Microbiota and Autism: A Review on Oral and Gut Microbiome Analysis Through 16S rRNA Sequencing
Abstract
1. Introduction
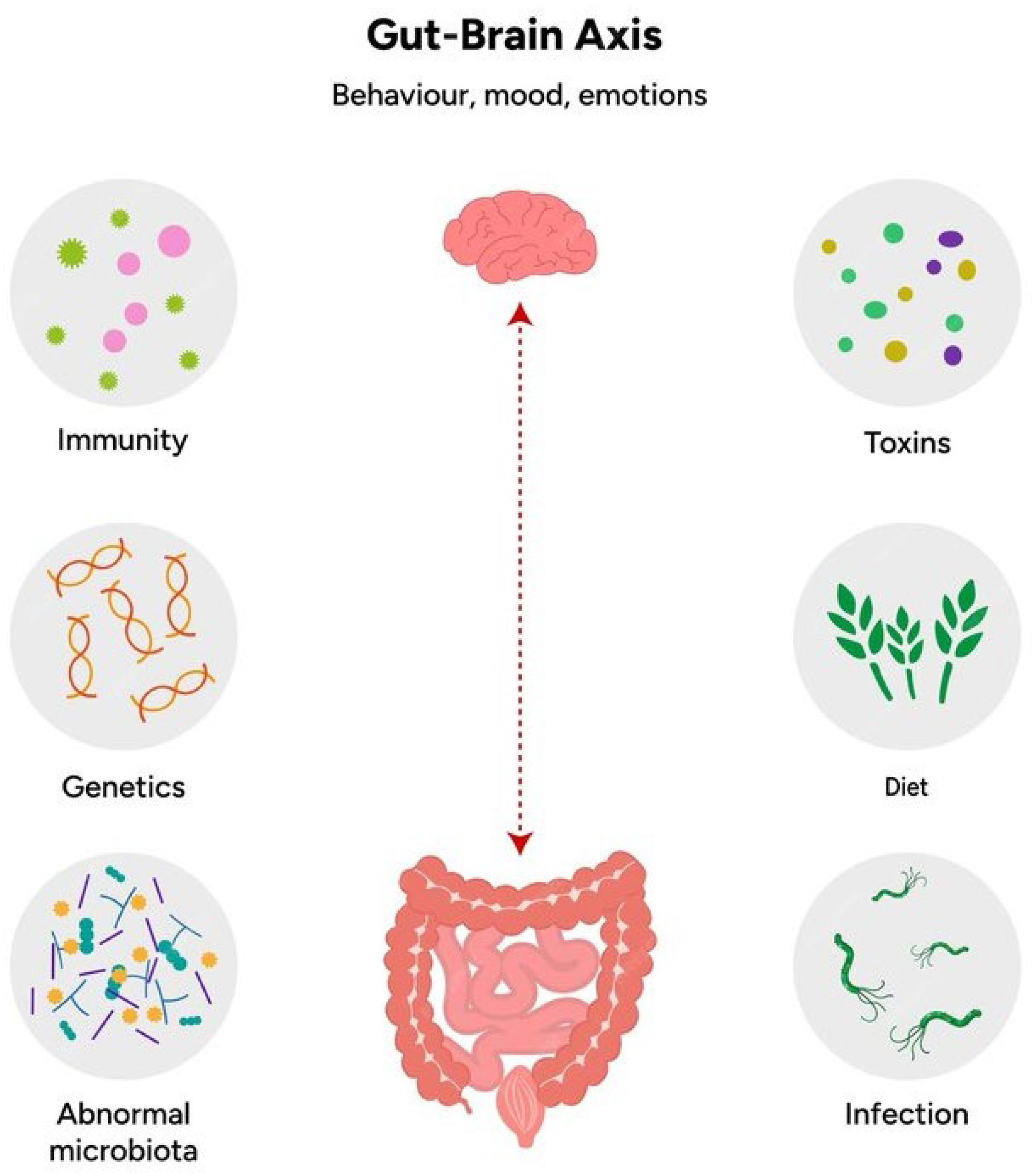
2. The Gut-Brain Axis and ASD
- ▪
- Neural pathways: where the the vagus nerve plays a central role in transmitting signals from the gut to the brain, transporting sensory and microbial-derived information. In ASD, disruptions in vagal signaling due to microbial imbalances may contribute to altered brain function and behavior. In addition, the gastrointestinal tract possesses an intrinsic embedded nervous system, the Enteric Nervous System (ENS), which consists of a very large number of neurons (more than one hundred million in humans, corresponding, roughly, to the number of neurons in the spinal cord), collected in numerous bundles of efferent and afferent fibers that connect gut and brain bidirectionally through the vagus nerve. The ENS, also known as the “second brain”, is made up of internal intestinal neurons and glial cells that innervate several laminae proper to the intramuscular mucosa. This intrinsic network of neurons allows the gastrointestinal tract to partially maintain its function without any input from the CNS, while the ENS can receive sensory input from the Autonomic Nervous System (ANS) and transmit information to it.
