batra, S.K.; Nasser, M.W.; Jain, M. Mucins as Potential Biomarkers for Early Detection of Cancer. Cancers 2023, 15, 1640. [Google Scholar] [CrossRef]
- Rachagani, S.; Torres, M.P.; Moniaux, N.; Batra, S.K. Current status of mucins in the diagnosis and therapy of cancer. Biofactors 2009, 35, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Boyce, R.W.G.; Abd El-Rehim, D.; Kurien, T.; Green, A.R.; Paish, E.C.; Robertson, J.F.R.; Ellis, I.O. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod. Pathol. 2005, 18, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Horinouchi, M.; Saitou, M.; Higashi, M.; Nomoto, M.; Goto, M.; Yonezawa, S. Mucin expression profile in pancreatic cancer and the precursor lesions. J. Hepatobiliary Pancreat. Surg. 2007, 14, 243–254. [Google Scholar] [CrossRef]
- Boonla, C.; Wongkham, S.; Sheehan, J.K.; Wongkham, C.; Bhudhisawasdi, V.; Tepsiri, N.; Pairojkul, C. Prognostic value of serum MUC5AC mucin in patients with cholangiocarcinoma. Cancer 2003, 98, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Wongkham, S.; Sheehan, J.K.; Boonla, C.; Patrakitkomjorn, S.; Howard, M.; Kirkham, S.; Sripa, B.; Wongkham, C.; Bhudhisawasdi, V. Serum MUC5AC mucin as a potential marker for cholangiocarcinoma. Cancer Lett. 2003, 195, 93–99. [Google Scholar] [CrossRef]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef]
- Devine, P.L.; McKenzie, I.F.C. Mucins: Structure, function, and associations with malignancy. BioEssays 1992, 14, 619–625. [Google Scholar] [CrossRef]
- Gendler, S.J.; Lancaster, C.A.; Taylor-Papadimitrou, J.; Duhig, T.; Peat, N.; Burchell, J.; Pemberton, L.; Lalani, E.N.; Wilson, D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J. Biol. Chem. 1990, 265, 15286–15293. [Google Scholar] [CrossRef] [PubMed]
- Martin Pinzon, S.; Seeberger, P.; Silva Varon, D. Mucins and Pathogenic Mucin-Like Molecules Are Immunomodulators During Infection and Targets for Diagnostics and Vaccines. Front. Chem. 2019, 7, 710. [Google Scholar]
- Moniaux, N. Structural organization and classification of the human mucin genes. Front. Biosci. 2001, 6, d1192. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, V.P. MUC16 mutation is associated with tumor grade, clinical features, and prognosis in glioma patients. Cancer Genet. 2023, 270–271, 22–30. [Google Scholar] [CrossRef]
- Boltin, D.; Perets, T.T.; Vilkin, A.; Niv, Y. Mucin function in inflamatory bowl disease: An update. J. Clin. Gastroenterol. 2013, 47, 106–111. [Google Scholar] [CrossRef]
- Gum, J.R., Jr.; Hicks, J.W.; Toribara, N.W.; Siddiki, B.; Kim, Y.S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 1994, 269, 2440–2446. [Google Scholar] [CrossRef]
- Timpte, C.S.; Eckhardt, A.E.; Abernethy, J.L.; Hill, R.L. Porcine submaxillary gland apomucin contains tandemly repeated, identical sequences of 81 residues. J. Biol. Chem. 1988, 263, 1081–1088. [Google Scholar] [CrossRef]
- Desseyn, J.-L.; Aubert, J.-P.; Porchet, N.; Laine, A. Evolution of the Large Secreted Gel-Forming Mucins. Mol. Biol. Evol. 2000, 17, 1175–1184. [Google Scholar] [CrossRef]
- Pigny, P.; Guyonnet-Duperat, V.; Hill, A.S.; Pratt, W.S.; Galiegue-Zouitina, S.; d’Hooge, M.C.; Laine, A.; Van-Seuningen, I.; Degand, P.; Gum, J.R.; et al. Human mucin genes assigned to 11p15.5: Identification and organization of a cluster of genes. Genomics 1996, 38, 340–352. [Google Scholar] [CrossRef]
- Martínez-Sáez, N.; Peregrina, J.M.; Corzana, F. Principles of mucin structure: Implications for the rational design of cancer vaccines derived from MUC1-glycopeptides. Chem. Soc. Rev. 2017, 46, 7154–7175. [Google Scholar] [CrossRef]
- Bengal10 Mucin Structure. Available online: https://commons.wikimedia.org/wiki/File:Mucin_Structure.svg (accessed on 16 September 2024).
