The Gut–Lung Axis During Ethanol Exposure and a Pseudomonas aeruginosa Bacterial Challenge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chronic-Binge Ethanol-Feeding Model with and Without P. aeruginosa Nasal Inoculation
2.2. Shotgun Metagenomics Sequencing, Bioinformatics, and Statistical Analyses of Bacterial gDNA from Cecal Contents
2.3. Isolation of Immune Cells from Mesenteric Lymph Nodes (MLNs)
2.4. Isolation of Immune Cells from Whole Blood
2.5. Isolation of Lung Immune Cells
2.6. Flow Cytometry Analysis of MLN, Lung, and Whole-Blood Immune Cells
2.7. RT-qPCR
2.8. Cecal Secretory IgA (SIgA) Enzyme-Linked Immunoassay (ELISA)
2.9. Immunohistochemical Analysis of Proximal Colon Tissue
2.10. Statistical Analysis
3. Results
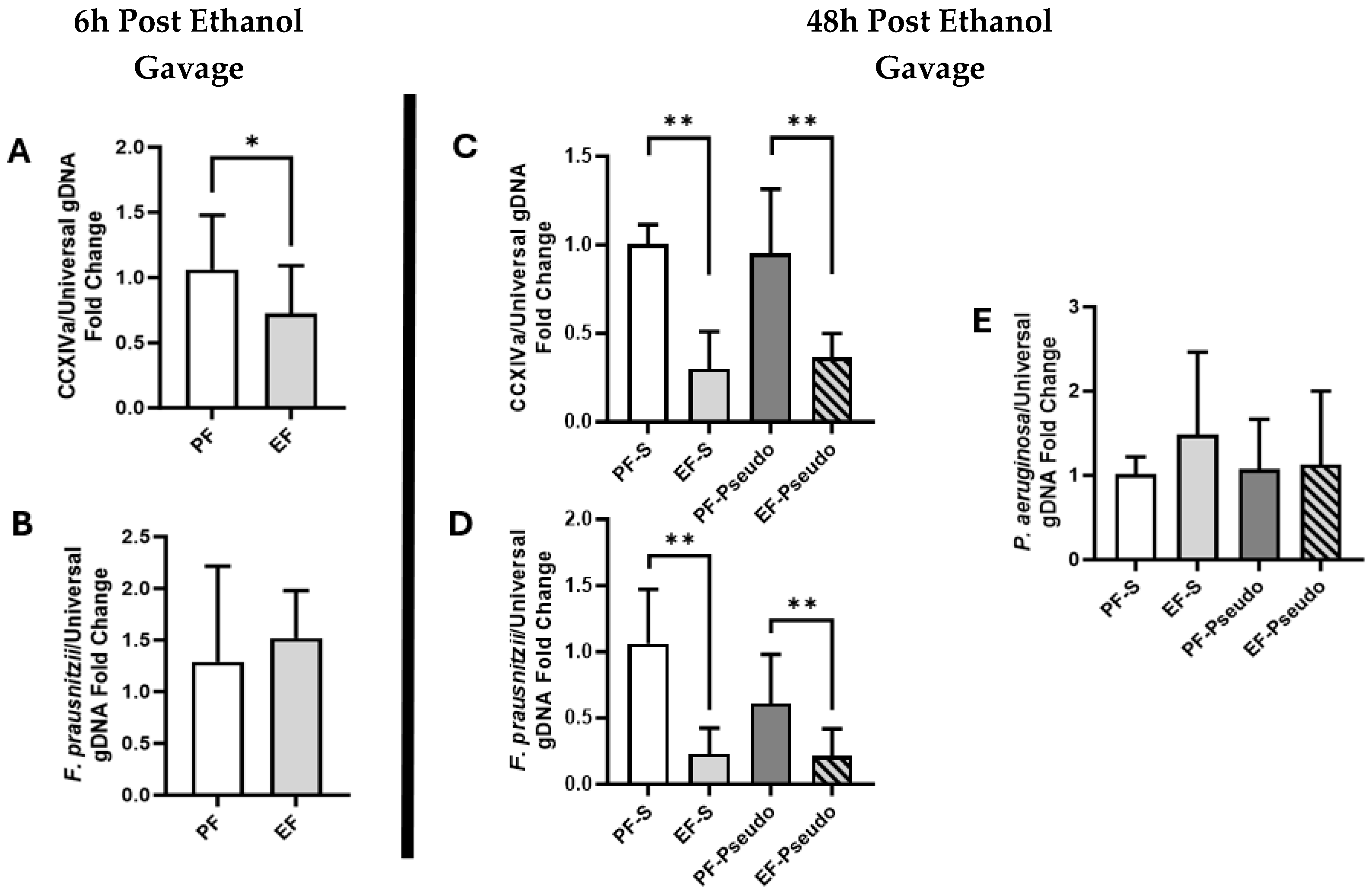
3.1. Ethanol Exposure Alters Gut Microbiota, Suppressing Butyrate-Producing Members and Altering Bacteria Metabolic Pathways
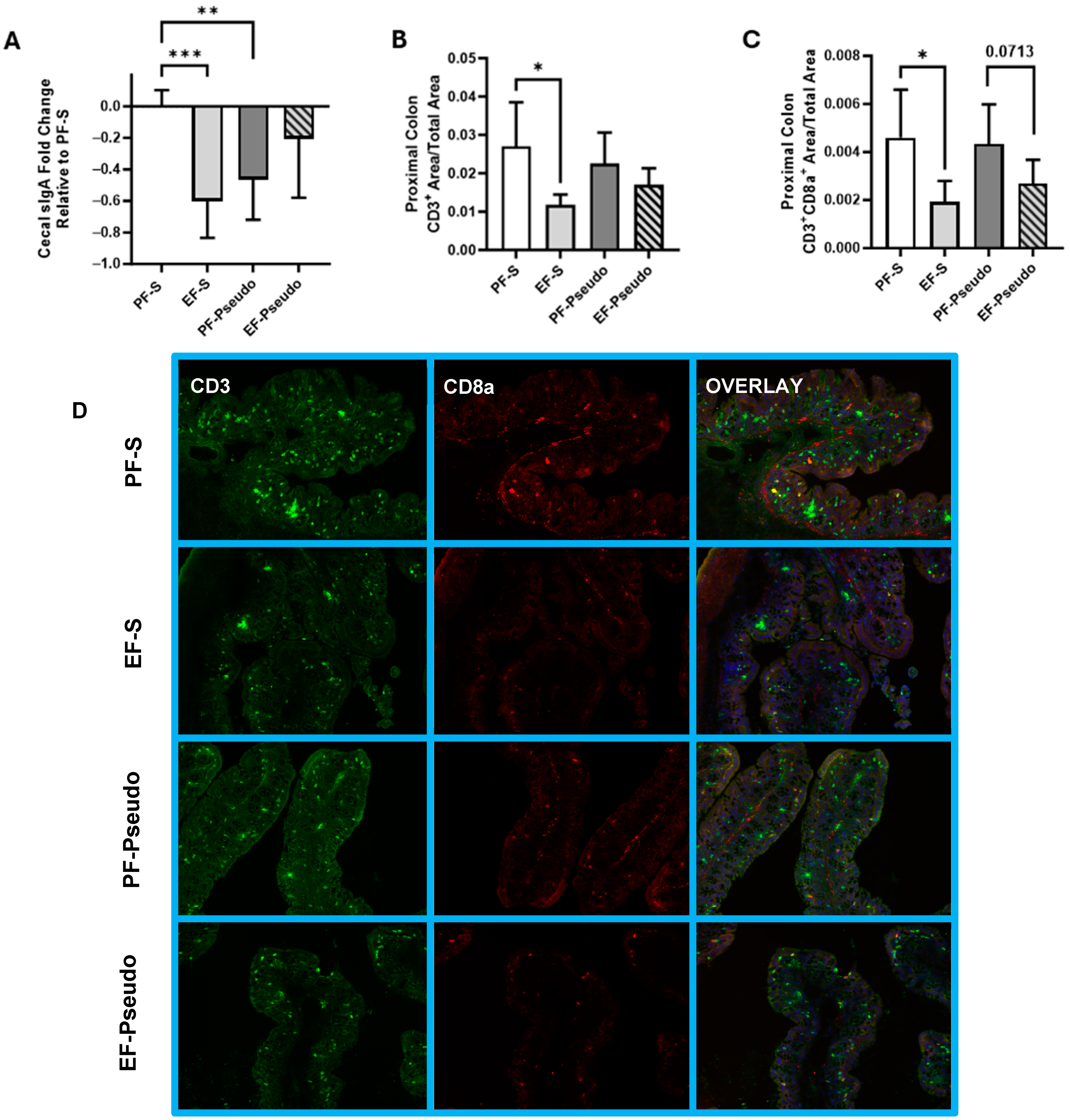
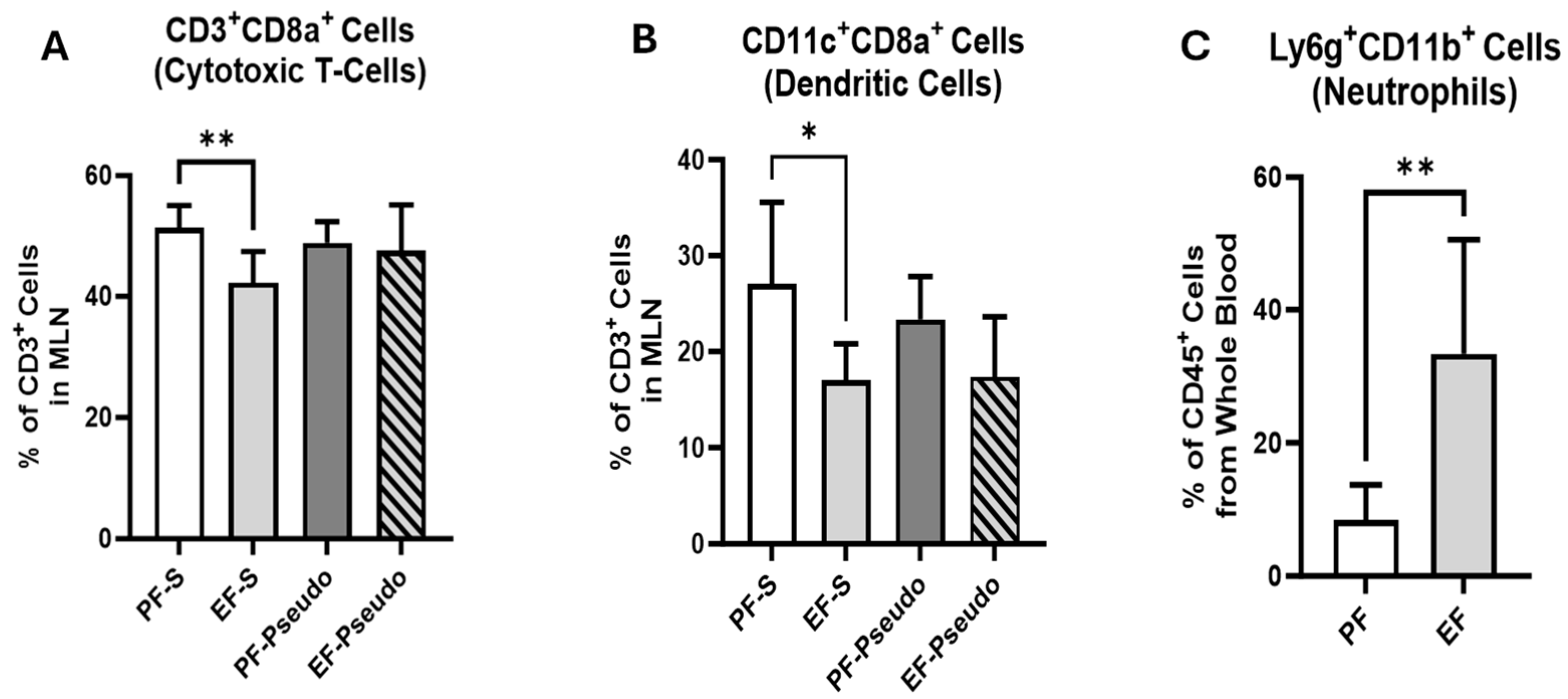
3.2. Ethanol-Induced Disruptions to the Gut Immune Environment Persist at 48 h Post Exposure
3.3. Ethanol Exposure Increases Lung Immune-Cell Infiltrate and Lipocalin 2 (LCN2) Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samuelson, D.R.; Shellito, J.E.; Maffei, V.J.; Tague, E.D.; Campagna, S.R.; Blanchard, E.E.; Luo, M.; Taylor, C.M.; Ronis, M.J.J.; Molina, P.E.; et al. Alcohol-associated intestinal dysbiosis impairs pulmonary host defense against Klebsiella pneumoniae. PLoS Pathog. 2017, 13, e1006426. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.T.; Han, Y.; Shapiro, D.; West, G.; Fiocchi, C.; Cresci, G.A.M. The Postbiotic Butyrate Mitigates Gut Mucosal Disruption Caused by Acute Ethanol Exposure. Int. J. Mol. Sci. 2024, 25, 1665. [Google Scholar] [CrossRef] [PubMed]
- Viennois, E.; Gewirtz, A.T.; Chassaing, B. Chronic Inflammatory Diseases: Are We Ready for Microbiota-Based Dietary Intervention? Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.A.; Gubatan, J. Gut Microbiome–Based Therapeutics in Inflammatory Bowel Disease. Clin. Transl. Discov. 2023, 3, e182. [Google Scholar] [CrossRef]