- ▪
- Immune system modulation: a significant portion of the body’s immune cells reside in the gut-associated lymphoid tissue. Dysbiosis, or an imbalance in gut microbiota, can lead to immune dysregulation, chronic inflammation, and the release of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). These cytokines can cross the blood-brain barrier (BBB) and influence neuroinflammation, which is commonly observed in ASD.
- ▪
- Endocrine pathways: the gut microbiota influences the production of several hormones and neurotransmitters that affect the CNS. For example, gut bacteria produce SCFAs such as butyrate, propionate, and acetate, which have neuroactive properties. Dysbiosis in ASD patients has been associated with altered SCFA levels, which can impact brain development and function.
- ▪
- Microbial metabolites: the gut microbiota produces a wide range of metabolites, including SCFAs and neurotransmitters such as serotonin and gamma-aminobutyric acid (GABA). These metabolites can modulate the brain through systemic circulation, affecting cognitive function, mood, and behavior. Dysbiosis can disrupt the production of these key metabolites, exacerbating symptoms of ASD [28].
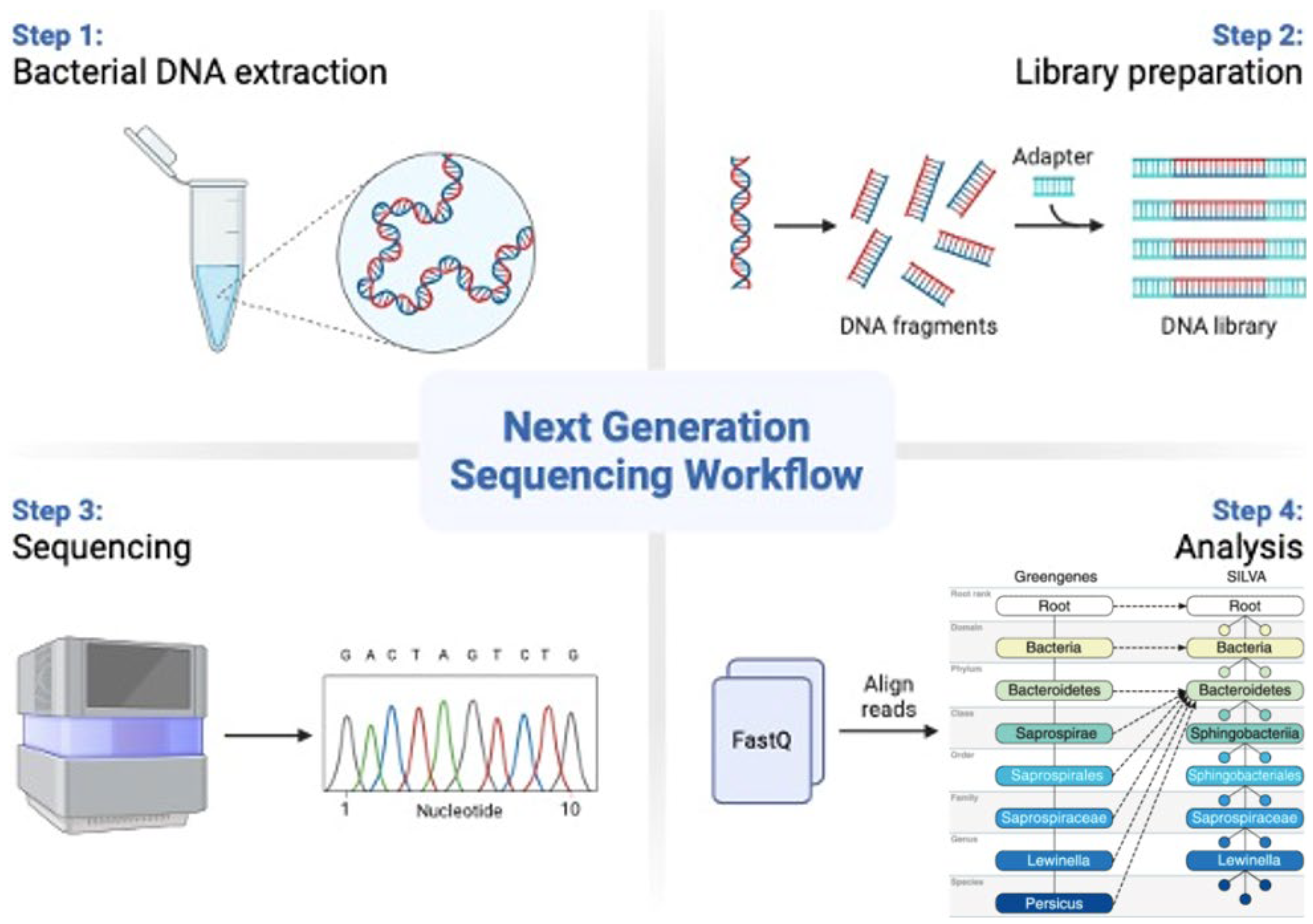
3. 16S rRNA Sequencing in Microbiome Studies
Characteristics of 16S rRNA Methodology
4. Gut Microbiota and ASD
5. Oral Microbiota and ASD
6. The Microbiota-Immune System Interaction
7. Therapeutic Strategies for Patients with ASD and Altered Microbiota
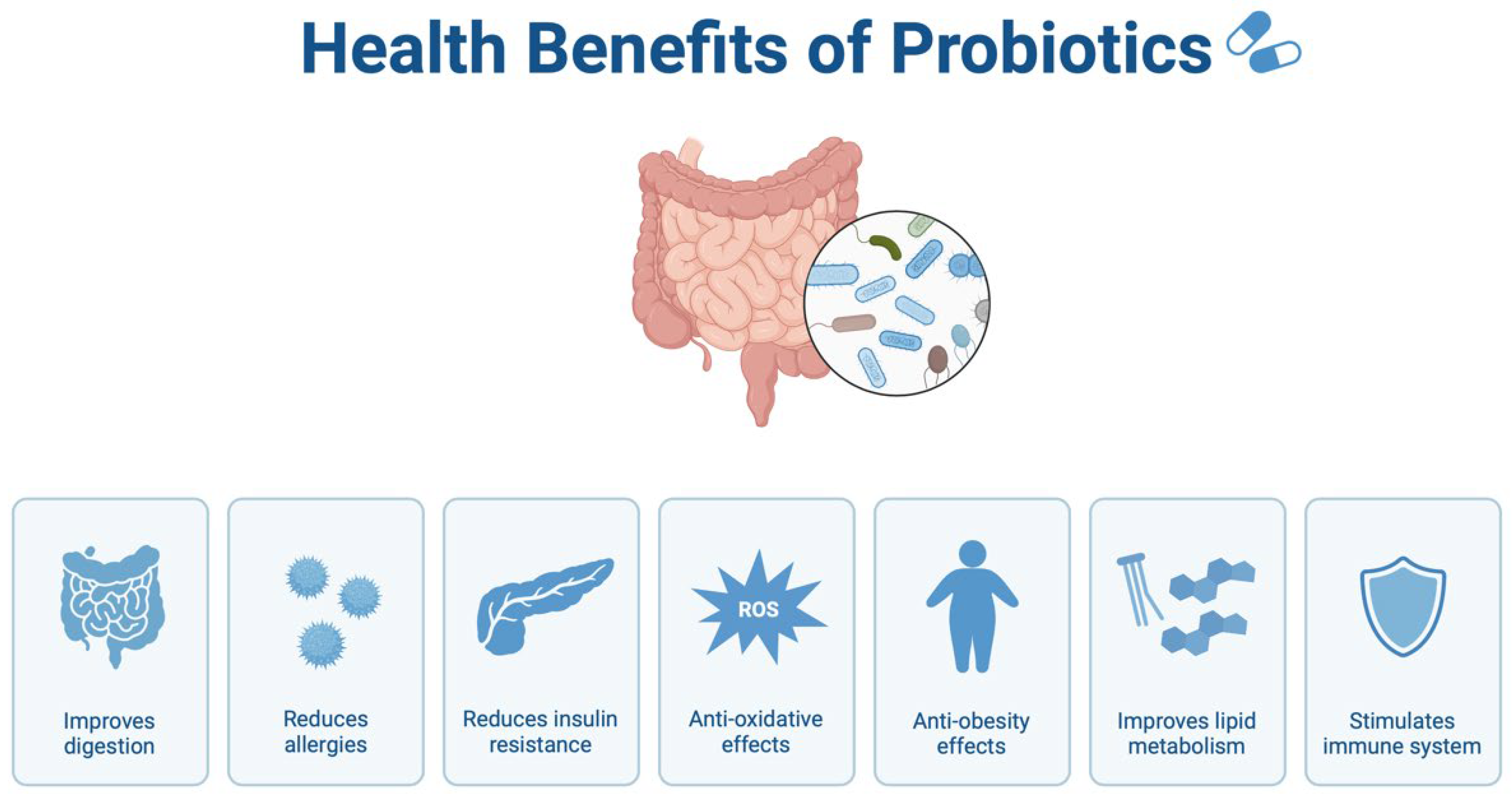
7.1. Probiotics and Prebiotics
7.2. Dietary Interventions
7.3. Fecal Microbiota Transplantation
7.4. Emerging Biomarkers and Novel Therapeutic Approaches in ASD
8. AI-Enhanced Bioinformatics and Multi-Omics Integration in ASD Research
8.1. Advances in Bioinformatics and AI for MGB-Targeted Clinical Trials
8.2. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
List of Abbreviations
| ANS | Autonomic Nervous System |
| AI | Artificial intelligence |
| ASD | Autism spectrum disorder |
| BBB | Blood-brain barrier |
| cfRNA | circulating free RNA |
| CNS | Central Nervous System |
| ENS | Enteric Nervous System |
| EV | extracellular vesicles |
| FMT | Fecal microbiota transplantation |
| FOS | Fructo-oligosaccharides |
| GABA | Gamma-aminobutyric acid |
| GFCF | Gluten-free, casein-free |
| GOS | Galacto-oligosaccharides |
| IBD | Inflammatory bowel diseases |
| IL-6 | Interleukin-6 |
| LPS | Lipopolysaccharides |
| miRNA | microRNA |
| MGB | Microbiota-gut-brain |
| ML | machine learning |
| MTT | Microbiota transfer therapy |
| NGS | Next Generation Sequencing |
| rRNA | Ribosomal RNA |
| SCFAs | Short-chain fatty acids |
| TNF-α | Tumor necrosis factor-alpha |
References