- BruceBlaus Respiratory Epithelium. Available online: https://commons.wikimedia.org/wiki/File:Blausen_0766_RespiratoryEpithelium-es.png (accessed on 16 September 2024).
- Rosen, Y. Metastatic Ovarian Adenocarcinoma-Pleural Fluid Cell Block CA125 Case 168. Available online: https://commons.wikimedia.org/wiki/File:Metastatic_ovarian_adenocarcnioma-_Pleural_fluid_cell_block_CA125_Case_168_(5493910931).jpg (accessed on 16 September 2024).
- Suzuki, T.; Conant, A.; Curow, C.; Alexander, A.; Loffe, Y.; Unternaehrer, J.J. Types of Epithelial-Mesenchymal Transition. Available online: https://commons.wikimedia.org/wiki/File:Types_of_epithelial-mesenchymal_transition.jpg (accessed on 16 September 2024).
- Zina Deretsky, N.S.F. Ulcer-Causing Bacterium (H. pylori) Crossing Mucus Layer of Stomach. Available online: https://commons.wikimedia.org/wiki/File:Ulcer-causing_Bacterium_(H.Pylori)_Crossing_Mucus_Layer_of_Stomach_(4822021538).jpg (accessed on 16 September 2024).
- Carraway, K.L.; Ramsauer, V.P.; Haq, B.; Carothers Carraway, C.A. Cell signaling through membrane mucins. BioEssays 2003, 25, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Gum, J.R.; Ho, J.J.L.; Pratt, W.S.; Hicks, J.W.; Hill, A.S.; Vinall, L.E.; Roberton, A.M.; Swallow, D.M.; Kim, Y.S. MUC3 Human Intestinal Mucin. J. Biol. Chem. 1997, 272, 26678–26686. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Sanders, A.; Handa, A.; Vora, A.; Cardona, M.R.; Brouwer, C.; Mukherjee, P. Molecular crosstalk between MUC1 and STAT3 influences the anti-proliferative effect of Napabucasin in epithelial cancers. Sci. Rep. 2024, 14, 3178. [Google Scholar] [CrossRef] [PubMed]
- Carraway, K.L. Multiple facets of sialomucin complex/MUC4, a membrane mucin and ErbB2 ligand, in tumors and tissues (Y2K update). Front. Biosci. 2000, 5, d95. [Google Scholar]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Jepson, S.; Komatsu, M.; Haq, B.; Arango, M.E.; Huang, D.; Carraway, C.A.C.; Carraway, K.L. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27kip, but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene 2002, 21, 7524–7532. [Google Scholar] [CrossRef]
- Av, K. Web of Science Search. Available online: https://www.webofscience.com/ (accessed on 7 October 2023).
- Jonckheere, N.; Skrypek, N.; Van Seuningen, I. Mucins and tumor resistance to chemotherapeutic drugs. Biochim. Biophys. Acta 2014, 1846, 142–151. [Google Scholar] [CrossRef]
- Theodoropoulos, G.; Carraway, K.L. Molecular signaling in the regulation of mucins. J. Cell Biochem. 2007, 102, 1103–1116. [Google Scholar] [CrossRef]
- Brown, D.V.; Daniel, P.M.; D’Abaco, G.M.; Gogos, A.; Ng, W.; Morokoff, A.P.; Mantamadiotis, T. Coexpression analysis of CD133 and CD44 identifies proneural and mesenchymal subtypes of glioblastoma multiforme. Oncotarget 2015, 6, 6267–6280. [Google Scholar] [CrossRef]
- Lin, H.; Liu, C.; Hu, A.; Zhang, D.; Yang, H.; Mao, Y. Understanding the immunosuppressive microenvironment of glioma: Mechanistic insights and clinical perspectives. J. Hematol. Oncol. 2024, 17, 31. [Google Scholar]
- Wang, Q.; Li, P.; Li, A.; Jiang, W.; Wang, H.; Wang, J.; Xie, K. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J. Exp. Clin. Cancer Res. 2012, 31, 97. [Google Scholar] [CrossRef] [PubMed]
- Machado, G.C.; Ferrer, V.P. MUC1 and MUC4 expression are inversely correlated and trigger immunological response and transport pathways in gliomas. medRxiv 2024. [Google Scholar] [CrossRef]
- Quesnel, A.; Coles, N.; Polvikoski, T.M.; Karagiannis, G.S.; Angione, C.; Islam, M.; Khundakar, A.A.; Filippou, P.S. The diagnostic and prognostic potential of the EGFR/MUC4/MMP9 axis in glioma patients. Sci. Rep. 2022, 12, 19868. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Liu, J.; Liu, Q. MUC21 induces the viability and migration of glioblastoma via the STAT3/AKT pathway. Exp. Ther. Med. 2022, 23, 331. [Google Scholar] [CrossRef]
