Examining Erythrocytes as Potential Blood Biomarkers for Autism Spectrum Disorder: Their Relationship to Symptom Severity and Adaptive Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. ASD Symptom Assessment
2.3. Assessment of Adaptive Behavior
2.4. Blood Processing
2.5. Erythrocyte Deformability Measurements
2.6. Erythrocyte Nitric Oxide Production
2.7. Assessment of Erythrocyte Osmotic Resistance
2.8. Erythrocyte Membrane Isolation and Na,K-ATPase Enzyme Kinetics Measurements
2.9. Statistical Analyses
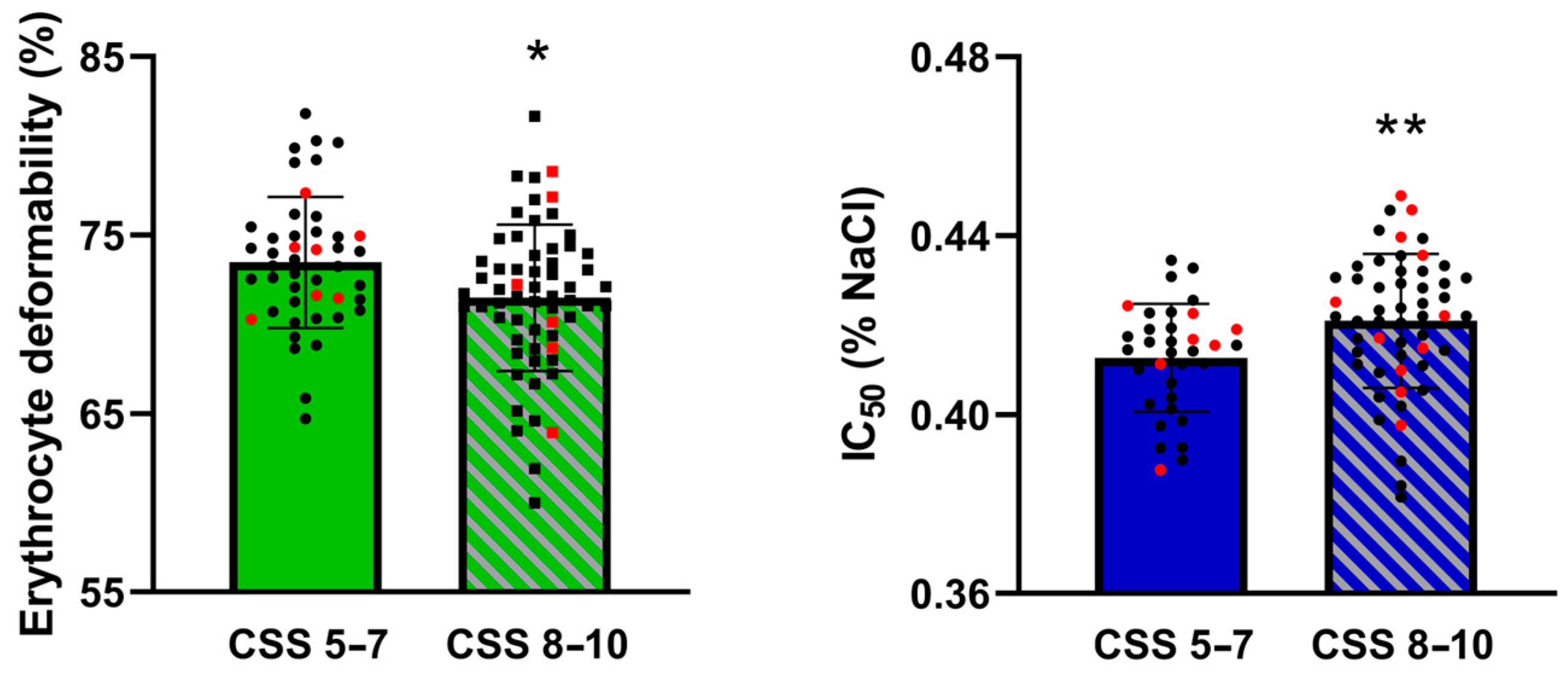
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Muskens, J.B.; Velders, F.P.; Staal, W.G. Medical Comorbidities in Children and Adolescents with Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorders: A Systematic Review. Eur. Child. Adolesc. Psychiatry 2017, 26, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Yip, B.H.K.; Windham, G.C.; Sourander, A.; Francis, R.; Yoffe, R.; Glasson, E.; Mahjani, B.; Suominen, A.; Leonard, H.; et al. Association of Genetic and Environmental Factors With Autism in a 5-Country Cohort. JAMA Psychiatry 2019, 76, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Bölte, S.; Girdler, S.; Marschik, P.B. The Contribution of Environmental Exposure to the Etiology of Autism Spectrum Disorder. Cell Mol. Life Sci. 2018, 76, 1275–1297. [Google Scholar] [CrossRef] [PubMed]
- Hultman, C.M.; Sandin, S.; Levine, S.Z.; Lichtenstein, P.; Reichenberg, A. Advancing Paternal Age and Risk of Autism: New Evidence from a Population-Based Study and a Meta-Analysis of Epidemiological Studies. Mol. Psychiatry 2011, 16, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Slama, S.; Bahia, W.; Soltani, I.; Gaddour, N.; Ferchichi, S. Risk Factors in Autism Spectrum Disorder: A Tunisian Case-Control Study. Saudi J. Biol. Sci. 2022, 29, 2749–2755. [Google Scholar] [CrossRef]
- Tseng, P.-T.; Chen, Y.-W.; Stubbs, B.; Carvalho, A.F.; Whiteley, P.; Tang, C.-H.; Yang, W.-C.; Chen, T.-Y.; Li, D.-J.; Chu, C.-S.; et al. Maternal Breastfeeding and Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis. Nutr. Neurosci. 2019, 22, 354–362. [Google Scholar] [CrossRef]
- Yenkoyan, K.; Mkhitaryan, M.; Bjørklund, G. Environmental Risk Factors in Autism Spectrum Disorder: A Narrative Review. Curr. Med. Chem. 2024, 31, 2345–2360. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global Prevalence of Autism: A Systematic Review Update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Baron-Cohen, S. The Extreme Male Brain Theory of Autism. Trends Cogn. Sci. 2002, 6, 248–254. [Google Scholar] [CrossRef]
