Humanin-G Ameliorates Hemorrhage-Induced Acute Lung Injury in Mice Through AMPKα1-Dependent and -Independent Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Model of Hemorrhagic Shock
2.2. Preparation of PEGylated Humanin-G
2.3. Histopathologic Analysis
2.4. Lung Myeloperoxidase Assay
2.5. Lung Levels of ATP
2.6. Cytosol and Nuclear Extracts
2.7. Western Blot Analysis
2.8. Materials and Reagents
2.9. Statistical Analysis
3. Results
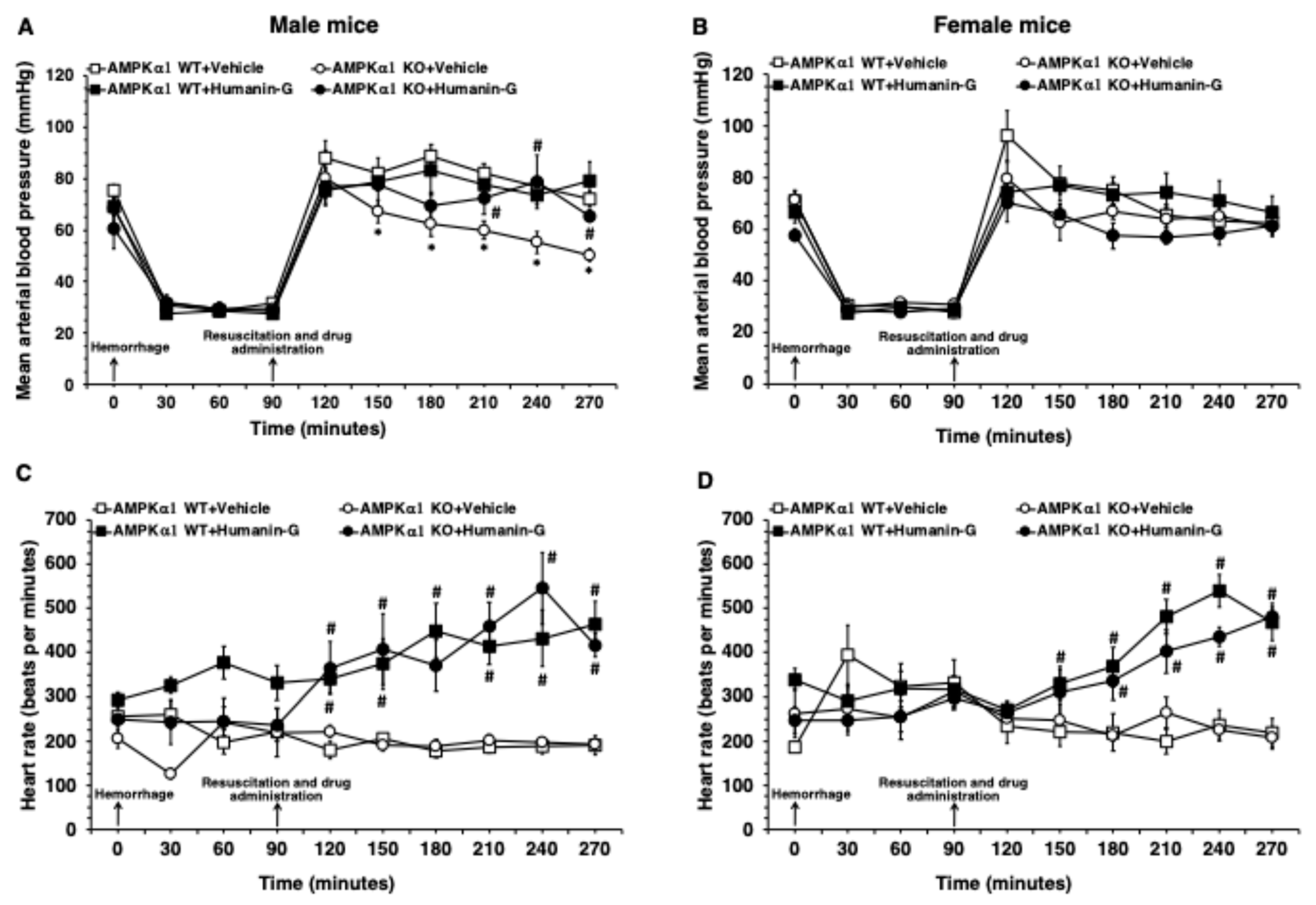
3.1. In Vivo Administration of PEGylated Humanin-G Ameliorates MABP in Male AMPKα1 KO After Hemorrhagic Shock
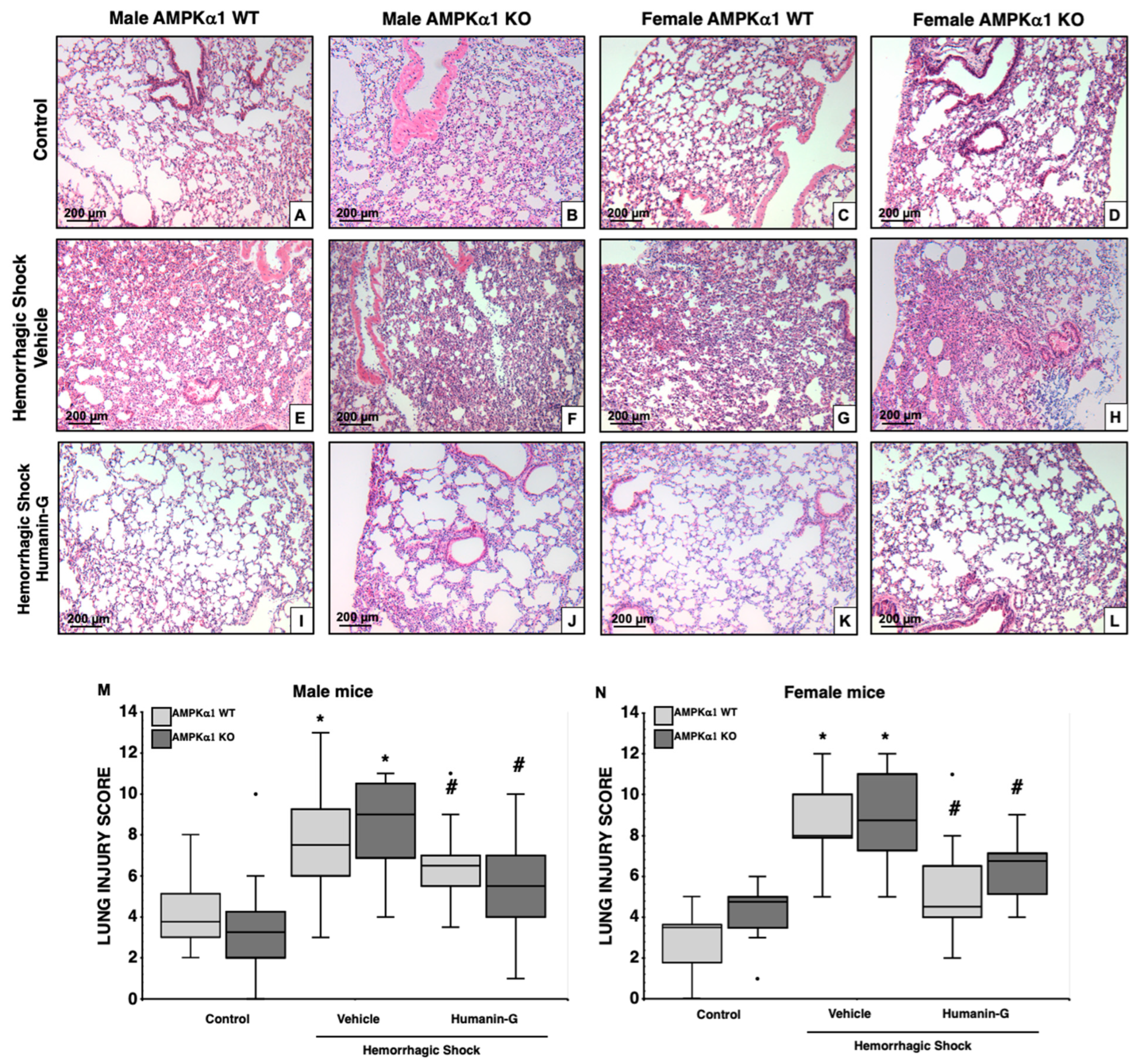
3.2. In Vivo Administration of PEGylated Humanin-G Improves Lung Architecture, but Not Neutrophil Infiltration, in an AMPKα1-Independent Manner
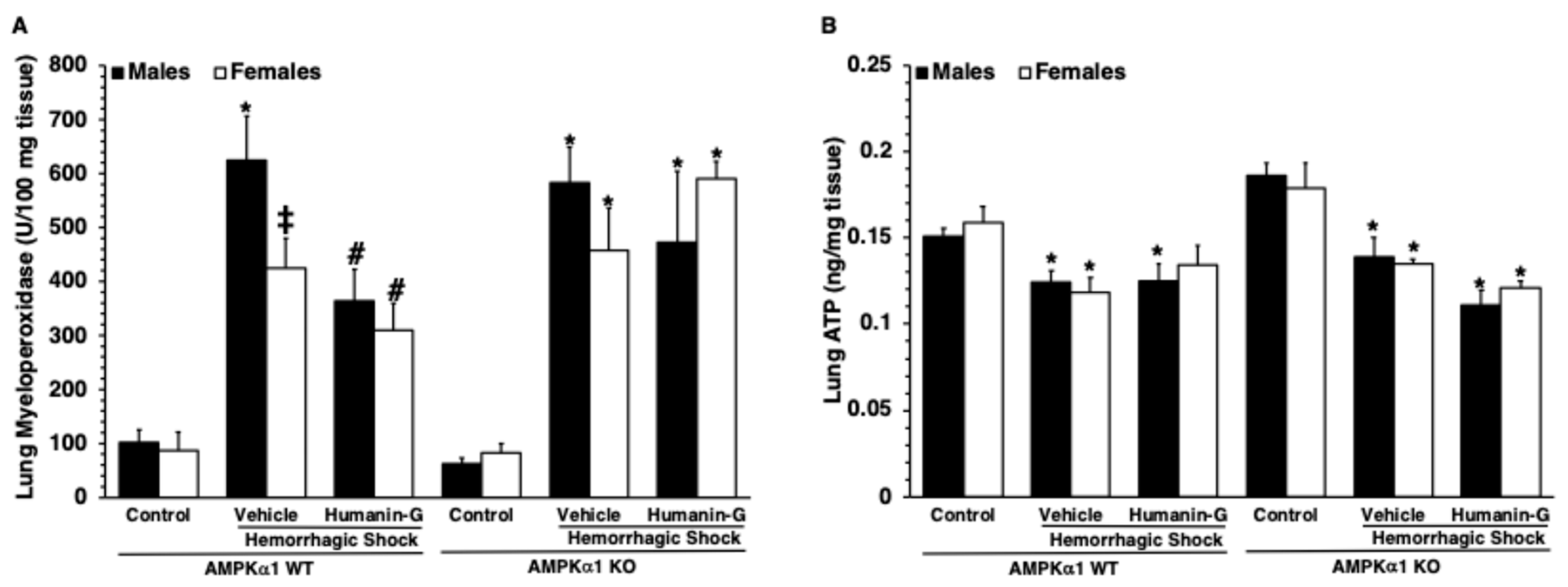
3.3. In Vivo Administration of PEGylated Humanin-G Did Not Modify Content of Lung ATP
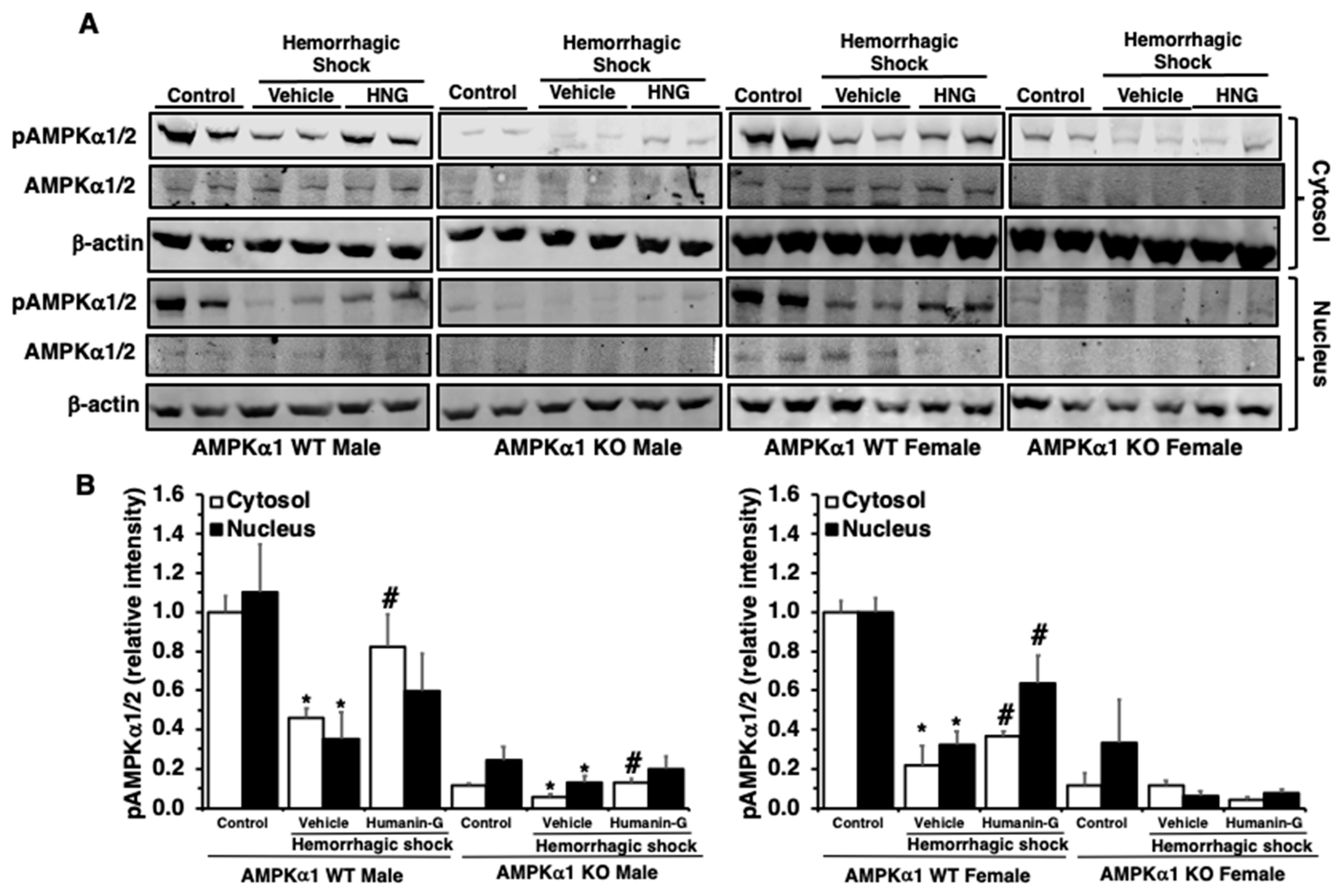
3.4. In Vivo Administration of PEGylated Humanin-G Restores Activation of AMPKα1 in the Lung
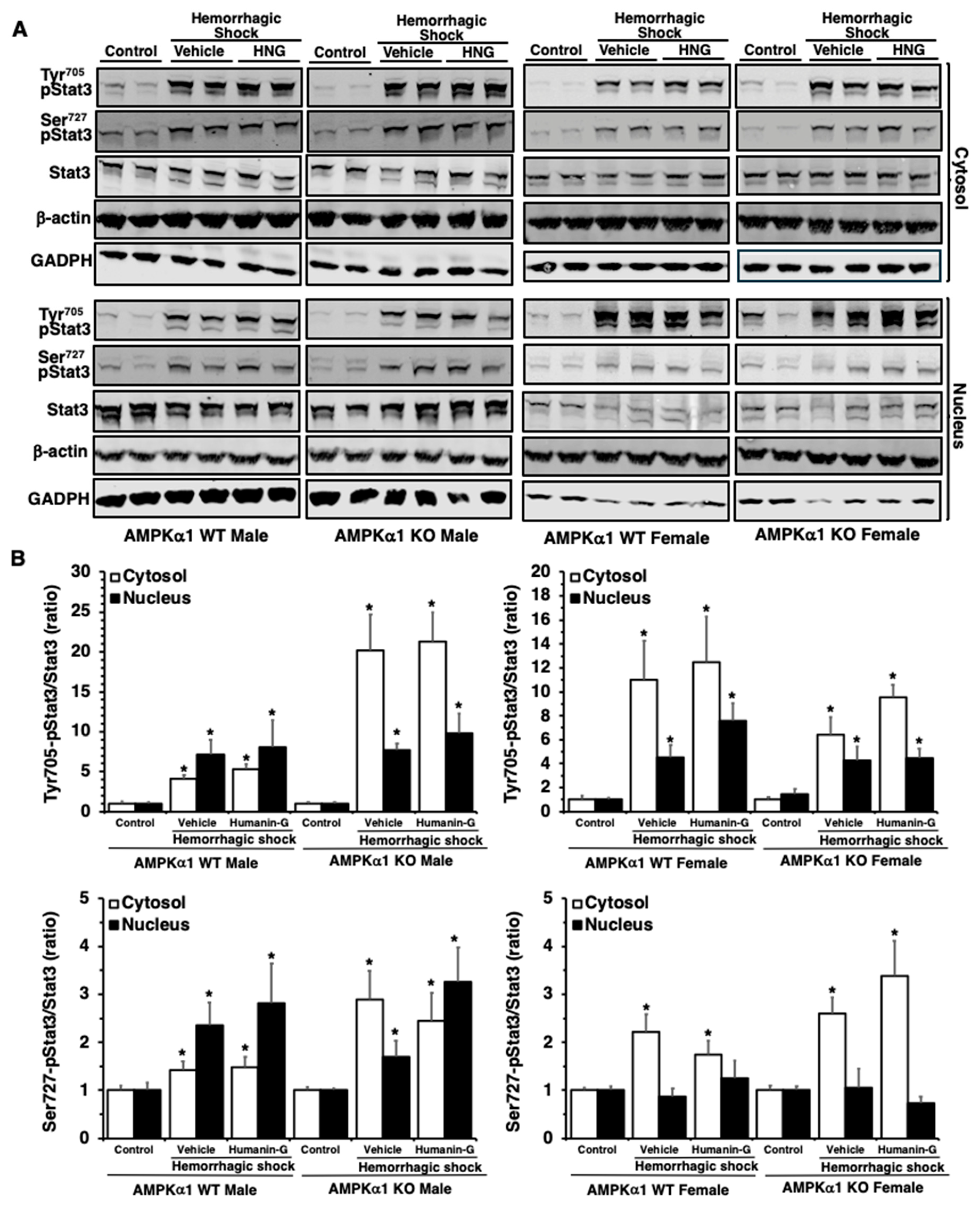
3.5. In Vivo Administration of PEGylated Humanin-G Does Not Modify Activation of STAT3 in the Lung
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eworuke, E.; Major, J.M.; Gilbert McClain, L.I. National incidence rates for Acute Respiratory Distress Syndrome (ARDS) and ARDS cause-specific factors in the United States (2006–2014). J. Crit. Care 2018, 47, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Kauvar, D.S.; Lefering, R.; Wade, C.E. Impact of Hemorrhage on Trauma