- Li, W.; Wu, C.; Yao, Y.; Dong, B.; Wei, Z.; Lv, X.; Zhang, J.; Xu, Y. MUC4 modulates human glioblastoma cell proliferation and invasion by upregulating EGFR expression. Neurosci. Lett. 2014, 566, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.; Dong, J.J.; Xie, T.; Guan, X. Integrative Analysis of MUC4 to Prognosis and Immune Infiltration in Pan-Cancer: Friend or Foe? Front. Cell Dev. Biol. 2021, 9, 695544. [Google Scholar] [CrossRef]
- Huang, K.; Liu, X.; Li, Y.; Wang, Q.; Zhou, J.; Wang, Y.; Dong, F.; Yang, C.; Sun, Z.; Fang, C.; et al. Genome-Wide CRISPR-Cas9 Screening Identifies NF-κB/E2F6 Responsible for EGFRvIII-Associated Temozolomide Resistance in Glioblastoma. Adv. Sci. 2019, 6, 1900782. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef]
- Machado, G.C.; Ferrer, V.P. MUC17 mutations and methylation are associated with poor prognosis in adult-type diffuse glioma patients. J. Neurol. Sci. 2023, 452, 120762. [Google Scholar] [CrossRef]
- Bhatia, R.; Gautam, S.K.; Cannon, A.; Thompson, C.; Hall, B.R.; Aithal, A.; Banerjee, K.; Jain, M.; Solheim, J.C.; Kumar, S.; et al. Cancer-associated mucins: Role in immune modulation and metastasis. Cancer Metastasis Rev. 2019, 38, 223–236. [Google Scholar] [CrossRef]
- Demetriou, A.N.; Chow, F.; Craig, D.W.; Webb, M.G.; Ormond, D.R.; Battiste, J.; Chakravarti, A.; Colman, H.; Villano, J.L.; Schneider, B.P.; et al. Profiling the molecular and clinical landscape of glioblastoma utilizing the Oncology Research Information Exchange Network brain cancer database. Neurooncol Adv. 2024, 6, vdae046. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Zhao, J.X.; Fang, Z.Y.; Cui, X.T.; Su, D.Y.; Liu, X.; Zhou, J.H.; Wang, G.X.; Qiu, Z.J.; Liu, S.Z.; et al. MUC1 promotes glioblastoma progression and TMZ resistance by stabilizing EGFRvIII. Pharmacol. Res. 2023, 187, 106606. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Seo, Y.; Chowdhury, T.; Yu, H.J.; Lee, C.E.; Kim, K.-M.; Kang, H.; Kim, H.J.; Park, S.-J.; Kim, K. Inhibition of MUC1 exerts cell-cycle arrest and telomerase suppression in glioblastoma cells. Sci. Rep. 2020, 10, 18238. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, L. MUC15 promotes growth and invasion of glioma cells by activating Raf/MEK/ERK pathway. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Yawata, T.; Higashi, Y.; Kawanishi, Y.; Nakajo, T.; Fukui, N.; Fukuda, H.; Ueba, T. CD146 is highly expressed in glioma stem cells and acts as a cell cycle regulator. J. Neurooncol 2019, 144, 21–32. [Google Scholar] [CrossRef]
- Asif, S.; Fatima, R.; Krc, R.; Bennett, J.; Raza, S. Comparative proteogenomic characterization of glioblastoma. CNS Oncol. 2019, 8, Cns37. [Google Scholar] [CrossRef]
- Cordone, I.; Masi, S.; Summa, V.; Carosi, M.; Vidiri, A.; Fabi, A.; Pasquale, A.; Conti, L.; Rosito, I.; Carapella, C.M.; et al. Overexpression of syndecan-1, MUC-1, and putative stem cell markers in breast cancer leptomeningeal metastasis: A cerebrospinal fluid flow cytometry study. Breast Cancer Res. 2017, 19, 46. [Google Scholar] [CrossRef]
- Harrop, C.A.; Thornton, D.J.; McGuckin, M.A. Detecting, Visualising, and Quantifying Mucins; Humana Press: Totowa, NJ, USA, 2012; pp. 49–66. [Google Scholar]
- Albrecht, H.; Carraway, K.L., 3rd. MUC1 and MUC4: Switching the emphasis from large to small. Cancer Biother. Radiopharm. 2011, 26, 261–271. [Google Scholar]
- Zhang, S.; Zhang, W.; Xiao, Y.; Qin, T.; Yue, Y.; Qian, W.; Shen, X.; Ma, Q.; Wang, Z. Targeting MUC15 Protein in Cancer: Molecular Mechanisms and Therapeutic Perspectives. Curr. Cancer Drug Targets 2020, 20, 647–653. [Google Scholar] [CrossRef]