- Robinson, E.B.; Lichtenstein, P.; Anckarsäter, H.; Happé, F.; Ronald, A. Examining and Interpreting the Female Protective Effect against Autistic Behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 5258–5262. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; Frye, R.E.; James, S.J. Evidence of Oxidative Damage and Inflammation Associated with Low Glutathione Redox Status in the Autism Brain. Transl. Psychiatry 2012, 2, e134. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Lionnard, L.; Singh, I.; Karim, M.A.; Chajra, H.; Frechet, M.; Kissa, K.; Racine, V.; Ammanamanchi, A.; McCarty, P.J.; et al. Mitochondrial Morphology Is Associated with Respiratory Chain Uncoupling in Autism Spectrum Disorder. Transl. Psychiatry 2021, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, J.; Zhang, H.; Khan, N.U.; Zhang, J.; Tang, X.; Cao, X.; Shen, L. Oxidative Stress in Autism Spectrum Disorder—Current Progress of Mechanisms and Biomarkers. Front. Psychiatry 2022, 13, 813304. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Narayan, C.L.; Mahesan, A.; Kamila, G.; Kapoor, S.; Chaturvedi, P.K.; Scaria, V.; Velpandian, T.; Jauhari, P.; Chakrabarty, B.; et al. Transmethylation and Oxidative Biomarkers in Children with Autism Spectrum Disorder: A Cross Sectional Study. J. Autism Dev. Disord. 2024. [Google Scholar] [CrossRef]
- Johnson, A.J.; Shankland, E.; Richards, T.; Corrigan, N.; Shusterman, D.; Edden, R.; Estes, A.; St. John, T.; Dager, S.; Kleinhans, N.M. Relationships between GABA, Glutamate, and GABA/Glutamate and Social and Olfactory Processing in Children with Autism Spectrum Disorder. Psychiatry Res. Neuroimaging 2023, 336, 111745. [Google Scholar] [CrossRef]
- Bjørklund, G.; Kern, J.; Urbina, M.; Saad, K.; El-Houfey, A.; Geier, D.; Chirumbolo, S.; Geier, M.; Mehta, J.; Aaseth, J. Cerebral Hypoperfusion in Autism Spectrum Disorder. Acta Neurobiol. Exp. 2018, 78, 21–29. [Google Scholar] [CrossRef]
- Bibic, A.; Sordia, T.; Henningsson, E.; Knutsson, L.; Ståhlberg, F.; Wirestam, R. Effects of Red Blood Cells with Reduced Deformability on Cerebral Blood Flow and Vascular Water Transport: Measurements in Rats Using Time-Resolved Pulsed Arterial Spin Labelling at 9.4 T. Eur. Radiol. Exp. 2021, 5, 53. [Google Scholar] [CrossRef]
- Zhang, H.; Sumbria, R.K.; Chang, R.; Sun, J.; Cribbs, D.H.; Holmes, T.C.; Fisher, M.J.; Xu, X. Erythrocyte–Brain Endothelial Interactions Induce Microglial Responses and Cerebral Microhemorrhages in Vivo. J. Neuroinflamm. 2023, 20, 265. [Google Scholar] [CrossRef]
- Ciccoli, L.; De Felice, C.; Paccagnini, E.; Leoncini, S.; Pecorelli, A.; Signorini, C.; Belmonte, G.; Guerranti, R.; Cortelazzo, A.; Gentile, M.; et al. Erythrocyte Shape Abnormalities, Membrane Oxidative Damage, and β-Actin Alterations: An Unrecognized Triad in Classical Autism. Mediat. Inflamm. 2013, 2013, 432616. [Google Scholar] [CrossRef]
- Bolotta, A.; Battistelli, M.; Falcieri, E.; Ghezzo, A.; Manara, M.C.; Manfredini, S.; Marini, M.; Posar, A.; Visconti, P.; Abruzzo, P.M. Oxidative Stress in Autistic Children Alters Erythrocyte Shape in the Absence of Quantitative Protein Alterations and of Loss of Membrane Phospholipid Asymmetry. Oxidative Med. Cell. Longev. 2018, 2018, e6430601. [Google Scholar] [CrossRef]