- Rosenfeld, C.S. Microbiome Disturbances and Autism Spectrum Disorders. Drug Metab. Dispos. 2015, 43, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Hsiao, E.Y. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol. Psychiatry. 2017, 81, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180, Erratum in Nature 2011, 474, 666; Erratum in Nature 2014, 506, 516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iliodromiti, Z.; Triantafyllou, A.R.; Tsaousi, M.; Pouliakis, A.; Petropoulou, C.; Sokou, R.; Volaki, P.; Boutsikou, T.; Iacovidou, N. Gut Microbiome and Neurodevelopmental Disorders: A Link Yet to Be Disclosed. Microorganisms 2023, 11, 487. [Google Scholar] [CrossRef]
- Faienza, M.F.; Urbano, F.; Anaclerio, F.; Moscogiuri, L.A.; Konstantinidou, F.; Stuppia, L.; Gatta, V. Exploring Maternal Diet-Epigenetic-Gut Microbiome Crosstalk as an Intervention Strategy to Counter Early Obesity Programming. Curr. Issues Mol. Biol. 2024, 46, 4358–4378. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2018, 1420, 5–25. [Google Scholar] [CrossRef]
- Mulle, J.G.; Sharp, W.G.; Cubells, J.F. The gut microbiome: A new frontier in autism research. Curr. Psychiatry Rep. 2013, 15, 337. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Padua, D.; Tillisch, K. Altered brain-gut axis in autism: Comorbidity or causative mechanisms? BioEssays 2014, 36, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: Pluses, perils, and pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef]
- Finegold, S.M.; Molitoris, D.; Song, Y.; Liu, C.; Vaisanen, M.L.; Bolte, E.; McTeague, M.; Sandler, R.; Wexler, H.; Marlowe, E.M.; et al. Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2002, 35 (Suppl. S1), S6–S16. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism–Comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- De Theije, C.G.; Wu, J.; Koelink, P.J.; Korte-Bouws, G.A.; Borre, Y.; Kas, M.J.; da Silva, S.L.; Korte, S.M.; Olivier, B.; Garssen, J.; et al. Autistic-like behavioural and neurochemical changes in a mouse model of food allergy. Behav. Brain Res. 2014, 261, 265–274. [Google Scholar] [CrossRef]
- MacFabe, D.F. Short-chain fatty acid fermentation products of the gut microbiome: Implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2012, 23, 19260. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Mark Welch, J.L.; Rossetti, B.J.; Rieken, C.W.; Dewhirst, F.E.; Borisy, G.G. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA 2016, 113, E791–E800. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Yamazaki, K. Can oral bacteria affect the microbiome of the gut? J. Oral. Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Diaz Heijtz, R.; Gressens, P.; Swann, J.R. Targeting microbial metabolites to treat autism. Nat. Med. 2022, 28, 448–450. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Luna, R.A.; Savidge, T.C.; Williams, K.C. The Brain-Gut-Microbiome Axis: What Role Does It Play in Autism Spectrum Disorder? Curr. Dev. Disord Rep. 2016, 3, 75–81. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry. 2018, 9, 44. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Argou-Cardozo, I.; Zeidán-Chuliá, F. Clostridium Bacteria and Autism Spectrum Conditions: A Systematic Review and Hypothetical Contribution of Environmental Glyphosate Levels. Med. Sci. 2018, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Mazuryk, J.; Klepacka, K.; Kutner, W.; Sharma, P.S. Glyphosate: Impact on the microbiota-gut-brain axis and the immune-nervous system, and clinical cases of multiorgan toxicity. Ecotoxicol. Environ. Saf. 2024, 271, 115965. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.; Olson, C.; Hsiao, E. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Finegold, S.M. State of the art; microbiology in health and disease. Intest. Bact. Flora Autism 2011, 52, 367–368. [Google Scholar]
- Eisenhofer, R.; Minich, J.J.; Marotz, C.; Cooper, A.; Knight, R.; Weyrich, L.S. Contamination in Low Microbial Biomass Microbiome Studies: Issues and Recommendations. Trends Microbiol. 2019, 27, 105–117. [Google Scholar] [CrossRef]
- Claesson, M.J.; O’Sullivan, O.; Wang, Q.; Nikkilä, J.; Marchesi, J.R.; Smidt, H.; De Vos, W.M.; Paul Ross, R.; O’Toole, P.W. Comparative Analysis of Pyrosequencing and a Phylogenetic Microarray for Exploring Microbial Community Structures in the Human Distal Intestine. PLoS ONE 2009, 4, e6669. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Minelli, M.; Anaclerio, F.; Calisi, D.; Onofrj, M.; Antonucci, I.; Gatta, V.; Stuppia, L. Application of Metagenomics Sequencing in a Patient with Dementia: A New Case Report. Genes 2024, 15, 1089. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Abuljadayel, D.; Alotibi, A.; Algothmi, K.; Basingab, F.; Alhazmi, S.; Almuhammadi, A.; Alharthi, A.; Alyoubi, R.; Bahieldin, A. Gut microbiota of children with autism spectrum disorder and healthy siblings: A comparative study. Exp. Ther. Med. 2024, 28, 430. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 2013, 4, 42. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Liu, X. The microbiota-gut-brain axis and neurodevelopmental disorders. Protein Cell 2023, 14, 762–775. [Google Scholar] [CrossRef]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef]
- Zuffa, S.; Schimmel, P.; Gonzalez-Santana, A.; Belzer, C.; Knol, J.; Bölte, S.; Falck-Ytter, T.; Forssberg, H.; Swann, J.; Diaz Heijtz, R. Early-life differences in the gut microbiota composition and functionality of infants at elevated likelihood of developing autism spectrum disorder. Transl. Psychiatry. 2023, 13, 257. [Google Scholar] [CrossRef]