- Osti, D.; Del Bene, M.; Rappa, G.; Santos, M.; Matafora, V.; Richichi, C.; Faletti, S.; Beznoussenko, G.V.; Mironov, A.; Bachi, A.; et al. Clinical Significance of Extracellular Vesicles in Plasma from Glioblastoma Patients. Clin. Cancer Res. 2019, 25, 266–276. [Google Scholar] [CrossRef]
- Heimberger, A.B.; McGary, E.C.; Suki, D.; Ruiz, M.; Wang, H.; Fuller, G.N.; Bar-Eli, M. Loss of the AP-2alpha transcription factor is associated with the grade of human gliomas. Clin. Cancer Res. 2005, 11, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Kamata-Sakurai, M.; Denda-Nagai, K.; Itoh, T.; Okada, K.; Ishii-Schrade, K.; Iguchi, A.; Sugiura, D.; Irimura, T. Mucin 21/epiglycanin modulates cell adhesion. J. Biol. Chem. 2010, 285, 21233–21240. [Google Scholar] [CrossRef] [PubMed]
- GongSun, X.; Zhao, Y.; Jiang, B.; Xin, Z.; Shi, M.; Song, L.; Qin, Q.; Wang, Q.; Liu, X. Inhibition of MUC1-C regulates metabolism by AKT pathway in esophageal squamous cell carcinoma. J. Cell Physiol. 2019, 234, 12019–12028. [Google Scholar] [CrossRef] [PubMed]
- Pino, V.; Ramsauer, V.P.; Salas, P.; Carraway, C.A.C.; Carraway, K.L. Membrane Mucin Muc4 Induces Density-dependent Changes in ERK Activation in Mammary Epithelial and Tumor Cells. J. Biol. Chem. 2006, 281, 29411–29420. [Google Scholar] [CrossRef]
- von Mering, C.; Huynen, M.; Jaeggi, D.; Schmidt, S.; Bork, P.; Snel, B. STRING: A database of predicted functional associations between proteins. Nucleic Acids Res. 2003, 31, 258–261. [Google Scholar] [CrossRef]
- Lam, W.K.J.; Chan, K.C.A. Plasma DNA for early cancer detection-opportunities and challenges. Expert. Rev. Mol. Diagn. 2019, 19, 5–7. [Google Scholar] [CrossRef]
- Tang, Q.; Cheng, J.; Cao, X.; Surowy, H.; Burwinkel, B. Blood-based DNA methylation as biomarker for breast cancer: A systematic review. Clin. Epigenet. 2016, 8, 115. [Google Scholar] [CrossRef]
- Giuntoli, R.L., 2nd; Rodriguez, G.C.; Whitaker, R.S.; Dodge, R.; Voynow, J.A. Mucin gene expression in ovarian cancers. Cancer Res. 1998, 58, 5546–5550. [Google Scholar]
- Jonckheere, N.; Vincent, A.; Neve, B.; Van Seuningen, I. Mucin expression, epigenetic regulation and patient survival: A toolkit of prognostic biomarkers in epithelial cancers. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188538. [Google Scholar] [CrossRef]
- Lakshmanan, I.; Ponnusamy, M.P.; Macha, M.A.; Haridas, D.; Majhi, P.D.; Kaur, S.; Jain, M.; Batra, S.K.; Ganti, A.K. Mucins in lung cancer: Diagnostic, prognostic, and therapeutic implications. J. Thorac. Oncol. 2015, 10, 19–27. [Google Scholar] [CrossRef]
- Byrd, J.C.; Bresalier, R.S. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Krishn, S.R.; Kaur, S.; Smith, L.M.; Johansson, S.L.; Jain, M.; Patel, A.; Gautam, S.K.; Hollingsworth, M.A.; Mandel, U.; Clausen, H.; et al. Mucins and associated glycan signatures in colon adenoma-carcinoma sequence: Prospective pathological implication(s) for early diagnosis of colon cancer. Cancer Lett. 2016, 374, 304–314. [Google Scholar] [CrossRef]
- Yan, P.S.; Ho, S.B.; Itzkowitz, S.H.; Byrd, J.C.; Siddiqui, B.; Kim, Y.S. Expression of native and deglycosylated colon cancer mucin antigens in normal and malignant epithelial tissues. Lab. Investig. 1990, 63, 698–706. [Google Scholar] [PubMed]
- Mikkelsen, V.E.; Solheim, O.; Salvesen, Ø.; Torp, S.H. The histological representativeness of glioblastoma tissue samples. Acta Neurochir. 2021, 163, 1911–1920. [Google Scholar] [CrossRef]
- Aithal, A.; Rauth, S.; Kshirsagar, P.; Shah, A.; Lakshmanan, I.; Junker, W.M.; Jain, M.; Ponnusamy, M.P.; Batra, S.K. MUC16 as a novel target for cancer therapy. Expert. Opin. Ther. Targets 2018, 22, 675–686. [Google Scholar] [CrossRef]
- Atlas, H.P. Cell Type Markers. In The Human Protein Atlas; The Human Protein Atlas: Stockholm, Sweden; Available online: https://www.proteinatlas.org/ (accessed on 15 October 2024).