- László, A.; Novák, Z.; Szőllősi-Varga, I.; Hai, D.Q.; Vetró, Á.; Kovács, A. Blood Lipid Peroxidation, Antioxidant Enzyme Activities and Hemorheological Changes in Autistic Children. Ideggyogy. Sz. 2013, 66, 23–28. [Google Scholar] [PubMed]
- Jasenovec, T.; Radosinska, D.; Jansakova, K.; Kopcikova, M.; Tomova, A.; Snurikova, D.; Vrbjar, N.; Radosinska, J. Alterations in Antioxidant Status and Erythrocyte Properties in Children with Autism Spectrum Disorder. Antioxidants 2023, 12, 2054. [Google Scholar] [CrossRef] [PubMed]
- Radosinska, J.; Vrbjar, N. Erythrocyte Deformability and Na,K-ATPase Activity in Various Pathophysiological Situations and Their Protection by Selected Nutritional Antioxidants in Humans. Int. J. Mol. Sci. 2021, 22, 11924. [Google Scholar] [CrossRef] [PubMed]
- Sprague, R.S.; Bowles, E.A.; Achilleus, D.; Ellsworth, M.L. Erythrocytes as Controllers of Perfusion Distribution in the Microvasculature of Skeletal Muscle. Acta Physiol. 2011, 202, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Rodriguez-Mateos, A.; Sansone, R.; Kuhnle, G.G.C.; Thasian-Sivarajah, S.; Krenz, T.; Horn, P.; Krisp, C.; Wolters, D.; Heiß, C.; et al. Human Red Blood Cells at Work: Identification and Visualization of Erythrocytic eNOS Activity in Health and Disease. Blood 2012, 120, 4229–4237. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Detterich, J.A.; Connes, P. Nitric Oxide, Vasodilation and the Red Blood Cell. Biorheology 2014, 51, 121–134. [Google Scholar] [CrossRef]
- Söğüt, S.; Zoroğlu, S.S.; Ozyurt, H.; Yilmaz, H.R.; Ozuğurlu, F.; Sivasli, E.; Yetkin, O.; Yanik, M.; Tutkun, H.; Savaş, H.A.; et al. Changes in Nitric Oxide Levels and Antioxidant Enzyme Activities May Have a Role in the Pathophysiological Mechanisms Involved in Autism. Clin. Chim. Acta 2003, 331, 111–117. [Google Scholar] [CrossRef]
- Tostes, M.H.F.S.; Teixeira, H.C.; Gattaz, W.F.; Brandão, M.a.F.; Raposo, N.R.B. Altered Neurotrophin, Neuropeptide, Cytokines and Nitric Oxide Levels in Autism. Pharmacopsychiatry 2012, 45, 241–243. [Google Scholar] [CrossRef]
- Zoroğlu, S.S.; Yürekli, M.; Meram, I.; Söğüt, S.; Tutkun, H.; Yetkin, O.; Sivasli, E.; Savaş, H.A.; Yanik, M.; Herken, H.; et al. Pathophysiological Role of Nitric Oxide and Adrenomedullin in Autism. Cell Biochem. Funct. 2003, 21, 55–60. [Google Scholar] [CrossRef]
- Radosinska, J.; Vrbjar, N. The Role of Red Blood Cell Deformability and Na,K-ATPase Function in Selected Risk Factors of Cardiovascular Diseases in Humans: Focus on Hypertension, Diabetes Mellitus and Hypercholesterolemia. Physiol. Res. 2016, 65, S43–S54. [Google Scholar] [CrossRef]
