The Functional Role and Prognostic Significance of TIM-3 Expression on NK Cells in the Diagnostic Bone Marrows in Acute Myeloid Leukemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. scRNA-Seq Data Analysis of HAVCR2 (+) and HAVCR2 (−) NK Cells in AML
2.2. Clinical Cohort
2.3. Flow Cytometric Analysis of TIM-3 and Cytotoxic Molecules Expression on NK Cells in Fresh BM Samples
2.4. In Vitro NK Cell Stimulation and Cell-Killing Activity Testing
2.5. Sorting of TIM-3+ and TIM-3− NK Cells and In Vitro NK Cell Function Testing
2.6. Definitions and Statistical Analysis
3. Results
3.1. scRNA-Seq Data Indicated the Positive Correlation of Transcript Levels Between HAVCR2 and Cytotoxic Molecules in the BM NK Cells
3.2. TIM-3 Expression Correlated with PFP and GZMB Expression in Fresh BM NK Cells
3.3. The Relationship Between TIM-3 Expression and PFP, GZMB and IFN-γ Levels in NK Cells After In Vitro Stimulation
3.4. In Vitro NK-Cell-Killing Capacity of AML Patients Was Not Related to TIM-3 Expression
3.5. In Vitro NK-Cell-Killing Capacity of AML Patients Positively Correlated with PFP and GZMB Levels
3.6. Outcomes and Clinical Characteristics of Follow-Up Patients
3.7. High TIM-3 and Low GZMB Levels of NK Cells at Diagnosis Predicted Poorer RFS in AML
3.8. Low GZMB Levels in TIM-3+ NK Cells Predicted Poorer RFS Superior to GZMB Levels in Total NK Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiNardo, C.D.; Erba, H.P.; Freeman, S.D.; Wei, A.H. Acute myeloid leukaemia. Lancet 2023, 401, 2073–2086. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Ofran, Y.; Boissel, N.; Brunet Mauri, S.; Craddock, C.; Janssen, J.; Wierzbowska, A.; Buske, C. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Kekre, N.; Atkins, H.; Imsirovic, H.; Hutton, B.; Coyle, D.; Thavorn, K. Phase-Based and Lifetime Health System Costs of Care for Patients Diagnosed with Leukemia and Lymphoma: A Population-Based Descriptive Study. Curr. Oncol. 2024, 31, 4192–4208. [Google Scholar] [CrossRef]
- Newell, L.F.; Cook, R.J. Advances in acute myeloid leukemia. BMJ 2021, 375, n2026. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Bhansali, R.S.; Pratz, K.W.; Lai, C. Recent advances in targeted therapies in acute myeloid leukemia. J. Hematol. Oncol. 2023, 16, 29. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, G.; Cai, X.; Liu, Y.; Qian, B.; Li, D. Global, regional, and national burden of acute myeloid leukemia, 1990-2021: A systematic analysis for the global burden of disease study 2021. Biomark. Res. 2024, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Wu, C.J. Dynamics and specificities of T cells in cancer immunotherapy. Nat. Rev. Cancer 2023, 23, 295–316. [Google