- Morton, J.T.; Jin, D.-M.; Mills, R.H.; Shao, Y.; Rahman, G.; McDonald, D.; Zhu, Q.; Balaban, M.; Jiang, Y.; Cantrell, K.; et al. Multi-level analysis of the gut–brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat. Neurosci. 2023, 26, 1208–1217. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Coretti, L.; Paparo, L.; Riccio, M.P.; Amato, F.; Cuomo, M.; Natale, A.; Borrelli, L.; Corrado, G.; Comegna, M.; Buommino, E.; et al. Gut Microbiota Features in Young Children With Autism Spectrum Disorders. Front. Microbiol. 2018, 9, 3146. [Google Scholar] [CrossRef]
- Zhou, L.; Foster, J.A. Psychobiotics and the gut-brain axis: In the pursuit of happiness. Neuropsychiatr. Dis. Treat. 2015, 11, 715–723. [Google Scholar]
- Yang, Y.; Jobin, C. Microbial imbalance and intestinal pathologies: Connections and contributions. Dis. Model. Mech. 2014, 7, 1131–1142. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Taniya, M.A.; Chung, H.-J.; Al Mamun, A.; Alam, S.; Aziz, A.; Emon, N.U.; Islam, M.; Hong, S.-T.S.; Podder, B.R.; Mimi, A.A.; et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 915701. [Google Scholar] [CrossRef]
- Rook, G.A.W.; Raison, C.L.; Lowry, C.A. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin. Exp. Immunol. 2014, 177, 1–12. [Google Scholar] [CrossRef]
- Yap, C.X.; Henders, A.K.; Alvares, G.A.; Wood, D.L.A.; Krause, L.; Tyson, G.W.; Restuadi, R.; Wallace, L.; McLaren, T.; Hansell, N.K.; et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 2021, 184, 5916–5931.e17. [Google Scholar] [CrossRef]
- MacFabe, D.F. Enteric short-chain fatty acids: Microbial messengers of metabolism, mitochondria, and mind: Implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2015, 26, 28177. [Google Scholar] [CrossRef] [PubMed]
- Griffen, A.L.; Beall, C.J.; Campbell, J.H.; Firestone, N.D.; Kumar, P.S.; Yang, Z.K.; Podar, M.; Leys, E.J. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2011, 6, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.G.; Sarkar, S.; Umar, S.; Lee, S.T.M.; Thomas, S.M. The Contribution of the Human Oral Microbiome to Oral Disease: A Review. Microorganisms 2023, 11, 318. [Google Scholar] [CrossRef]
- Sureda, A.; Daglia, M.; Castilla, S.A.; Sanadgol, N.; Khan, H.; Belwal, T.; Jeandet, P.; Marchese, A.; Pistollato, F.; Forbes-Hernandez, T.; et al. Oral microbiota and Alzheimer’s disease: Do all roads lead to Rome? Pharmacol. Res. 2020, 151, 104582. [Google Scholar] [CrossRef]
- Bello-Corral, L.; Alves-Gomes, L.; Fernández-Fernández, J.A.; Fernández-García, D.; Casado-Verdejo, I.; Sánchez-Valdeón, L. Implications of gut and oral microbiota in neuroinflammatory responses in Alzheimer’s disease. Life Sci. 2023, 333, 122132. [Google Scholar] [CrossRef]