- Brusnic, O.; Onisor, D.; Boicean, A.; Hasegan, A.; Ichim, C.; Guzun, A.; Chicea, R.; Todor, S.B.; Vintila, B.I.; Anderco, P.; et al. Fecal Microbiota Transplantation: Insights into Colon Carcinogenesis and Immune Regulation. J. Clin. Med. 2024, 13, 6578. [Google Scholar] [CrossRef]
- Khan, M.A.S.; Chang, S.L. Alcohol and the Brain–Gut Axis: The Involvement of Microglia and Enteric Glia in the Process of Neuro-Enteric Inflammation. Cells 2023, 12, 2475. [Google Scholar] [CrossRef]
- Vanuytsel, T.; Tack, J.; Farre, R. The Role of Intestinal Permeability in Gastrointestinal Disorders and Current Methods of Evaluation. Front. Nutr. 2021, 8, 717925. [Google Scholar] [CrossRef]
- Cresci, G.A.; Bush, K.; Nagy, L.E. Tributyrin Supplementation Protects Mice from Acute Ethanol-Induced Gut Injury. Alcohol Clin. Exp. Res. 2014, 38, 1489–1501. [Google Scholar] [CrossRef]
- Cresci, G.A.; Glueck, B.; McMullen, M.R.; Xin, W.; Allende, D.; Nagy, L.E. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J. Gastroenterol. Hepatol. 2017, 32, 1587–1597. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Glueck, B.; Han, Y.; Mohammad, M.A.; Cresci, G.A.M. A Designer Synbiotic Attenuates Chronic-Binge Ethanol-Induced Gut-Liver Injury in Mice. Nutrients 2019, 11, 97. [Google Scholar] [CrossRef]
- Han, Y.; Glueck, B.; Shapiro, D.; Miller, A.; Roychowdhury, S.; Cresci, G.A.M. Dietary Synbiotic Supplementation Protects Barrier Integrity of Hepatocytes and Liver Sinusoidal Endothelium in a Mouse Model of Chronic-Binge Ethanol Exposure. Nutrients 2020, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.A.; Cinco, I.R.; Doratt, B.M.; Blanton, M.B.; Hoagland, C.; Newman, N.; Davies, M.; Grant, K.A.; Messaoudi, I. Chronic alcohol consumption dysregulates innate immune response to SARS-CoV-2 in the lung. Lancet 2023, 97, 104812. [Google Scholar] [CrossRef] [PubMed]