Scholar] [CrossRef]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Ruggeri, L.; Mancusi, A.; Burchielli, E.; Perruccio, K.; Aversa, F.; Martelli, M.F.; Velardi, A. Natural killer cell recognition of missing self and haploidentical hematopoietic transplantation. Semin. Cancer Biol. 2006, 16, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Della Chiesa, M.; Carlomagno, S.; Quatrini, L.; Munari, E.; Vacca, P.; Tumino, N.; Mariotti, F.R.; Mingari, M.C.; Pende, D.; et al. Inhibitory Receptors and Checkpoints in Human NK Cells, Implications for the Immunotherapy of Cancer. Front. Immunol. 2020, 11, 2156. [Google Scholar] [CrossRef] [PubMed]
- Borgeaud, M.; Sandoval, J.; Obeid, M.; Banna, G.; Michielin, O.; Addeo, A.; Friedlaender, A. Novel targets for immune-checkpoint inhibition in cancer. Cancer Treat. Rev. 2023, 120, 102614. [Google Scholar] [CrossRef]
- Rakova, J.; Truxova, I.; Holicek, P.; Salek, C.; Hensler, M.; Kasikova, L.; Pasulka, J.; Holubova, M.; Kovar, M.; Lysak, D.; et al. TIM-3 levels correlate with enhanced NK cell cytotoxicity and improved clinical outcome in AML patients. Oncoimmunology 2021, 10, 1889822. [Google Scholar] [CrossRef]
- Judge, S.J.; Dunai, C.; Aguilar, E.G.; Vick, S.C.; Sturgill, I.R.; Khuat, L.T.; Stoffel, K.M.; Van Dyke, J.; Longo, D.L.; Darrow, M.A.; et al. Minimal PD-1 expression in mouse and human NK cells under diverse conditions. J. Clin. Investig. 2020, 130, 3051–3068. [Google Scholar] [CrossRef]
- Ndhlovu, L.C.; Lopez-Vergès, S.; Barbour, J.D.; Jones, R.B.; Jha, A.R.; Long, B.R.; Schoeffler, E.C.; Fujita, T.; Nixon, D.F.; Lanier, L.L. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012, 119, 3734–3743. [Google Scholar] [CrossRef]
- Folgiero, V.; Cifaldi, L.; Li Pira, G.; Goffredo, B.M.; Vinti, L.; Locatelli, F. TIM-3/Gal-9 interaction induces IFNγ-dependent IDO1 expression in acute myeloid leukemia blast cells. J. Hematol. Oncol. 2015, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- van Galen, P.; Hovestadt, V.; Wadsworth Ii, M.H.; Hughes, T.K.; Griffin, G.K.; Battaglia, S.; Verga, J.A.; Stephansky, J.; Pastika, T.J.; Lombardi Story, J.; et al. Single-Cell RNA-Seq Reveals AML Hierarchies Relevant to Disease Progression and Immunity. Cell 2019, 176, 1265–1281.e24. [Google Scholar] [CrossRef]
- Guo, R.; Lü, M.; Cao, F.; Wu, G.; Gao, F.; Pang, H.; Li, Y.; Zhang, Y.; Xing, H.; Liang, C.; et al. Single-cell map of diverse immune phenotypes in the acute myeloid leukemia microenvironment. Biomark. Res. 2021, 9, 15. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Chen, W.M.; Hao, Y.; Li, L.D.; Li, J.Y.; Sun, K.; Shi, Z.Y.; Jiang, H.; Jiang, Q.; et al. Usefulness of KIT mutant transcript levels for monitoring measurable residual disease in t (8;21) acute myeloid leukemia. Hematol. Oncol. 2024, 42, e3264. [Google Scholar] [CrossRef]