- Boland, J.L.; Zhou, Q.; Iasonos, A.E.; O’Cearbhaill, R.E.; Konner, J.; Callahan, M.; Friedman, C.; Aghajanian, C.; Sabbatini, P.; Zamarin, D.; et al. Utility of serum CA-125 monitoring in patients with ovarian cancer undergoing immune checkpoint inhibitor therapy. Gynecol. Oncol. 2020, 158, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.C.; Kumar, D.; Jaggi, M. Mucins in ovarian cancer diagnosis and therapy. J. Ovarian Res. 2009, 2, 21. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Xu, F.; Berchuck, A.; van Haaften-Day, C.; Havrilesky, L.J.; de Bruijn, H.W.; van der Zee, A.G.; Woolas, R.P.; Jacobs, I.J.; et al. Combining multiple serum tumor markers improves detection of stage I epithelial ovarian cancer. Gynecol. Oncol. 2007, 107, 526–531. [Google Scholar] [CrossRef]
- Croce, M.V.; Isla-Larrain, M.T.; Demichelis, S.O.; Gori, J.R.; Price, M.R.; Segal-Eiras, A. Tissue and serum MUC1 mucin detection in breast cancer patients. Breast Cancer Res. Treat. 2003, 81, 195–207. [Google Scholar] [CrossRef]
- Simon, M.J.; Iliff, J.J. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta 2016, 1862, 442–451. [Google Scholar] [CrossRef]
- Moniaux, N.; Andrianifahanana, M.; Brand, R.E.; Batra, S.K. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br. J. Cancer 2004, 91, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lipert, R.J.; Jain, M.; Kaur, S.; Chakraboty, S.; Torres, M.P.; Batra, S.K.; Brand, R.E.; Porter, M.D. Detection of the potential pancreatic cancer marker MUC4 in serum using surface-enhanced Raman scattering. Anal. Chem. 2011, 83, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Oborski, M.J.; Hwang, M.; Lieberman, F.S.; Mountz, J.M. Malignant gliomas: Current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag. Res. 2014, 6, 149–170. [Google Scholar] [PubMed]
- Wirsching, H.G.; Galanis, E.; Weller, M. Glioblastoma. Handb. Clin. Neurol. 2016, 134, 381–397. [Google Scholar]
- Shankar, G.M.; Balaj, L.; Stott, S.L.; Nahed, B.; Carter, B.S. Liquid biopsy for brain tumors. Expert. Rev. Mol. Diagn. 2017, 17, 943–947. [Google Scholar] [CrossRef]
- Idrees, B.S.; Teng, G.; Israr, A.; Zaib, H.; Jamil, Y.; Bilal, M.; Bashir, S.; Khan, M.N.; Wang, Q. Comparison of whole blood and serum samples of breast cancer based on laser-induced breakdown spectroscopy with machine learning. Biomed. Opt. Express 2023, 14, 2492–2509. [Google Scholar] [CrossRef]
- Kawa, I.A.; Masood, A.; Amin, S.; Mustafa, M.F.; Rashid, F. Clinical Perspective of Posttranslational Modifications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–68. [Google Scholar]
- Litvinov, S.V.; Hilkens, J. The epithelial sialomucin, episialin, is sialylated during recycling. J. Biol. Chem. 1993, 268, 21364–21371. [Google Scholar] [CrossRef]
- Thingstad, T.; Vos, H.L.; Hilkens, J. Biosynthesis and shedding of epiglycanin: A mucin-type glycoprotein of the mouse TA3Ha mammary carcinoma cell. Biochem. J. 2001, 353 Pt 1, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef]
- Erickson, A.; Krauze, A.V. Proposed Framework for Mucin Analysis as Precision Biomarkers in Glioma Illustrating Potential Steps That May Be Carried out Concurrently or Sequentially. Available online: https://BioRender.com (accessed on 15 October 2024).