- Malacco, N.L.S.d.O.; Souza, J.A.M.; Martins, F.R.B.; Rachid, M.A.; Simplicio, J.A.; Tirapelli, C.R.; Sabino, A.d.P.; Queiroz-Junior, C.M.; Goes, G.R.; Vieira, L.Q.; et al. Chronic ethanol consumption compromises neutrophil function in acute pulmonary Aspergillus fumigatus infection. eLife 2020, 9, e58855. [Google Scholar] [CrossRef] [PubMed]
- Santilli, A.; Shapiro, D.; Han, Y.; Sangwan, N.; Cresci, G.A.M. Tributyrin Supplementation Rescues Chronic–Binge Ethanol-Induced Oxidative Stress in the Gut–Lung Axis in Mice. Antioxidants 2024, 13, 472. [Google Scholar] [CrossRef]
- Crews, F.T.; Sarkar, D.K.; Qin, L.; Zou, J.; Boyadjieva, N.; Vetreno, R.P. Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol Res. 2015, 37, 331–351. [Google Scholar]
- Raval, N.R.; Angarita, G.; Matuskey, D.; Miller, R.; Drake, L.R.; Kapinos, M.; Nabulsi, N.; Huang, Y.; Carson, R.E.; O’Malley, S.S.; et al. Imaging the brain’s immune response to alcohol with [11C]PBR28 TSPO Positron Emission Tomography. Mol. Psychiatry 2023, 28, 3384–3390. [Google Scholar] [CrossRef]
- Day, A.W.; Kumamoto, C.A. Gut Microbiome Dysbiosis in Alcoholism: Consequences for Health and Recovery. Front. Cell. Infect. Microbiol. 2022, 12, 840164. [Google Scholar] [CrossRef]
- Bjørkhaug, S.T.; Aanes, H.; Neupane, S.P.; Bramness, J.G.; Malvik, S.; Henriksen, C.; Skar, V.; Medhus, A.W.; Valeur, J. Characterization of Gut Microbiota Composition and Functions in Patients with Chronic Alcohol Overconsumption. Gut Microbes 2019, 10, 663–675. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, H.; Han, M.; Su, L.; Cui, X.; Li, D.; Liu, L.; Wang, C.; Yang, F. Alcohol-Induced Gut Microbiome Dysbiosis Enhances the Colonization of Klebsiella Pneumoniae on the Mouse Intestinal Tract. mSystems 2024, 9, e0005224. [Google Scholar] [CrossRef]
- Lowe, P.P.; Gyongyosi, B.; Satishchandran, A.; Iracheta-Vellve, A.; Ambade, A.; Kodys, K.; Catalano, D.; Ward, D.V.; Szabo, G. Alcohol-Related Changes in the Intestinal Microbiome Influence Neutrophil Infiltration, Inflammation and Steatosis in Early Alcoholic Hepatitis in Mice. PLoS ONE 2017, 12, e0174544. [Google Scholar]