- Qin, Y.Z.; Zhao, T.; Zhu, H.H.; Wang, J.; Jia, J.S.; Lai, Y.Y.; Zhao, X.S.; Shi, H.X.; Liu, Y.R.; Jiang, H.; et al. High EVI1 Expression Predicts Poor Outcomes in Adult Acute Myeloid Leukemia Patients with Intermediate Cytogenetic Risk Receiving Chemotherapy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Yang, S.; Zhao, T.; Hu, L.; Qin, Y.; Jia, J.; Wang, J.; Lu, S.; Jiang, H.; Zhang, X.; et al. Comparison of efficacy between homoharringtonine, aclarubicin, cytarabine (HAA) and idarubicin, cytarabine (IA) regimens as induction therapy in patients with de novo core binding factor acute myeloid leukemia. Ann. Hematol. 2023, 102, 2695–2705. [Google Scholar] [CrossRef]
- Wang, L.Y.; Li, Y.; Jiang, Q.; Jiang, H.; Wang, Y.; Xu, L.P.; Zhang, X.H.; Liu, K.Y.; Tang, F.F. Clinical characteristics and prognosis of acute myeloid leukemia patients with Runt-related transcription factor 1 mutation: A single-center retrospective analysis. Hematol. Oncol. 2024, 42, e3256. [Google Scholar] [CrossRef]
- Zhang, X.H.; Chen, J.; Han, M.Z.; Huang, H.; Jiang, E.L.; Jiang, M.; Lai, Y.R.; Liu, D.H.; Liu, Q.F.; Liu, T.; et al. The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J. Hematol. Oncol. 2021, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Wang, J.; Zhang, Q.; Wang, H.; Li, C.; Xu, X.; Zhai, Z. Clinical implications of aberrant PD-1 expression for acute leukemia prognosis. Eur. J. Med. Res. 2023, 28, 383. [Google Scholar] [CrossRef]
- Sadeghi, M.; Khodakarami, A.; Ahmadi, A.; Fathi, M.; Gholizadeh Navashenaq, J.; Mohammadi, H.; Yousefi, M.; Hojjat-Farsangi, M.; Movasaghpour Akbari, A.A.; Jadidi-Niaragh, F. The prognostic and therapeutic potentials of CTLA-4 in hematological malignancies. Expert Opin. Ther. Targets 2022, 26, 1057–1071. [Google Scholar] [CrossRef]
- Ferraro, F.; Miller, C.A.; Christensen, K.A.; Helton, N.M.; O’Laughlin, M.; Fronick, C.C.; Fulton, R.S.; Kohlschmidt, J.; Eisfeld, A.K.; Bloomfield, C.D.; et al. Immunosuppression and outcomes in adult patients with de novo acute myeloid leukemia with normal karyotypes. Proc. Natl. Acad. Sci. USA 2021, 118, e2116427118. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, Y.; Lai, W.; Tan, J.; Zheng, X.; Zha, X.; Li, Y.; Chen, S. Higher PD-1/Tim-3 expression on IFN-γ+ T cells is associated with poor prognosis in patients with acute myeloid leukemia. Cancer Biol. Ther. 2023, 24, 2278229. [Google Scholar] [CrossRef] [PubMed]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Anderson, D.E.; Bregoli, L.; Hastings, W.D.; Kassam, N.; Lei, C.; Chandwaskar, R.; Karman, J.; Su, E.W.; Hirashima, M.; et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 2007, 318, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Phong, B.L.; Avery, L.; Sumpter, T.L.; Gorman, J.V.; Watkins, S.C.; Colgan, J.D.; Kane, L.P. Tim-3 enhances FcεRI-proximal signaling to modulate mast cell activation. J. Exp. Med. 2015, 212, 2289–2304. [Google Scholar] [CrossRef] [PubMed]