Outcome: An Overview of Epidemiology, Clinical Presentations, and Therapeutic Considerations. J. Trauma 2006, 60, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, R.; Beekley, A.; Caset, L.; Salim, A.; Martin, M. The impact of advanced age on trauma triage decisions and outcomes: A statewide analysis. Am. J. Surg. 2009, 172, 571–575. [Google Scholar] [CrossRef]
- Dewar, D.C.; Tarrant, S.M.; King, K.L.; Balogh, Z.J. Changes in the epidemiology and prediction of multiple-organ failure after injury. J. Trauma Acute Care Surg. 2013, 74, 774–779. [Google Scholar] [CrossRef]
- Angele, M.K.; Schneider, C.P.; Chaudry, I.H. Bench-to-bedside review: Latest results in hemorrhagic shock. Crit. Care 2008, 12, 218. [Google Scholar] [CrossRef]
- Liu, T.; Xie, J.; Yang, F.; Chen, J.J.; Li, Z.F.; Yi, C.L.; Gao, W.; Bai, X.J. The influence of sex on outcomes in trauma patients: A meta-analysis. Am. J. Surg. 2015, 210, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.L.; Nathens, A.B.; Frankel, H.L.; Vanek, S.L.; Moore, E.E.; Maier, R.V.; Minei, J.P.; Inflammation and the Host Response to Injury Investigators. Characterization of the gender dimorphism after injury and hemorrhagic shock: Are hormonal differences responsible? Crit. Care Med. 2008, 36, 1838–1845. [Google Scholar] [CrossRef]
- Kamran, M.; Kamal, E.; Momen, M. Association of gender with outcomes in critically ill patients. Crit. Care 2012, 16, R92. [Google Scholar]