- Ghezzo, A.; Visconti, P.; Abruzzo, P.M.; Bolotta, A.; Ferreri, C.; Gobbi, G.; Malisardi, G.; Manfredini, S.; Marini, M.; Nanetti, L.; et al. Oxidative Stress and Erythrocyte Membrane Alterations in Children with Autism: Correlation with Clinical Features. PLoS ONE 2013, 8, e66418. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, D.A.; Brinkmann, C.; Bloch, W.; Grau, M. Increase in Red Blood Cell-Nitric Oxide Synthase Dependent Nitric Oxide Production during Red Blood Cell Aging in Health and Disease: A Study on Age Dependent Changes of Rheologic and Enzymatic Properties in Red Blood Cells. PLoS ONE 2015, 10, e0125206. [Google Scholar] [CrossRef] [PubMed]
- Tomschi, F.; Bloch, W.; Grau, M. Impact of Type of Sport, Gender and Age on Red Blood Cell Deformability of Elite Athletes. Int. J. Sports Med. 2017, 40, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Jasenovec, T.; Radosinska, D.; Celusakova, H.; Filcikova, D.; Babinska, K.; Ostatnikova, D.; Radosinska, J. Erythrocyte Deformability in Children with Autism Spectrum Disorder: Correlation with Clinical Features. Physiol. Res. 2019, 68, S307–S313. [Google Scholar] [CrossRef] [PubMed]
- Gotham, K.; Pickles, A.; Lord, C. Standardizing ADOS Scores for a Measure of Severity in Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 693–705. [Google Scholar] [CrossRef]
- Sparrow, S.S.; Saulnier, C.A.; Cicchetti, D.V.; Doll, E.A. Vineland-3: Vineland Adaptive Behavior Scales; PsychCorp: Bloomington, MN, USA, 2016. [Google Scholar]
- Radosinska, J.; Jasenovec, T.; Puzserova, A.; Krajcir, J.; Lacekova, J.; Kucerova, K.; Kalnovicova, T.; Tothova, L.; Kovacicova, I.; Vrbjar, N. Promotion of Whole Blood Rheology after Vitamin C Supplementation: Focus on Red Blood Cells 1. Can. J. Physiol. Pharmacol. 2019, 97, 837–843. [Google Scholar] [CrossRef]
- Jasenovec, T.; Radosinska, D.; Kollarova, M.; Balis, P.; Ferenczyova, K.; Kalocayova, B.; Bartekova, M.; Tothova, L.; Radosinska, J. Beneficial Effect of Quercetin on Erythrocyte Properties in Type 2 Diabetic Rats. Molecules 2021, 26, 4868. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Taussky, H.H.; Shorr, E. A Microcolorimetric Method for the Determination of Inorganic Phosphorus. J. Biol. Chem. 1953, 202, 675–685. [Google Scholar] [CrossRef]
- Ji, L.; Chauhan, A.; Brown, W.T.; Chauhan, V. Increased Activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase in the Frontal Cortex and Cerebellum of Autistic Individuals. Life Sci. 2009, 85, 788–793. [Google Scholar] [CrossRef]
- El-Ansary, A.; Bjørklund, G.; Khemakhem, A.M.; Al-Ayadhi, L.; Chirumbolo, S.; Ben Bacha, A. Metabolism-Associated Markers and Childhood Autism Rating Scales (CARS) as a Measure of Autism Severity. J. Mol. Neurosci. 2018, 65, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.G.; Stern, A. Effects of Physiologic Concentrations of Lactate, Pyruvate and Ascorbate on Glucose Metabolism in Unstressed and Oxidatively Stressed Human Red Blood Cells. Biochem. Pharmacol. 1983, 32, 2891–2902. [Google Scholar] [CrossRef] [PubMed]