- Ausejo-Mauleon, I.; Nuin, S.; Alonso, M.M. The rise of TIM-3: A promising immune target in diffuse midline gliomas. Clin. Transl. Med. 2024, 14, e1536. [Google Scholar] [CrossRef]
- Li, H.; Wu, K.; Tao, K.; Chen, L.; Zheng, Q.; Lu, X.; Liu, J.; Shi, L.; Liu, C.; Wang, G.; et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012, 56, 1342–1351. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, X.; Xia, X.; Zhang, C.; Liang, X.; Gao, L.; Zhang, X.; Ma, C. Ectopic expression of TIM-3 in lung cancers: A potential independent prognostic factor for patients with NSCLC. Am. J. Clin. Pathol. 2012, 137, 978–985. [Google Scholar] [CrossRef]
- Kikushige, Y.; Shima, T.; Takayanagi, S.; Urata, S.; Miyamoto, T.; Iwasaki, H.; Takenaka, K.; Teshima, T.; Tanaka, T.; Inagaki, Y.; et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell 2010, 7, 708–717. [Google Scholar] [CrossRef]
- Sakoda, T.; Kikushige, Y.; Miyamoto, T.; Irifune, H.; Harada, T.; Hatakeyama, K.; Kunisaki, Y.; Kato, K.; Akashi, K. TIM-3 signaling hijacks the canonical Wnt/β-catenin pathway to maintain cancer stemness in acute myeloid leukemia. Blood Adv. 2023, 7, 2053–2065. [Google Scholar] [CrossRef]
- Klaihmon, P.; Luanpitpong, S.; Kang, X.; Issaragrisil, S. Anti-TIM3 chimeric antigen receptor-natural killer cells from engineered induced pluripotent stem cells effectively target acute myeloid leukemia cells. Cancer Cell Int. 2023, 23, 297. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Ando, K.; Rauzy, O.; Turgut, M.; Wang, M.C.; Cairoli, R.; Hou, H.A.; Kwong, Y.L.; Arnan, M.; Meers, S.; et al. Sabatolimab plus hypomethylating agents in previously untreated patients with higher-risk myelodysplastic syndromes (STIMULUS-MDS1): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Haematol. 2024, 11, e38–e50. [Google Scholar] [CrossRef]
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Traer, E.; Scholl, S.; Garcia-Manero, G.; Vey, N.; Wermke, M.; Janssen, J.; et al. Phase Ib study of sabatolimab (MBG453), a novel immunotherapy targeting TIM-3 antibody, in combination with decitabine or azacitidine in high- or very high-risk myelodysplastic syndromes. Am. J. Hematol. 2024, 99, E32–E36. [Google Scholar] [CrossRef]
- Yu, X.; Lang, B.; Chen, X.; Tian, Y.; Qian, S.; Zhang, Z.; Fu, Y.; Xu, J.; Han, X.; Ding, H.; et al. The inhibitory receptor Tim-3 fails to suppress IFN-γ production via the NFAT pathway in NK-cell, unlike that in CD4(+) T cells. BMC Immunol. 2021, 22, 25. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Rahmati, A.; Bigam, S.; Elahi, S. Galectin-9 promotes natural killer cells activity via interaction with CD44. Front. Immunol. 2023, 14, 1131379. [Google Scholar] [CrossRef]