- Haider, A.H.; Crompton, J.G.; Oyetunji, T.; Stevens, K.A.; Efron, D.T.; Kieninger, A.N.; Chang, D.C.; Cornwell, E.E., 3rd; Haut, E.R. Females have fewer complications and lower mortality following trauma than similarly injured males: A risk adjusted analysis of adults in the National Trauma Data Bank. Surgery 2009, 146, 308–315. [Google Scholar] [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Carré, J.E.; Orban, J.C.; Re, L.; Felsmann, K.; Iffert, W.; Bauer, M.; Suliman, H.B.; Piantadosi, C.A.; Mayhew, T.M.; Breen, P.; et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am. J. Respir. Crit. Care Med. 2010, 182, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Carling, D.; Viollet, B. Beyond energy homeostasis: The expanding role of AMP-activated protein kinase in regulating metabolism. Cell Metab. 2015, 21, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef]
- Inata, Y.; Kikuchi, S.; Samraj, R.S.; Hake, P.W.; O’Connor, M.; Ledford, J.R.; O’Connor, J.; Lahni, P.; Wolfe, V.; Piraino, G.; et al. Autophagy and mitochondrial biogenesis impairment contributes to age-dependent liver injury in experimental sepsis: Dysregulation of AMP-activated protein kinase pathway. FASEB J. 2018, 32, 728–741. [Google Scholar] [CrossRef]
- Matsiukevich, D.; Piraino, G.; Klingbeil, L.R.; Hake, P.W.; Wolfe, V.; O’Connor, M.; Zingarelli, B. The AMPK activator AICAR ameliorates age-dependent myocardial injury in murine hemorrhagic shock. Shock 2017, 47, 70–78. [Google Scholar] [CrossRef]
- Matsiukevich, D.; Piraino, G.; Lahni, P.; Hake, P.W.; Wolfe, V.; O’Connor, M.; James, J.; Zingarelli, B. Metformin ameliorates gender-and age-dependent hemodynamic instability and myocardial injury in murine hemorrhagic shock. Biochim. Biophys. Acta 2017, 1863, 2680–2691. [Google Scholar] [CrossRef]