- Bailey, K.L.; Samuelson, D.R.; Wyatt, T.A. Alcohol use disorder: A pre-existing condition for COVID-19? Alcohol 2021, 90, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.B.; Johansen, M.O.; Riddersholm, S.J.; Weinreich, U.M. The association between alcohol consumption and pulmonary function: A scoping review. Eur. Res. Rev. 2023, 33, 230233. [Google Scholar] [CrossRef] [PubMed]
- Simou, E.; Britton, J.; Leonardi-Bee, J. Alcohol and the risk of pneumonia: A systematic review and meta-analysis. BMJ Open 2018, 8, e022344. [Google Scholar] [CrossRef] [PubMed]
- Simou, E.; Leonardi-Bee, J.; Britton, J. The Effect of Alcohol Consumption on the Risk of ARDS: A Systematic Review and Meta-Analysis. Chest 2018, 154, 58–68. [Google Scholar] [CrossRef]
- Guo, F.; Zheng, K.; Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. The Lieber-DeCarli Diet-A Flagship Model for Experimental Alcoholic Liver Disease. Alcohol Clin. Exp. Res. 2018, 42, 1828–1840. [Google Scholar] [CrossRef]
- Poole, L.G.; Beier, J.I.; Torres-Gonzales, E.; Schlueter, C.F.; Hudson, S.V.; Artis, A.; Warner, N.L.; Nguyen-Ho, C.T.; Dolin, C.E.; Ritzenthaler, J.D.; et al. Chronic + Binge Alcohol Exposure Promotes Inflammation and Alters Airway Mechanics in the Lung. Alcohol 2018, 80, 53–63. [Google Scholar] [CrossRef]
- Fazzeli, H.; Akbari, R.; Moghim, S.; Narimani, T.; Arabestani, M.R.; Ghoddousi, A.R. Pseudomonas Aeruginosa Infections in Patients, Hospital Means, and Personnel’s Specimens. J. Res. Med. Sci. 2012, 17, 332. [Google Scholar]
- Qiu, Z.; Sheridan, B.S. Isolating Lymphocytes from the Mouse Small Intestinal Immune System. J. Vis. Exp. 2018, 132, 57281. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.M.; Stewart, C.S.; Flint, H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov. comb. nov. Int. J. Syst. Evol. Microbiol. 2020, 52, 2141–2146. [Google Scholar] [CrossRef]