- Tilton, W.M.; Seaman, C.; Carriero, D.; Piomelli, S. Regulation of Glycolysis in the Erythrocyte: Role of the Lactate/Pyruvate and NAD/NADH Ratios. J. Lab. Clin. Med. 1991, 118, 146–152. [Google Scholar] [PubMed]
- Evtugina, N.G.; Peshkova, A.D.; Khabirova, A.I.; Andrianova, I.A.; Abdullayeva, S.; Ayombil, F.; Shepeliuk, T.; Grishchuk, E.L.; Ataullakhanov, F.I.; Litvinov, R.I.; et al. Activation of Piezo1 Channels in Compressed Red Blood Cells Augments Platelet-Driven Contraction of Blood Clots. J. Thromb. Haemost. 2023, 21, 2418–2429. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, S.M.; Lukacs, V.; Ranade, S.S.; Chien, S.; Bandell, M.; Patapoutian, A. Piezo1 Links Mechanical Forces to Red Blood Cell Volume. eLife 2015, 4, e07370. [Google Scholar] [CrossRef]
- Bae, C.; Gnanasambandam, R.; Nicolai, C.; Sachs, F.; Gottlieb, P.A. Xerocytosis Is Caused by Mutations That Alter the Kinetics of the Mechanosensitive Channel PIEZO1. Proc. Natl. Acad. Sci. USA 2013, 110, E1162–E1168. [Google Scholar] [CrossRef]
- Shahidullah, M.; Rosales, J.L.; Delamere, N. Activation of Piezo1 Increases Na,K-ATPase-Mediated Ion Transport in Mouse Lens. Int. J. Mol. Sci. 2022, 23, 12870. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhu, T.; Qu, Y.; Mu, D. Blood Glutamate Levels in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158688. [Google Scholar] [CrossRef]
- Makhro, A.; Wang, J.; Vogel, J.; Boldyrev, A.A.; Gassmann, M.; Kaestner, L.; Bogdanova, A. Functional NMDA Receptors in Rat Erythrocytes. Am. J. Physiol.-Cell Physiol. 2010, 298, C1315–C1325. [Google Scholar] [CrossRef]
- Gunes, S.; Ekinci, O.; Celik, T. Iron Deficiency Parameters in Autism Spectrum Disorder: Clinical Correlates and Associated Factors. Ital. J. Pediatr. 2017, 43, 86. [Google Scholar] [CrossRef]
- Sudnitsyna, J.; Skverchinskaya, E.; Dobrylko, I.I.; Nikitina, E.; Gambaryan, S.; Mindukshev, I. Microvesicle Formation Induced by Oxidative Stress in Human Erythrocytes. Antioxidants 2020, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Kuhad, A.; Bhandari, R. Nitric Oxide Pathway as a Plausible Therapeutic Target in Autism Spectrum Disorders. Expert. Opin. Ther. Targets 2022, 26, 659–679. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Meguid, N.A.; El-Ansary, A.; El-Bana, M.A.; Dadar, M.; Aaseth, J.; Hemimi, M.; Osredkar, J.; Chirumbolo, S. Diagnostic and Severity-Tracking Biomarkers for Autism Spectrum Disorder. J. Mol. Neurosci. 2018, 66, 492–511. [Google Scholar] [CrossRef] [PubMed]
- Sweeten, T.L.; Posey, D.J.; Shankar, S.; McDougle, C.J. High Nitric Oxide Production in Autistic Disorder: A Possible Role for Interferon-γ. Biol. Psychiatry 2004, 55, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Priya, M.D.; Geetha, A. A Biochemical Study on the Level of Proteins and Their Percentage of Nitration in the Hair and Nail of Autistic Children. Clin. Chim. Acta 2011, 412, 1036–1042. [Google Scholar] [CrossRef]