- Jung, M.; Klotzek, S.; Lewandowski, M.; Fleischhacker, M.; Jung, K. Changes in concentration of DNA in serum and plasma during storage of blood samples. Clin. Chem. 2003, 49 Pt 1, 1028–1029. [Google Scholar] [CrossRef]
- Cordova, C.; Syeda, M.M.; Corless, B.; Wiggins, J.M.; Patel, A.; Kurz, S.C.; Delara, M.; Sawaged, Z.; Utate, M.; Placantonakis, D.; et al. Plasma cell-free circulating tumor DNA (ctDNA) detection in longitudinally followed glioblastoma patients using TERT promoter mutation-specific droplet digital PCR assays. J. Clin. Oncol. 2019, 37 (Suppl. S15), 2026. [Google Scholar] [CrossRef]
- Au, K.L.K.; Latonas, S.; Shameli, A.; Auer, I.; Hahn, C. Cerebrospinal Fluid Flow Cytometry: Utility in Central Nervous System Lymphoma Diagnosis. Can. J. Neurol. Sci. 2020, 47, 382–388. [Google Scholar] [CrossRef]
- Akers, J.C.; Hua, W.; Li, H.; Ramakrishnan, V.; Yang, Z.; Quan, K.; Zhu, W.; Li, J.; Figueroa, J.; Hirshman, B.R.; et al. A cerebrospinal fluid microRNA signature as biomarker for glioblastoma. Oncotarget 2017, 8, 68769–68779. [Google Scholar] [CrossRef]
- Zubair, A.; Orlando, D.J. Ommaya Reservoir; StatsPearls Publishing: Florida, FL, USA, 2023. [Google Scholar]
- Rickert, C.A.; Lutz, T.M.; Marczynski, M.; Lieleg, O. Several Sterilization Strategies Maintain the Functionality of Mucin Glycoproteins. Macromol. Biosci. 2020, 20, 2000090. [Google Scholar] [CrossRef] [PubMed]
- Debailleul, V.; Laine, A.; Huet, G.; Mathon, P.; d’Hooghe, M.C.; Aubert, J.P.; Porchet, N. Human mucin genes MUC2, MUC3, MUC4, MUC5AC, MUC5B, and MUC6 express stable and extremely large mRNAs and exhibit a variable length polymorphism. An improved method to analyze large mRNAs. J. Biol. Chem. 1998, 273, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Blood Biomarker Signature in Glioma. Available online: https://clinicaltrials.gov/study/NCT03698201?cond=Glioma%20of%20Brain&term=biomarker&rank=1 (accessed on 1 December 2024).
- Visual Study of Molecular Genotype in Glioma. Available online: https://clinicaltrials.gov/study/NCT03750890?cond=Glioma%20of%20Brain&term=biomarker&rank=2 (accessed on 1 December 2024).
- Glioma Microenvironment an Exploratory Study. Available online: https://clinicaltrials.gov/study/NCT03189420?cond=Glioma%20of%20Brain&rank=5 (accessed on 1 December 2024).
- Clinical Trials. Available online: https://clinicaltrials.gov/ (accessed on 1 December 2024).
- Bevacizumab and Temozolomide Following Radiation and Chemotherapy for Newly Diagnosed Glioblastoma Multiforme. Available online: https://clinicaltrials.gov/study/NCT00590681?cond=Glioblastoma&aggFilters=results:with&page=2&rank=20 (accessed on 29 November 2024).
- Biomarker Tools. Available online: https://analysistools.cancer.gov/biomarkerTools/ (accessed on 1 December 2024).
- Temozolomide 12 Cycles Versus 6 Cycles of Standard First-Line Treatment in Patients with Glioblastoma. Available online: https://clinicaltrials.gov/study/NCT02209948?cond=Glioblastoma&term=biomarker&aggFilters=results:with&page=2&rank=13 (accessed on 1 December 2024).