- Solari, E.; Marcozzi, C.; Negrini, D.; Moriondo, A. Interplay between Gut Lymphatic Vessels and Microbiota. Cells 2021, 10, 2584. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Sun, Q.; Sun, H.; Liu, N.; Liu, Q.; Ma, J.; Wang, P.; Hu, C.; Wu, J.; et al. Mesenteric Lymph Duct Drainage Attenuates Lung Inflammatory Injury and Inhibits Endothelial Cell Apoptosis in Septic Rats. BioMed Res. Int. 2020, 2020, 3049302. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, X.; Chatterjee, V.; Wu, M.H.; Yuan, S.Y. The gut–lung axis in systemic inflammation role of mesenteric lymph as a conduit. Am. J. Respir. Cell Mol. Biol. 2021, 64, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of Dietary and Microbial Vitamin b Family in the Regulation of Host Immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef]
- Martin, P.R.; Singleton, C.K.; Hiller-Sturmhöfel, S. The Role of Thiamine Deficiency in Alcoholic Brain Disease. Alcohol Res. Health 2003, 27, 134–142. [Google Scholar]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Wang, R.X.; Lee, J.S.; Campbell, E.L.; Colgan, S.P. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc. Natl. Acad. Sci. USA 2020, 117, 11648–11657. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Anshory, M.; Effendi, R.M.R.A.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Nijsten, T.E.C.; Nouwen, J.L.; Thio, H.B. Butyrate Properties in Immune-Related Diseases: Friend or Foe? Fermentation 2023, 9, 205. [Google Scholar] [CrossRef]
- Ma, T.Y.; Nguyen, D.; Bui, V.; Nguyen, H.; Hoa, N. Ethanol modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 276, G965–G974. [Google Scholar] [CrossRef]
- Elamin, E.E.; Masclee, A.A.; Dekker, J.; Pieters, H.J.; Jonkers, D.M. Short-Chain Fatty Acids Activate AMP-Activated Protein Kinase and Ameliorate Ethanol-Induced Intestinal Barrier Dysfunction in Caco-2 Cell Monolayers. J. Nutr. 2013, 143, 1872–1881. [Google Scholar] [PubMed]
- Dang, A.T.; Begka, C.; Pattaroni, C.; Caley, L.R.; Floto, R.A.; Peckham, D.G.; Marsland, B.J. Butyrate Regulates Neutrophil Homeostasis and Impairs Early Antimicrobial Activity in the Lung. Mucosal Immunol. 2023, 16, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.O.; Castro, P.R.; Moser, R.; Ferreira, C.M.; Quesniaux, V.F.J.; Vinolo, M.A.R.; Ryffel, B. Butyrate: Connecting the Gut-Lung Axis to the Management of Pulmonary Disorders. Front. Nutr. 2022, 9, 1011732. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]