- McNerlan, S.E.; Rea, I.M.; Alexander, H.D.; Morris, T.C. Changes in natural killer cells, the CD57CD8 subset, and related cytokines in healthy aging. J. Clin. Immunol. 1998, 18, 31–38. [Google Scholar] [CrossRef]
- Datar, I.; Sanmamed, M.F.; Wang, J.; Henick, B.S.; Choi, J.; Badri, T.; Dong, W.; Mani, N.; Toki, M.; Mejías, L.D.; et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non-Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4663–4673. [Google Scholar] [CrossRef]
- Wu, H.; Tang, T.; Deng, H.; Chen, D.; Zhang, C.; Luo, J.; Chen, S.; Zhang, P.; Yang, J.; Dong, L.; et al. Immune checkpoint molecule Tim-3 promotes NKT cell apoptosis and predicts poorer prognosis in Sepsis. Clin. Immunol. 2023, 254, 109249. [Google Scholar] [CrossRef]
- Wang, H.; Cao, K.; Liu, S.; Xu, Y.; Tang, L. Tim-3 Expression Causes NK Cell Dysfunction in Type 2 Diabetes Patients. Front. Immunol. 2022, 13, 852436. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Lian, J.; Yang, H.; Li, F.; Zhao, S.; Qi, Y.; Zhang, Y.; Huang, L. TNF-α-induced Tim-3 expression marks the dysfunction of infiltrating natural killer cells in human esophageal cancer. J. Transl. Med. 2019, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Q.; Yang, J.; Li, X.; Xian, L.; Li, W.; Lin, T.; Cheng, J.; Lin, Q.; Xu, X.; et al. Increased TIGIT expressing NK cells with dysfunctional phenotype in AML patients correlated with poor prognosis. Cancer Immunol. Immunother. CII 2022, 71, 277–287. [Google Scholar] [CrossRef]
- Firouzi, J.; Hajifathali, A.; Azimi, M.; Parvini, N.; Ghaemi, F.; Shayan Asl, N.; Hedayati Asl, A.A.; Safa, M.; Ebrahimi, M. Hsp70, in Combination with IL-15 and PD-1 Blocker, Interferes with The Induction of Cytotoxic NK Cells in Relapsed Acute Myeloid Leukemia Patients. Cell J. 2023, 25, 92–101. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Y.; Tan, L.; Yu, W.; Chen, D.; Lu, C.; He, J.; Wu, G.; Liu, X.; Zhang, Y. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int. Immunopharmacol. 2015, 29, 635–641. [Google Scholar] [CrossRef]
- Yu, L.; Liu, X.; Wang, X.; Yan, F.; Wang, P.; Jiang, Y.; Du, J.; Yang, Z. TIGIT(+) TIM-3(+) NK cells are correlated with NK cell exhaustion and disease progression in patients with hepatitis B virus-related hepatocellular carcinoma. Oncoimmunology 2021, 10, 1942673. [Google Scholar] [CrossRef]
- Gros, A.; Robbins, P.F.; Yao, X.; Li, Y.F.; Turcotte, S.; Tran, E.; Wunderlich, J.R.; Mixon, A.; Farid, S.; Dudley, M.E.; et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Investig. 2014, 124, 2246–2259. [Google Scholar] [CrossRef]