- Ganguly, K.; Rauth, S.; Marimuthu, S.; Kumar, S.; Batra, S.K. Unraveling mucin domains in cancer and metastasis: When protectors become predators. Cancer Metastasis Rev. 2020, 39, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Goyette, M.A.; Stevens, L.E.; DePinho, C.R.; Seehawer, M.; Nishida, J.; Li, Z.; Wilde, C.M.; Li, R.; Qiu, X.; Pyke, A.L.; et al. Cancer-stromal cell interactions in breast cancer brain metastases induce glycocalyx-mediated resistance to HER2-targeting therapies. Proc. Natl. Acad. Sci. USA 2024, 121, e2322688121. [Google Scholar] [CrossRef]
- Chaudhary, S.; Siddiqui, J.A.; Appadurai, M.I.; Maurya, S.K.; Murakonda, S.P.; Blowers, E.; Swanson, B.J.; Nasser, M.W.; Batra, S.K.; Lakshmanan, I.; et al. Dissecting the MUC5AC/ANXA2 signaling axis: Implications for brain metastasis in lung adenocarcinoma. Exp. Mol. Med. 2024, 56, 1450–1460. [Google Scholar] [CrossRef]
- Detappe, A.; Mathieu, C.; Jin, C.; Agius, M.P.; Diringer, M.C.; Tran, V.L.; Pivot, X.; Lux, F.; Tillement, O.; Kufe, D.; et al. Anti-MUC1-C Antibody-Conjugated Nanoparticles Potentiate the Efficacy of Fractionated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1380–1389. [Google Scholar] [CrossRef]
- Maji, S.; Chaudhary, P.; Akopova, I.; Nguyen, P.M.; Hare, R.J.; Gryczynski, I.; Vishwanatha, J.K. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Mol. Cancer Res. 2017, 15, 93–105. [Google Scholar] [CrossRef]
- Coletto, E.; Savva, G.M.; Latousakis, D.; Pontifex, M.; Crost, E.H.; Vaux, L.; Telatin, A.; Bergstrom, K.; Vauzour, D.; Juge, N. Role of mucin glycosylation in the gut microbiota-brain axis of core 3 O-glycan deficient mice. Sci. Rep. 2023, 13, 13982. [Google Scholar] [CrossRef] [PubMed]

| Title | Year | Study Type | Disease Entity | Association | Sample Type |
|---|---|---|---|---|---|
| Profiling the molecular and clinical landscape of glioblastoma utilizing the oncology research information exchange network brain cancer database (MUC17) [57] | 2024 | Primary | GBM | Prognosis | Tissue |
| MUC1 promotes glioblastoma progression and TMZ resistance by stabilizing EGFRVIII [58] | 2023 | Primary | GBM | Resistance | Cell lines |
| MUC16 mutation is associated with tumor grade, clinical features, and prognosis in glioma patients [24] | 2023 | Primary | Glioma | Prognosis | Tissue |
| MUC17 mutations and methylation are associated with poor prognosis in adult-type diffuse glioma patients [55] | 2023 | Primary | Diffuse Glioma | Prognosis | Tissue |
| Mucins as Potential Biomarkers for Early Detection of Cancer [13] | 2023 | Review ** | Various | Detection | Various |
| The diagnostic and prognostic potential of the EGFR/MUC4/MMP9 axis in glioma patients [49] | 2022 | Primary | Glioma | Diagnosis/Prognosis | Tissue |
| MUC21 induces the viability and migration of glioblastoma via the STAT3/AKT pathway [50] | 2022 | Primary | GBM | Progression | Tissue |
| Integrative Analysis of MUC4 to Prognosis and Immune Infiltration in Pan-Cancer: Friend or Foe? [52] | 2021 | Primary | Various | Expression/Prognosis | Tissue |
| Inhibition of MUC1 exerts cell-cycle arrest and telomerase suppression in glioblastoma cells [59] | 2020 | Primary | GBM | Resistance | Tissue |
| MUC15 promotes growth and invasion of glioma cells by activating Raf/MEK/ERK pathway [60] | 2020 | Primary | Glioma | Progression | Cell Lines |
| CD146 is highly expressed in glioma stem cells and acts as a cell cycle regulator (MUC18) [61] | 2019 | Primary | Glioma | Resistance | Cell Lines |
| Comparative proteogenomic characterization of glioblastoma (MUC19) [62] | 2019 | Primary | Various | Expression | Tissue |
| Overexpression of syndecan-1, MUC-1, and putative stem cell markers in breast cancer leptomeningeal metastasis: a cerebrospinal fluid flow cytometry study [63] | 2017 | Primary | Breast | CSF Expression | CSF |
| MUC4 modulates human glioblastoma cell proliferation and invasion by upregulating EGFR expression [51] | 2014 | Primary | GBM | Proliferation/Invasion | Cell Lines/Tissues |
| Detecting, visualising, and quantifying mucins [64] | 2012 | Review ** | Various | Detection | Various |
| MUC1 and MUC4: Switching the Emphasis from Large to Small [65] | 2011 | Review ** | Various | Prognosis | Various |
| Setting | Tissue | Blood | CSF | Results | Rationale for Clinical Implication |
|---|---|---|---|---|---|