- Yao, L.; Fu, H.; Bai, L.; Deng, W.; Xie, F.; Li, Y.; Zhang, R.; Xu, X.; Wang, T.; Lai, S.; et al. Saliva Nitrite Is Higher in Male Children with Autism Spectrum Disorder and Positively Correlated with Serum Nitrate. Redox Rep. 2021, 26, 124–133. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Ojha, S.K.; Kartawy, M.; Hamoudi, W.; Choudhary, A.; Stern, S.; Aran, A.; Amal, H. The NO Answer for Autism Spectrum Disorder. Adv. Sci. 2023, 10, 2205783. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Luo, S.; Zhao, K.; Gao, C.; Mei, D.; Duan, Y.; Hu, S. Restoration of nNOS Expression Rescues Autistic-Like Phenotypes Through Normalization of AMPA Receptor-Mediated Neurotransmission. Mol. Neurobiol. 2024, 61, 6599–6612. [Google Scholar] [CrossRef]
- Kuck, L.; Peart, J.N.; Simmonds, M.J. Piezo1 Regulates Shear-Dependent Nitric Oxide Production in Human Erythrocytes. Am. J. Physiol.-Heart Circ. Physiol. 2022, 323, H24–H37. [Google Scholar] [CrossRef]
- Korbut, R.; Gryglewski, R.J. Nitric Oxide from Polymorphonuclear Leukocytes Modulates Red Blood Cell Deformability in Vitro. Eur. J. Pharmacol. 1993, 234, 17–22. [Google Scholar] [CrossRef]
- Farag, F.; Sims, A.; Strudwick, K.; Carrasco, J.; Waters, A.; Ford, V.; Hopkins, J.; Whitlingum, G.; Absoud, M.; Kelly, V.B. Avoidant/Restrictive Food Intake Disorder and Autism Spectrum Disorder: Clinical Implications for Assessment and Management. Dev. Med. Child. Neurol. 2022, 64, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.; Barrett, C.; Shepherd, C.; Charles, J.; Kalivas, B. Pancytopenia Due to Restrictive Food Intake in an Autistic Adult. J. Investig. Med. High. Impact Case Rep. 2022, 10, 23247096221139260. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Wang, C.-W.; Chen, H.-C.; Tu, H.-P.; Chen, S.-C.; Hung, C.-H.; Kuo, C.-H. Gender Difference in the Associations among Heavy Metals with Red Blood Cell Hemogram. Int. J. Environ. Res. Public. Health 2021, 19, 189. [Google Scholar] [CrossRef] [PubMed]
- Natoli, K.; Brown, A.; Bent, C.A.; Luu, J.; Hudry, K. No Sex Differences in Core Autism Features, Social Functioning, Cognition or Co-Occurring Conditions in Young Autistic Children: A Systematic Review and Meta-Analysis. Res. Autism Spectr. Disord. 2023, 107, 102207. [Google Scholar] [CrossRef]
| Mean ± SD | |
|---|---|
| Erythrocyte Parameter | |
| Deformability (%) | 72.3 ± 4.1 |
| Osmotic resistance-IC50 (% NaCl) | 0.418 ± 0.014 |
| Mean cell volume (fl) | 77.1 ± 4.5 |
| Nitric oxide production (a.u.) | 986 ± 106 |
| Vmax (µM Pi/mg protein/h) | 2.66 ± 0.71 |
| KNa (mM NaCl) | 30.5 ± 13.7 |
| median (IQR) | |
| ADOS-2 parameter | |
| Social affect | 7 (6–8) |
| Repetitive and restricted behavior | 9 (7–10) |