- Marsh, P.D.; Martin, M.V. Oral Microbiology, 6th ed; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wade, W.G. The oral microbiome in health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120334. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef]
- Kunath, B.J.; De Rudder, C.; Laczny, C.C.; Letellier, E.; Wilmes, P. The oral–gut microbiome axis in health and disease. Nat. Rev. Microbiol. 2024, 22, 791–805. [Google Scholar] [CrossRef]
- Lewandowska-Pietruszka, Z.; Figlerowicz, M.; Mazur-Melewska, K. Microbiota in Autism Spectrum Disorder: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 16660. [Google Scholar] [CrossRef]
- Mussap, M.; Beretta, P.; Esposito, E.; Fanos, V. Once upon a Time Oral Microbiota: A Cinderella or a Protagonist in Autism Spectrum Disorder? Metabolites 2023, 13, 1183. [Google Scholar] [CrossRef]
- Iljazovic, A.; Roy, U.; Gálvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021, 14, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Vuillermin, P.J.; O’hely, M.; Collier, F.; Allen, K.J.; Tang, M.L.K.; Harrison, L.C.; Carlin, J.B.; Saffery, R.; Ranganathan, S.; Sly, P.D.; et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Marventano, I.; Guerini, F.R.; Mancuso, R.; Ceresa, L.; Zanzottera, M.; Rusconi, B.; Maggioni, E.; Tinelli, C.; Clerici, M. An Autistic Endophenotype Results in Complex Immune Dysfunction in Healthy Siblings of Autistic Children. Biol. Psychiatry 2009, 66, 978–984. [Google Scholar] [CrossRef]
- Mead, J.; Ashwood, P. Evidence supporting an altered immune response in ASD. Immunol. Lett. 2015, 163, 49–55. [Google Scholar] [CrossRef]
- Pendyala, G.; Chou, S.; Jung, Y.; Coiro, P.; Spartz, E.; Padmashri, R.; Li, M.; Dunaevsky, A. Maternal Immune Activation Causes Behavioral Impairments and Altered Cerebellar Cytokine and Synaptic Protein Expression. Neuropsychopharmacology 2017, 42, 1435–1446. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef]
- Sandler, R.H.; Finegold, S.M.; Bolte, E.R.; Buchanan, C.P.; Maxwell, A.P.; Väisänen, M.L.; Nelson, M.N.; Wexler, H.M. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child Neurol. 2000, 15, 429–435. [Google Scholar] [CrossRef]
- He, X.; Liu, W.; Tang, F.; Chen, X.; Song, G. Effects of Probiotics on Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients 2023, 15, 1415. [Google Scholar] [CrossRef]
- Rajanala, K.; Kumar, N.; Chamallamudi, M.R. Modulation of Gut-Brain Axis by Probiotics: A Promising Anti-depressant Approach. Curr. Neuropharmacol. 2021, 19, 990–1006. [Google Scholar] [CrossRef]
- Rau, S.; Gregg, A.; Yaceczko, S.; Limketkai, B. Prebiotics and Probiotics for Gastrointestinal Disorders. Nutrients 2024, 16, 778. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nature reviews. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Cauli, O.; Guevara-Gonzaléz, J.; Guevara-Campos, J.; González, L. The Effects of Probiotics and Prebiotics on Gastrointestinal and Behavioural Symptoms in Autism Spectrum Disorder. Curr. Clin. Pharmacol. 2022, 17, 166–173. [Google Scholar] [CrossRef]