| Case study 1: ovarian cancer | Increased expression levels of MUC13 in the tissue of ovarian cancer using immune-histochemistry [85] | Overexpression of MUC16 (CA-125) for detection of ovarian cancer [82] | Serum analysis is more common, but multiple marker analysis is more effective in early diagnosis and could implement CSF in addition to serum [86] | MUC16 is shed from the cell surfaces and enters circulation, leading to elevated levels of MUC16 in serum that are used for ovarian cancer detection | Non-invasive testing like blood sampling can be done more frequently to get a more specific prognosis |
| Case study 2: breast cancer | High levels of MUC1 were detected in tissue specimens of breast cancer patients [87] | MUC1 was detected in 86% of breast cancer specimens with at least one monoclonal antibody, with elevated levels in IgG and IgM [87] | MUC-1 overexpression was documented on all breast cancer CSF samples analyzed [88] | MUC1 is a promising biomarker and can be measured in tissue, blood, and CSF for the detection and prognosis of breast cancer | More specific information on tumor location could assist in resection and disease monitoring |
| Case study 3: pancreatic cancer | MUC17 is overexpressed in pancreatic cancer cells when compared with both the normal pancreas and pancreatitis tissues [89] | MUC4 can be used in the diagnosis and prognosis of pancreatic cancer using a SERS-based immunoassay [90] | MUC5AC has been extensively investigated in tissue and sera obtained from pancreatic cancer patients [13] | Mucins are best detected in the serum and tissue of patients with pancreatic cancer. Further analysis of mucins in CSF needs to be done for pancreatic patients | SERS immunoassays can be readily adapted to detect other cancer markers |
| Validation Aspect | Biomarkers | Goal | Approach | Data Type | Potential Methods | Clinical Validation |
|---|---|---|---|---|---|---|
| Discovery | Large sample sets tissue, serum, plasma analyzed (Proteins, metabolites or RNA) | Identify biomarkers | Omics and identify candidates (Measure levels) | Proteomics Metabolomics Levels measured Promising candidates | Gene Set Enrichment Analysis (GSEA), Ingenuity Pathways Analysis (IPA) | Blood Biomarkers in Glioma [107] and Visual Study of Molecular Genotype in Glioma Evolution [108] |
| Biological | Link to disease process, physiology, biological pathways | Relevance to the disease where employed | Pathophysiological relevance Correlation with clinical parameters Functional relevance | Progression Survival Biol process (e.g., Pathways) | STRING, Reactome, iPathway, PathVisio | Glioblastoma Microenvironment: An Exploratory Study [109] |
| Clinical | Small cohort to verify candidate markers correlating with disease stage, progression | Test in clinical settings | Disease vs healthy or vs other disease Clinical outcome Stats | Levels across disease stage PPV/NPV, ROC | ClinicalTrials.gov accessed 1 December 2024 [110] | Bevacizumab and Temozolomide Following Radiation and Chemotherapy for Newly Diagnosed Glioblastoma Multiforme [111] |
| Analytical | Small or large cohort to verify candidate markers correlating with disease stage, progression | Reliable and consistent | Sensitivity/specificity Level of detection Repeated test by other lab or same test, same sample type in independent cohort | How well is it Detected Inter/intra assay Limit of detection Comparison with healthy individuals | Biomarker Comparison Risk Stratification Advanced Analysis Mean Risk Stratification Means to Risk [112] | Temozolomide 12 Cycles Versus 6 Cycles of Standard First-line Treatment in Patients With Glioblastoma [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erickson, A.; Jackson, L.R.; Camphausen, K.; Krauze, A.V. Mucins as Precision Biomarkers in Glioma: Emerging Evidence for Their Potential in Biospecimen Analysis and Outcome Prediction. Biomedicines 2024, 12, 2806. https://doi.org/10.3390/biomedicines12122806
Erickson A, Jackson LR, Camphausen K, Krauze AV. Mucins as Precision Biomarkers in Glioma: Emerging Evidence for Their Potential in Biospecimen Analysis and Outcome Prediction. Biomedicines. 2024; 12(12):2806. https://doi.org/10.3390/biomedicines12122806
Chicago/Turabian StyleErickson, Anna, Luke R. Jackson, Kevin Camphausen, and Andra V. Krauze. 2024. "Mucins as Precision Biomarkers in Glioma: Emerging Evidence for Their Potential in Biospecimen Analysis and Outcome Prediction" Biomedicines 12, no. 12: 2806. https://doi.org/10.3390/biomedicines12122806
APA StyleErickson, A., Jackson, L. R., Camphausen, K., & Krauze, A. V. (2024). Mucins as Precision Biomarkers in Glioma: Emerging Evidence for Their Potential in Biospecimen Analysis and Outcome Prediction. Biomedicines, 12(12), 2806. https://doi.org/10.3390/biomedicines12122806