- Klingbeil, L.R.; Kim, P.; Piraino, G.; O’Connor, M.; Hake, P.W.; Wolfe, V.; Zingarelli, B. Age-dependent changes in AMPK metabolic pathways in the lung in a mouse model of hemorrhagic shock. Am. J. Respir. Cell Mol. Biol. 2017, 56, 585–596. [Google Scholar] [CrossRef]
- Lee, C.; Yen, K.; Cohen, P. Humanin: A harbinger of mitochondrial derived peptides? Trends Endocrinol. Metab. 2013, 24, 222–228. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Niikura, T.; Tajima, H.; Yasukawa, T.; Sudo, H.; Ito, Y.; Kita, Y.; Kawasumi, M.; Kouyama, K.; Doyu, M.; et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc. Natl. Acad. Sci. USA 2001, 98, 6336–6341. [Google Scholar] [CrossRef]
- Yen, K.; Lee, C.; Mehta, H.; Cohen, P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J. Mol. Endocrinol. 2013, 50, R11–R19. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.L.; Ammann, A.; Piraino, G.; Wolfe, V.; O’Connor, M.; Lahni, P.; Ziady, A.; Zingarelli, B. Protective effects of humanin-G in hemorrhagic shock in female mice via AMPKα1-independent mechanisms. Shock 2023, 60, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Bachar, A.R.; Scheffer, L.; Schroeder, A.S.; Nakamura, H.K.; Cobb, L.J.; Oh, Y.K.; Lerman, L.O.; Pagano, R.E.; Cohen, P.; Lerman, A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc. Res. 2010, 88, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Zhou, X.; Wang, H. S14G-humanin (HNG) protects