| Target | Primer | Sequence |
|---|---|---|
| Lipocalin 2 NM-008491 | LCN2 F | TGG CCC TGA GTG TCA TGT G |
| LCN2 R | CTC TTG TAG CTC ATA GAT GGT GC | |
| GAPDH NM_001289726 | GAPDH F | AGG TCG GTG TGA ACG GAT TTG |
| GAPDH R | TGT AGA CCA TGT AGT TGA GGT CA |
| Target | Primer | Sequence |
|---|---|---|
| Clostridium Cluster XIVa (CCXIVa) NR_104700 | CCXIVa F | ACT CCT ACG GGA GGC AGC |
| CCXIVa R | GCT TCT TAG TCA GGT ACC GTC AT | |
| Faecalibacterium prausnitzii NR_028961 | FPrau F | AGA TGG CCT CGC GTC CGA |
| FPrau R | CCG AAG ACC TTC TTC CTC C | |
| Pseudomonas aeruginosa NC_002516 | Pseudo F | CCT GAC CAT CCG TCG CCA CAA C |
| Pseudo R | CGC AGC AGG ATG CCG ACG CC | |
| Universal Bacterial 16s | Universal F | ACT CCT ACG GGA GGC AGC AG |
| Universal R | ATT ACC GCG GCT GCT GG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santilli, A.; Han, Y.; Yan, H.; Sangwan, N.; Cresci, G.A.M. The Gut–Lung Axis During Ethanol Exposure and a Pseudomonas aeruginosa Bacterial Challenge. Biomedicines 2024, 12, 2757. https://doi.org/10.3390/biomedicines12122757
Santilli A, Han Y, Yan H, Sangwan N, Cresci GAM. The Gut–Lung Axis During Ethanol Exposure and a Pseudomonas aeruginosa Bacterial Challenge. Biomedicines. 2024; 12(12):2757. https://doi.org/10.3390/biomedicines12122757
Chicago/Turabian StyleSantilli, Anthony, Yingchun Han, Hannah Yan, Naseer Sangwan, and Gail A. M. Cresci. 2024. "The Gut–Lung Axis During Ethanol Exposure and a Pseudomonas aeruginosa Bacterial Challenge" Biomedicines 12, no. 12: 2757. https://doi.org/10.3390/biomedicines12122757
APA StyleSantilli, A., Han, Y., Yan, H., Sangwan, N., & Cresci, G. A. M. (2024). The Gut–Lung Axis During Ethanol Exposure and a Pseudomonas aeruginosa Bacterial Challenge. Biomedicines, 12(12), 2757. https://doi.org/10.3390/biomedicines12122757