- Zafirovski, K.; Aleksoska, M.T.; Thomas, J.; Hanna, F. Impact of Gluten-Free and Casein-Free Diet on Behavioural Outcomes and Quality of Life of Autistic Children and Adolescents: A Scoping Review. Children 2024, 11, 862. [Google Scholar] [CrossRef]
- Baspinar, B.; Yardimci, H. Gluten-Free Casein-Free Diet for Autism Spectrum Disorders: Can It Be Effective in Solving Behavioural and Gastrointestinal Problems? Eurasian J. Med. 2020, 52, 292–297. [Google Scholar] [CrossRef]
- Knivsberg, A.; Reichelt, K.; Høien, T.; Nødland, M. A Randomised, Controlled Study of Dietary Intervention in Autistic Syndromes. Nutr. Neurosci. 2002, 5, 251–261. [Google Scholar] [CrossRef]
- Hu, C.; He, T.; Zou, B.; Li, H.; Zhao, J.; Hu, C.; Cui, J.; Huang, Z.; Shu, S.; Hao, Y. Fecal microbiota transplantation in a child with severe ASD comorbidities of gastrointestinal dysfunctions—A case report. Front. Psychiatry 2023, 14, 1219104. [Google Scholar] [CrossRef]
- Vendrik, K.E.W.; Ooijevaar, R.E.; De Jong, P.R.C.; Laman, J.D.; Van Oosten, B.W.; Van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, T. Fecal Microbiota Transplantation in Autism Spectrum Disorder. Neuropsychiatr. Dis. Treat. 2022, 18, 2905–2915. [Google Scholar] [CrossRef]
- Li, N.; Chen, H.; Cheng, Y.; Xu, F.; Ruan, G.; Ying, S.; Tang, W.; Chen, L.; Chen, M.; Lv, L.; et al. Fecal Microbiota Transplantation Relieves Gastrointestinal and Autism Symptoms by Improving the Gut Microbiota in an Open-Label Study. Front. Cell. Infect. Microbiol. 2021, 11, 759435. [Google Scholar] [CrossRef]
- Youngster, I.; Sauk, J.; Pindar, C.; Wilson, R.G.; Kaplan, J.L.; Smith, M.B.; Alm, E.J.; Gevers, D.; Russell, G.H.; Hohmann, E.L. Fecal Microbiota Transplant for Relapsing Clostridium difficile Infection Using a Frozen Inoculum From Unrelated Donors: A Randomized, Open-Label, Controlled Pilot Study. Clin. Infect. Dis. 2014, 58, 1515–1522. [Google Scholar] [CrossRef]
- Fregeac, J.; Colleaux, L.; Nguyen, L.S. The emerging roles of MicroRNAs in autism spectrum disorders. Neurosci. Biobehav. Rev. 2016, 71, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Salloum-Asfar, S.; Elsayed, A.K.; Elhag, S.F.; Abdulla, S.A. Circulating Non-Coding RNAs as a Signature of Autism Spectrum Disorder Symptomatology. Int. J. Mol. Sci. 2021, 22, 6549. [Google Scholar] [CrossRef] [PubMed]