retinal endothelial cells from UV-B-induced NLRP3 inflammation activation through inhibiting Egr-1. Inflamm. Res. 2021, 70, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Nashine, S.; Cohen, P.; Chwa, M.; Lu, S.; Nesburn, A.B.; Kuppermann, B.D.; Kenney, M.C. Humanin G (HNG) protects age-related macular degeneration (AMD) transmitochondrial ARPE-19 cybrids from mitochondrial and cellular damage. Cell Death Dis. 2017, 8, e2951. [Google Scholar] [CrossRef]
- Yen, K.; Wan, J.; Mehta, H.H.; Miller, B.; Christensen, A.; Levine, M.E.; Salomon, M.P.; Brandhorst, S.; Xiao, J.; Kim, S.-J.; et al. Humanin prevents age-related cognitive decline in mice and is associated with improved cognitive age in humans. Sci. Rep. 2018, 8, 14212. [Google Scholar] [CrossRef]
- Chai, G.; Duan, D.; Ma, R.; Shen, J.; Li, H.; Ma, Z.; Luo, Y.; Wang, L.; Qi, X.; Wang, Q.; et al. Humanin attenuates Alzheimer-like cognitive deficits and pathological changes induced by amyloid β-peptide in rats. Neurosci. Bull. 2014, 30, 923–935. [Google Scholar] [CrossRef]
- Xu, X.; Chua, C.; Gao, J.; Hamdy, R.; Chua, B. Humanin is a novel neuroprotective agent against stroke. Stroke 2006, 37, 2613–2619. [Google Scholar] [CrossRef]
- Kim, S.; Guerrero, N.; Wassef, G.; Xiao, J.; Mehta, H.H.; Cohen, P.; Yen, K. The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways and has age-dependent signaling differences in the hippocampus. Oncotarget 2016, 7, 46899–46912. [Google Scholar] [CrossRef]