| Calibrated severity score | 8 (7–9) |
| VABS-3 parameter | |
| Communication | |
| -Receptive | 5.5 (3–8) |
| -Expressive | 5 (3–8) |
| -Written | 11 (8–12) |
| Daily living skills | |
| -Personal | 9 (8–11) |
| -Domestic | 11 (9–12) |
| -Community | 9 (8–10) |
| Socialization | |
| -Interpersonal relationships | 8 (7–9) |
| -Play and leisure time | 8 (6–10) |
| -Coping skills | 8.5 (8–10) |
| ADOS-2/VABS-3 Tool | Erythrocyte Parameter | p Value (Unadjusted) | Spearman’s R | N | Significance (FDR = 0.2) | |
|---|---|---|---|---|---|---|
| VABS-3 | Written | Deformability | 0.029 | 0.34 | 41 | no |
| Community | Deformability | 0.007 | 0.41 | 41 | yes | |
| Expressive | Nitric oxide | 0.037 | 0.35 | 36 | no | |
| Personal | Nitric oxide | 0.020 | 0.39 | 36 | yes | |
| Domestic | Nitric oxide | 0.043 | 0.40 | 26 | no | |
| Interpersonal relationships | Nitric oxide | 0.038 | 0.35 | 36 | no | |
| Play and leisure time | Nitric oxide | 0.014 | 0.41 | 36 | yes | |
| ADOS-2 | Social affect | Nitric oxide | 0.038 | −0.25 | 70 | no |
| Calibrated severity score | Osmotic resistance | 0.006 | 0.29 | 89 | yes | |
| Repetitive and restricted behavior | Osmotic resistance | 0.009 | 0.27 | 89 | yes | |
| Repetitive and restricted behavior | Mean cell volume | 0.047 | −0.19 | 106 | no | |
| Calibrated severity score | Mean cell volume | 0.034 | −0.21 | 106 | no | |
| Repetitive and restricted behavior | KNa | 0.018 | −0.29 | 67 | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasenovec, T.; Radosinska, D.; Belica, I.; Raskova, B.; Puzserova, A.; Vrbjar, N.; Radosinska, J. Examining Erythrocytes as Potential Blood Biomarkers for Autism Spectrum Disorder: Their Relationship to Symptom Severity and Adaptive Behavior. Biomedicines 2024, 12, 2619. https://doi.org/10.3390/biomedicines12112619
Jasenovec T, Radosinska D, Belica I, Raskova B, Puzserova A, Vrbjar N, Radosinska J. Examining Erythrocytes as Potential Blood Biomarkers for Autism Spectrum Disorder: Their Relationship to Symptom Severity and Adaptive Behavior. Biomedicines. 2024; 12(11):2619. https://doi.org/10.3390/biomedicines12112619
Chicago/Turabian StyleJasenovec, Tomas, Dominika Radosinska, Ivan Belica, Barbara Raskova, Angelika Puzserova, Norbert Vrbjar, and Jana Radosinska. 2024. "Examining Erythrocytes as Potential Blood Biomarkers for Autism Spectrum Disorder: Their Relationship to Symptom Severity and Adaptive Behavior" Biomedicines 12, no. 11: 2619. https://doi.org/10.3390/biomedicines12112619
APA StyleJasenovec, T., Radosinska, D., Belica, I., Raskova, B., Puzserova, A., Vrbjar, N., & Radosinska, J. (2024). Examining Erythrocytes as Potential Blood Biomarkers for Autism Spectrum Disorder: Their Relationship to Symptom Severity and Adaptive Behavior. Biomedicines, 12(11), 2619. https://doi.org/10.3390/biomedicines12112619