- Horton, B.L.; Williams, J.B.; Cabanov, A.; Spranger, S.; Gajewski, T.F. Intratumoral CD8(+) T-cell Apoptosis Is a Major Component of T-cell Dysfunction and Impedes Antitumor Immunity. Cancer Immunol. Res. 2018, 6, 14–24. [Google Scholar] [CrossRef]
- Joller, N.; Anderson, A.C.; Kuchroo, V.K. LAG-3, TIM-3, and TIGIT: Distinct functions in immune regulation. Immunity 2024, 57, 206–222. [Google Scholar] [CrossRef]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- de Mingo Pulido, Á.; Hänggi, K.; Celias, D.P.; Gardner, A.; Li, J.; Batista-Bittencourt, B.; Mohamed, E.; Trillo-Tinoco, J.; Osunmakinde, O.; Peña, R.; et al. The inhibitory receptor TIM-3 limits activation of the cGAS-STING pathway in intra-tumoral dendritic cells by suppressing extracellular DNA uptake. Immunity 2021, 54, 1154–1167.e7. [Google Scholar] [CrossRef] [PubMed]
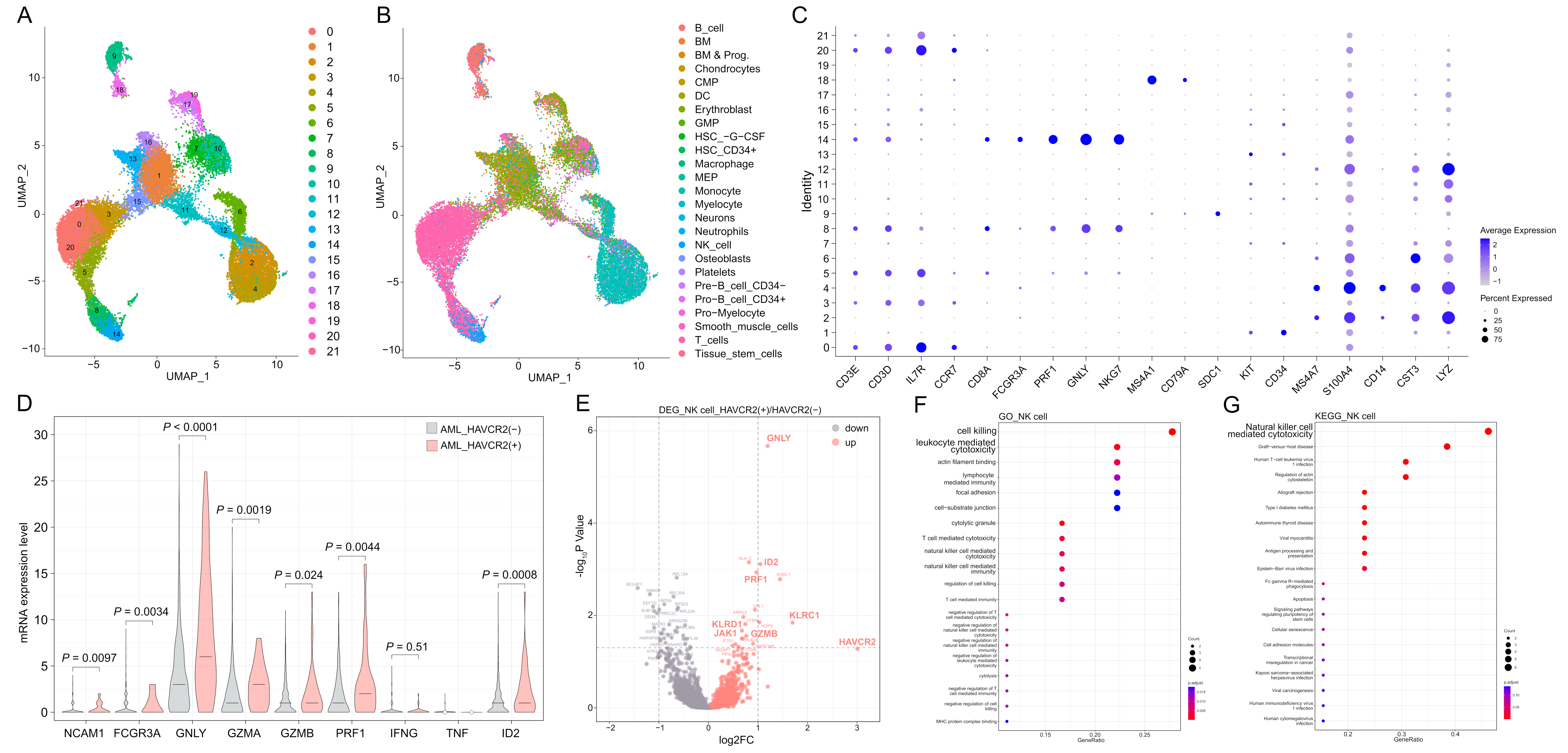


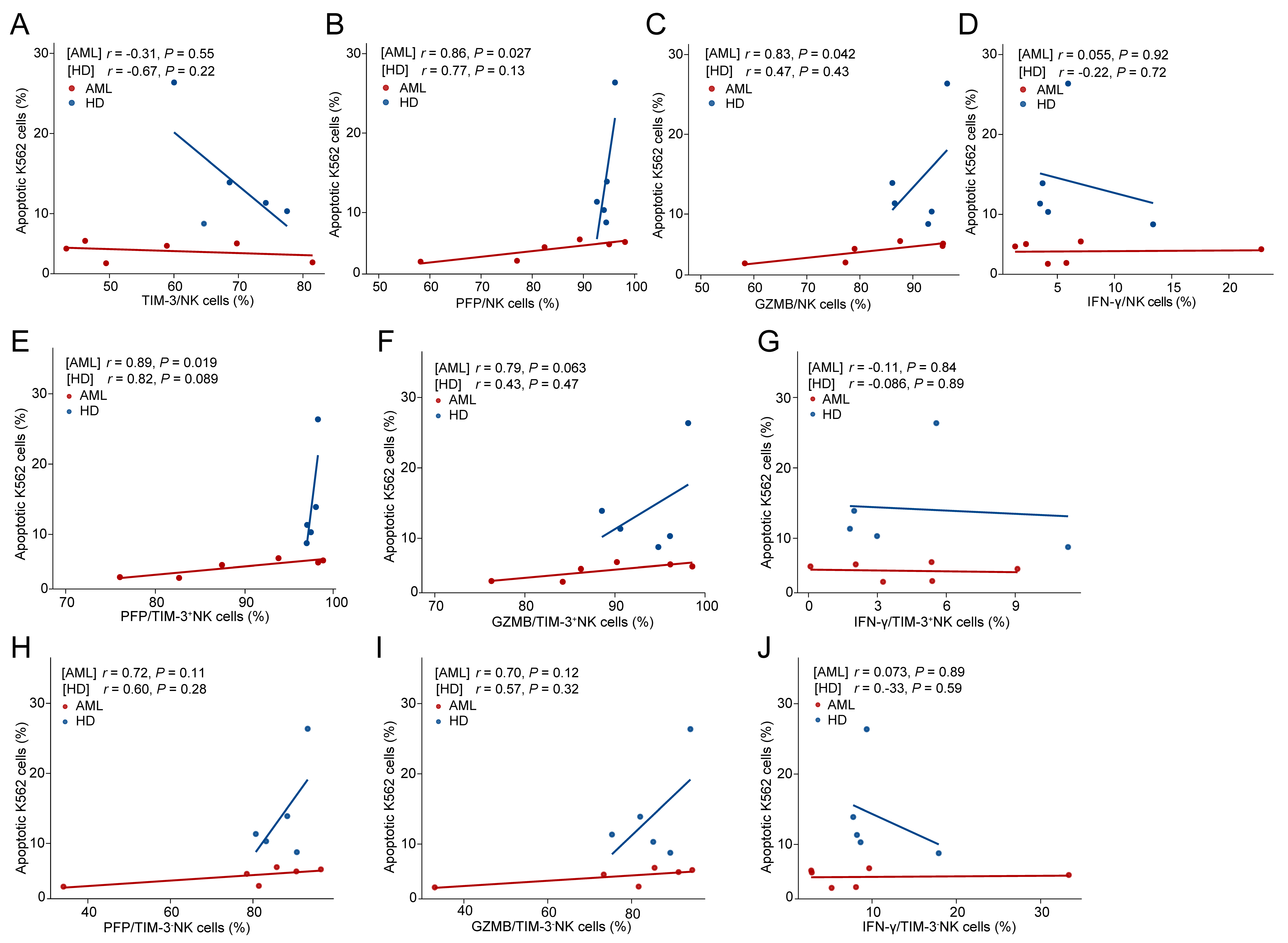
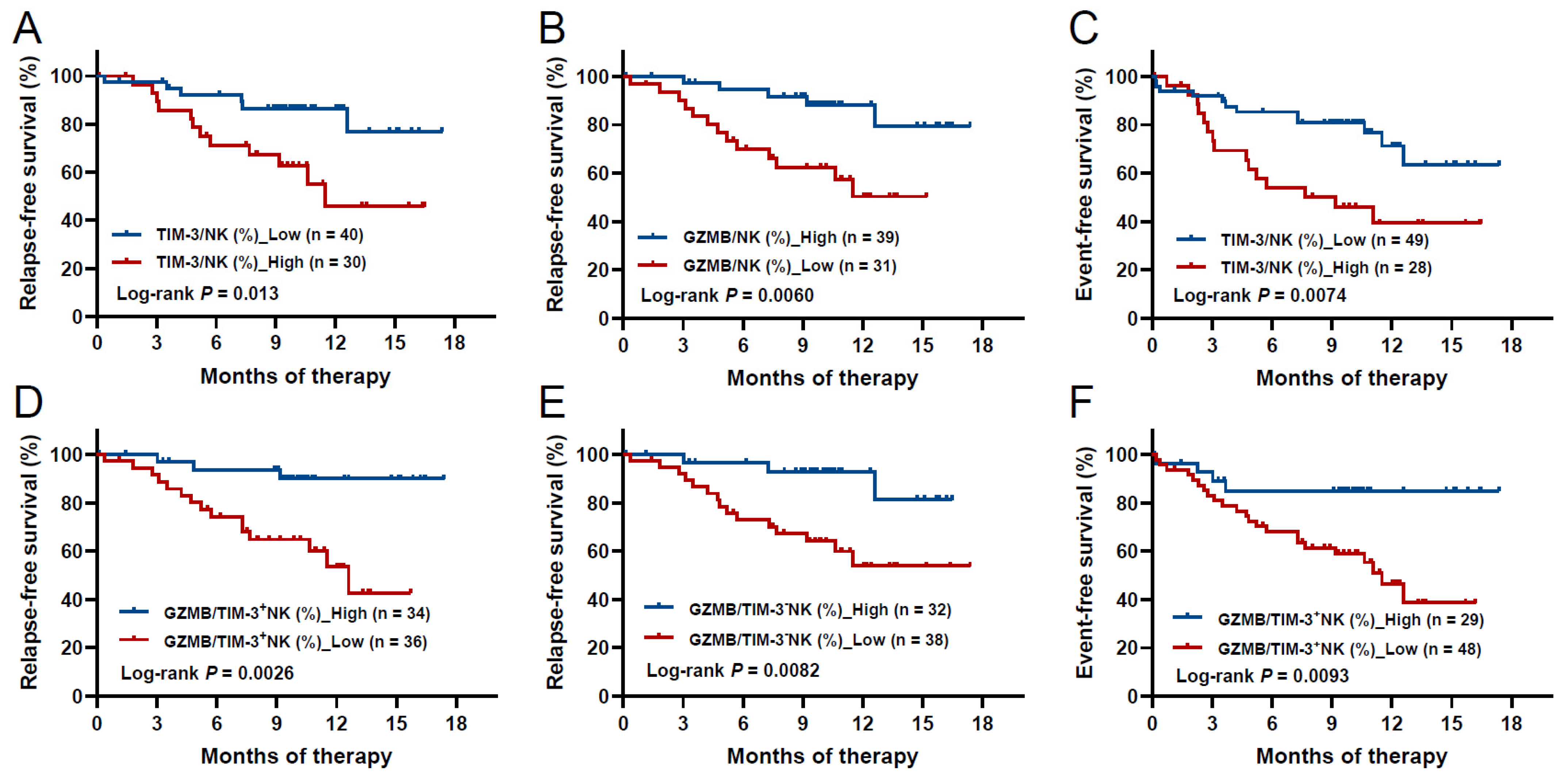
| Variables | Number of Patients or Median (Range) | TIM-3 Expression on NK Cells (%) | p Value |
|---|---|---|---|
| All | 105 | 68.9 (19.7–95.0) | |
| Age (y) | 47 (16–65) | 0.46 | |
| 15–45 | 49, 46.7% | 70.0 (21.8–95.0) | |
| 45–65 | 56, 53.3% | 68.1 (19.7–92.2) | |
| Gender | 0.85 | ||
| Male | 55, 52.4% | 69.1 (29.3–92.2) | |
| Female | 50, 47.6% | 67.7 (19.7–95.0) | |
| WBC count (×109/L) | 16.6 (1.2–460.0) | 0.69 | |
| ≤13 | 52, 50.0% | 69.5 (19.7–92.2) | |
| >13 | 52, 50.0% | 67.2 (32.6–95.0) | |
| Hemoglobin (g/L) | 87 (36–152) | 0.35 | |
| ≤90 | 56, 53.8% | 67.3 (19.7–95.0) | |
| >90 | 48, 46.2% | 71.1 (29.3–92.1) | |
| Platelet count (×109/L) | 40 (4–507) | 0.091 | |
| ≤45 | 54, 51.9% | 67.2 (19.7–92.1) | |
| >45 | 50, 48.1% | 73.3 (30.7–95.0) | |
| BM blast (%) | 61 (22–98) | 0.51 | |
| ≤60 (n = 50) | 50, 47.6% | 67.3 (21.8–92.1) | |
| >60 (n = 55) | 55, 52.4% | 73.4 (19.7–95.0) | |
| FAB subtypes | 0.75 | ||
| M0 | 3, 2.9% | 77.6 (49.6–82.5) | |
| M1 | 5, 4.8% | 78.7 (57.1–90.3) | |
| M2 | 64, 61.0% | 69.1 (21.8–92.2) | |
| M4 | 27, 25.7% | 65.5 (19.7–95.0) | |
| M5 | 5, 4.8% | 73.1 (60.4–87.2) | |
| M7 | 1, 1.0% | 55.9 | |
| ELN genetic risk classification (n = 96) * | 0.094 | ||
| Favorable | 44, 45.8% | 66.4 (21.8–91.3) | |
| Intermediate | 24, 25.0% | 75.6 (19.7–95.0) | |
| Adverse | 28, 29.2% | 74.7 (44.3–92.2) |
| Univariate Analysis | Multivariate Analysis | Multivariate Analysis * | ||||
|---|---|---|---|---|---|---|
| Variables | 2-Year RFS (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| TIM-3/NK (%) | 0.013 | 0.55 | 0.85 | |||
| ≤cutoff 72.0 (n = 40) | 77.0 (48.9–90.9) | - | - | |||
| >cutoff 72.0 (n = 30) | 45.8 (21.4–67.4) | - | - | |||