- Hoang, P.T.; Park, P.; Cobb, L.J.; Paharkova-Vatchkova, V.; Hakimi, M.; Cohen, P.; Lee, K.W. The neurosurvival factor humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in non-obese diabetic mice. Metabolism 2010, 59, 343–349. [Google Scholar] [CrossRef]
- Urban, C.; Hayes, H.V.; Piraino, G.; Wolfe, V.; Lahni, P.; O’Connor, M.; Phares, C.; Zingarelli, B. Colivelin, a synthetic derivative of humanin, ameliorates endothelial injury and glycocalyx shedding after sepsis in mice. Front. Immunol. 2022, 13, 984298. [Google Scholar] [CrossRef] [PubMed]
- Ackert-Bicknell, C.L.; Anderson, L.C.; Sheehan, S.; Hill, W.G.; Chang, B.; Churchill, G.A.; Chesler, E.J.; Korstanje, R.; Peters, L.L. Aging Research Using Mouse Models. Curr. Protoc. Mouse Biol. 2015, 5, 95–133. [Google Scholar] [CrossRef]
- Viollet, B.; Athea, Y.; Mounier, R.; Guigas, B.; Zarrinpashneh, E.; Horman, S.; Lantier, L.; Hebrard, S.; Devin-Leclerc, J.; Beauloye, C.; et al. AMPK: Lessons from transgenic and knockout animals. Front. Biosci. 2009, 14, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Ziady, A.G.; Kotlarchyk, M.; Bryant, L.; McShane, M.; Lee, Z. Bioluminescent imaging of reporter gene expression in the lungs of wildtype and model mice following the administration of PEG-stabilized DNA nanoparticles. Microsc. Res. Tech. 2010, 73, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Piraino, G.; O’Connor, M.; Hake, P.W.; Wolfe, V.; Lahni, P.; Zingarelli, B. Metformin Exerts Beneficial Effects in Hemorrhagic Shock in An AMPKα1-Independent Manner. Shock 2018, 49, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, R.H.; Huffman, D.M.; Calvert, J.W.; Jha, S.; Weinberg, Y.; Cui, L.; Nemkal, A.; Atzmon, G.; Klein, L.; Gundewar, S.; et al. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1940–1948. [Google Scholar] [CrossRef]