- Stott, J.; Wright, T.; Holmes, J.; Wilson, J.; Griffiths-Jones, S.; Foster, D.; Wright, B. A systematic review of non-coding RNA genes with differential expression profiles associated with autism spectrum disorders. PLoS ONE. 2023, 18, e0287131. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, W.; Zheng, Y. Recent Progress on Relevant microRNAs in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 5904. [Google Scholar] [CrossRef]
- Lei, X.; Xie, X.N.; Yang, J.X.; Li, Y.M. The emerging role of extracellular vesicles in the diagnosis and treatment of autism spectrum disorders. Psychiatry Res. 2024, 337, 115954. [Google Scholar] [CrossRef]
- Anastasescu, C.M.; Gheorman, V.; Stoicanescu, E.C.; Popescu, F.; Gheorman, V.; Udriștoiu, I. Immunological Biomarkers in Autism Spectrum Disorder: The Role of TNF-Alpha and Dependent Trends in Serum IL-6 and CXCL8. Life 2024, 14, 1201. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]

| Study | Study Population | Results | Clinical Correlations |
|---|---|---|---|
| Zuffa S. et al., 2023 [49] | 60 children (30 ASD, 30 controls) | Increased Prevotella Decreased Bifidobacterium | Associated with greater severity of behavioral symptoms |
| Kang et al., 2017 [51] | 18 children (18 ASD) | Increased Clostridium Reduced total microbial diversity | Related with gastrointestinal and behavioral symptoms |
| Strati et al., 2018 [52] | 40 children (20 ASD, 20 controls) | Increased Ruminococcus Decreased Bacteroides | Associated with dysbiosis and gastrointestinal symptoms |
| Coretti et al., 2017 [53] | 39 children (27 ASD, 12 controls) | Increased in Lactobacillus and Clostridium Decreased in Bifidobacterium | Related with gastrointestinal symptoms and behavioral changes |
| Liu et al., 2019 [28] | 90 children (60 ASD, 30 controls) | Increased Sutterella Decreased Prevotella | Related with gut dysbiosis and inflammation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaclerio, F.; Minelli, M.; Antonucci, I.; Gatta, V.; Stuppia, L. Microbiota and Autism: A Review on Oral and Gut Microbiome Analysis Through 16S rRNA Sequencing. Biomedicines 2024, 12, 2686. https://doi.org/10.3390/biomedicines12122686
Anaclerio F, Minelli M, Antonucci I, Gatta V, Stuppia L. Microbiota and Autism: A Review on Oral and Gut Microbiome Analysis Through 16S rRNA Sequencing. Biomedicines. 2024; 12(12):2686. https://doi.org/10.3390/biomedicines12122686
Chicago/Turabian StyleAnaclerio, Federico, Maria Minelli, Ivana Antonucci, Valentina Gatta, and Liborio Stuppia. 2024. "Microbiota and Autism: A Review on Oral and Gut Microbiome Analysis Through 16S rRNA Sequencing" Biomedicines 12, no. 12: 2686. https://doi.org/10.3390/biomedicines12122686
APA StyleAnaclerio, F., Minelli, M., Antonucci, I., Gatta, V., & Stuppia, L. (2024). Microbiota and Autism: A Review on Oral and Gut Microbiome Analysis Through 16S rRNA Sequencing. Biomedicines, 12(12), 2686. https://doi.org/10.3390/biomedicines12122686