| GZMB/NK (%) | 0.0060 | 0.0020 | 0.18 | |||
| ≤cutoff 77.2 (n = 39) | 50.4 (28.4–68.8) | 8.1 (2.2–30.7) | - | |||
| >cutoff 77.2 (n = 31) | 79.4 (51.9–92.2) | 1.0 | - | |||
| GZMB/TIM3+NK (%) * | 0.0026 * | 0.0032 | ||||
| ≤cutoff 84.0 (n = 36) | 42.8 (18.9–64.9) | 7.7 (2.0–30.0) | ||||
| >cutoff 84.0 (n = 34) | 89.9 (71.9–96.7) | 1.0 | ||||
| GZMB/TIM-3−NK (%) * | 0.0084 * | |||||
| ≤cutoff 72.0 (n = 38) | 53.9 (33.8–70.3) | 0.65 | ||||
| >cutoff 72.0 (n = 32) | 81.3 (44.7–94.8) | - | ||||
| Age (y) | 0.99 | - | ||||
| 15–45 (n = 36) | 68.6 (44.1–84.1) | |||||
| 46–65 (n = 34) | 61.9 (36.1–79.8) | |||||
| Gender | 0.66 | |||||
| Male (n = 37) | 73.8 (54.1–86.0) | |||||
| Female (n = 33) | 56.0 (27.8–76.8) | |||||
| WBC count (× 109/L) | 0.24 | |||||
| ≤13 (n = 36) | 72.8 (51.2–86.0) | |||||
| >13 (n = 34) | 44.7 (9.8–75.6) | |||||
| Hemoglobin (g/L) | 0.51 | |||||
| ≤90 (n = 34) | 59.2 (30.9–79.1) | |||||
| >90 (n = 36) | 67.8 (41.7–84.2) | |||||
| Platelet count (× 109/L) | 0.33 | |||||
| ≤45 (n = 37) | 64.4 (34.6–83.3) | |||||
| >45 (n = 33) | 63.8 (41.6–79.4) | |||||
| BM blast (%) | 0.43 | |||||
| ≤60 (n = 35) | 66.4 (39.3–83.6) | |||||
| >60 (n = 35) | 61.7 (35.2–80.0) | |||||
| ELN risk category by genetics (n = 67) | 0.11 | 0.0015 | 0.0018 | |||
| Favorable (n = 34) | 71.7 (41.7–88.1) | - | 1.0 | - | 1.0 | - |
| Intermediate (n = 16) | 55.1 (25.5–77.1) | 0.030 | 11.3 (2.9–44.9) | 0.0006 | 9.3 (2.6–32.4) | 0.0005 |
| Adverse (n = 17) | 59.2 (23.7–82.6) | 0.31 | 3.7 (1.1–12.9) | 0.040 | 4.6 (1.3–16.6) | 0.021 |
| Induction therapy | 0.54 | |||||
| IA/HAA (n = 47) | 56.1 (33.5–73.7) | - | ||||
| AA/CAG (n = 5) | 75.0 (12.8–96.1) | 0.67 | ||||
| Azacitidine + Venetoclax (n = 16) | 85.6 (53.3–96.2) | 0.21 | ||||
| Others (n = 2) | 100 | 0.47 | ||||
| CR after 1-course induction | 0.73 | |||||
| No (n = 11) | 70.0 (22.5–91.8) | |||||
| Yes (n = 59) | 63.9 (45.0–77.8) | |||||
| Consolidation therapy | 0.038 | 0.0020 | 0.0046 | |||
| Chemotherapy alone (n = 51) | 56.8 (35.0–73.8) | 12.9 (2.5–65.8) | 10.4 (2.1–52.7) | |||
| Allo-HSCT (n = 19) | 84.0 (46.8–96.0) | 1.0 | 1.0 | |||
| Univariate Analysis | Multivariate Analysis | Multivariate Analysis * | ||||
|---|---|---|---|---|---|---|
| Variables | 2-Year RFS (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| TIM-3/NK (%) | 0.0074 | 0.31 | 0.31 | |||
| ≤cutoff 75.0 (n = 49) | 63.4 (39.6–79.8) | - | - | |||
| >cutoff 75.0 (n = 28) | 39.3 (19.7–58.5) | - | - | |||
| GZMB/TIM3+NK (%) * | 0.0093 * | 0.072 | ||||
| ≤cutoff 87.95 (n = 48) | 38.8 (19.7–57.5) | - | ||||
| >cutoff 87.95 (n = 29) | 85.0 (64.7–94.1) | - | ||||
| Age (y) | 0.92 | |||||
| 15–45 (n = 39) | 60.9 (39.2–76.9) | |||||
| 46–65 (n = 38) | 50.8 (28.8–69.1) | |||||
| Gender | 0.66 | |||||