- Terashita, K.; Hashimoto, Y.; Niikura, T.; Tajima, H.; Yamagishi, Y.; Ishizaka, M.; Kawasumi, M.; Chiba, T.; Kanekura, K.; Yamada, M.; et al. Two serine residues distinctly regulate the rescue function of Humanin, an inhibiting factor of Alzheimer’s disease-related neurotoxicity: Functional potentiation by isomerization and dimerization. J. Neurochem. 2003, 85, 1521–1538. [Google Scholar] [CrossRef]
- Bloom, J.E.; Chan, W.; Kaye, D.M.; Stub, D. State of Shock: Contemporary Vasopressor and Inotrope Use in Cardiogenic Shock. J. Am. Heart. Assoc. 2023, 12, e029787. [Google Scholar] [CrossRef]
- Zarzaur, B.L.; Croce, M.A.; Fischer, P.E.; Magnotti, L.J.; Fabian, T.C. New vitals after injury: Shock index for the young and age x shock index for the old. J. Surg. Res. 2008, 147, 229–236. [Google Scholar] [CrossRef]
- Victorino, G.P.; Battistella, F.D.; Wisner, D.H. Does tachycardia correlate with hypotension after trauma? J. Am. Coll. Surg. 2003, 196, 679–684. [Google Scholar] [CrossRef]
- Majde, J.A. Animal models for hemorrhage and resuscitation research. J. Trauma 2003, 54, S100–S105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Cai, M.M.; Li, D.; Zhao, B.Y.; Zhou, S.S.; Wu, Z.R.; Shi, Y.J.; Su, L. S14G-humanin confers cardioprotective effects against chronic adrenergic and pressure overload-induced heart failure in mice. Heliyon 2023, 9, e21892. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.; Sun, J.L.; Jeong, J.H.; Jung, T.W. Humanin attenuates palmitate-induced hepatic lipid accumulation and insulin resistance via AMPK-mediated suppression of the mTOR pathway. Biochem. Biophys. Res. Commun. 2020, 526, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Matsuoka, M.; Hashimoto, Y. Humanin and the receptors for humanin. Mol. Neurobiol. 2010, 41, 22–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amman, A.M.; Wolfe, V.; Piraino, G.; Ziady, A.; Zingarelli, B. Humanin-G Ameliorates Hemorrhage-Induced Acute Lung Injury in Mice Through AMPKα1-Dependent and -Independent Mechanisms. Biomedicines 2024, 12, 2615. https://doi.org/10.3390/biomedicines12112615
Amman AM, Wolfe V, Piraino G, Ziady A, Zingarelli B. Humanin-G Ameliorates Hemorrhage-Induced Acute Lung Injury in Mice Through AMPKα1-Dependent and -Independent Mechanisms. Biomedicines. 2024; 12(11):2615. https://doi.org/10.3390/biomedicines12112615
Chicago/Turabian StyleAmman, Allison M., Vivian Wolfe, Giovanna Piraino, Assem Ziady, and Basilia Zingarelli. 2024. "Humanin-G Ameliorates Hemorrhage-Induced Acute Lung Injury in Mice Through AMPKα1-Dependent and -Independent Mechanisms" Biomedicines 12, no. 11: 2615. https://doi.org/10.3390/biomedicines12112615
APA StyleAmman, A. M., Wolfe, V., Piraino, G., Ziady, A., & Zingarelli, B. (2024). Humanin-G Ameliorates Hemorrhage-Induced Acute Lung Injury in Mice Through AMPKα1-Dependent and -Independent Mechanisms. Biomedicines, 12(11), 2615. https://doi.org/10.3390/biomedicines12112615