| Male (n = 40) | 65.6 (47.2–79.0) | |||||
| Female (n = 37) | 46.2 (23.1–66.4) | |||||
| WBC count (× 109/L) | 0.19 | 0.60 | 0.60 | |||
| ≤13 (n = 39) | 62.4 (41.7–77.6) | - | - | |||
| >13 (n = 38) | 38.1 (9.4–67.4) | - | - | |||
| Hemoglobin (g/L) | 0.79 | |||||
| ≤90 (n = 38) | 52.7 (28.1–72.4) | |||||
| >90 (n = 39) | 54.6 (31.5–72.8) | |||||
| Platelet count (× 109/L) | 0.035 | 0.19 | 0.19 | |||
| ≤45 (n = 37) | 62.3 (33.8–81.4) | - | - | |||
| >45 (n = 40) | 47.0 (27.8–64.1) | - | - | |||
| BM blast (%) | 0.088 | 0.67 | 0.67 | |||
| ≤60 (n = 36) | 60.2 (35.0–78.2) | - | - | |||
| >60 (n = 41) | 50.3 (29.1–68.2) | - | - | |||
| ELN risk category by genetics (n = 74) | 0.0044 | 0.0008 | 0.0008 | |||
| Favorable (n = 34) | 71.7 (41.7–88.1) | - | 1.0 | - | 1.0 | - |
| Intermediate (n = 18) | 43.0 (18.9–65.3) | 0.0011 | 4.2 (1.4–12.5) | 0.0087 | 4.2 (1.4–12.5) | 0.0087 |
| Adverse (n = 22) | 38.8 (15.1–62.2) | 0.0061 | 6.4 (2.2–18.4) | 0.0005 | 6.4 (2.2–18.4) | 0.0005 |
| Induction therapy | 0.0004 | 0.0006 | 0.0006 | |||
| IA/HAA (n = 49) | 50.4 (29.5–68.0) | - | 1.0 | - | 1.0 | - |
| AA/CAG (n = 5) | 75.0 (12.8–96.1) | 0.55 | - | 0.26 | - | 0.26 |
| Azacitidine + Venetoclax (n = 16) | 85.6 (53.3–96.2) | 0.13 | 0.1 (0.01–0.8) | 0.027 | 0.1 (0.01–0.8) | 0.027 |
| Other non-intensive chemotherapy regemens (n = 5) | 20.0 (0.8–58.2) | 0.0006 | 5.6 (1.7–18.2) | 0.0046 | 5.6 (1.7–18.2) | 0.0046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Shi, Z.-Y.; Xie, D.-H.; Wang, Y.-Z.; Jiang, H.; Jiang, Q.; Huang, X.-J.; Qin, Y.-Z. The Functional Role and Prognostic Significance of TIM-3 Expression on NK Cells in the Diagnostic Bone Marrows in Acute Myeloid Leukemia. Biomedicines 2024, 12, 2717. https://doi.org/10.3390/biomedicines12122717
Sun K, Shi Z-Y, Xie D-H, Wang Y-Z, Jiang H, Jiang Q, Huang X-J, Qin Y-Z. The Functional Role and Prognostic Significance of TIM-3 Expression on NK Cells in the Diagnostic Bone Marrows in Acute Myeloid Leukemia. Biomedicines. 2024; 12(12):2717. https://doi.org/10.3390/biomedicines12122717
Chicago/Turabian StyleSun, Kai, Zong-Yan Shi, Dai-Hong Xie, Ya-Zhe Wang, Hao Jiang, Qian Jiang, Xiao-Jun Huang, and Ya-Zhen Qin. 2024. "The Functional Role and Prognostic Significance of TIM-3 Expression on NK Cells in the Diagnostic Bone Marrows in Acute Myeloid Leukemia" Biomedicines 12, no. 12: 2717. https://doi.org/10.3390/biomedicines12122717
APA StyleSun, K., Shi, Z.-Y., Xie, D.-H., Wang, Y.-Z., Jiang, H., Jiang, Q., Huang, X.-J., & Qin, Y.-Z. (2024). The Functional Role and Prognostic Significance of TIM-3 Expression on NK Cells in the Diagnostic Bone Marrows in Acute Myeloid Leukemia. Biomedicines, 12(12), 2717. https://doi.org/10.3390/biomedicines12122717







