Neuroprotective Effects of Ascorbic Acid, Vanillic Acid, and Ferulic Acid in Dopaminergic Neurons of Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Care and Husbandry
2.2. Solution Preparation
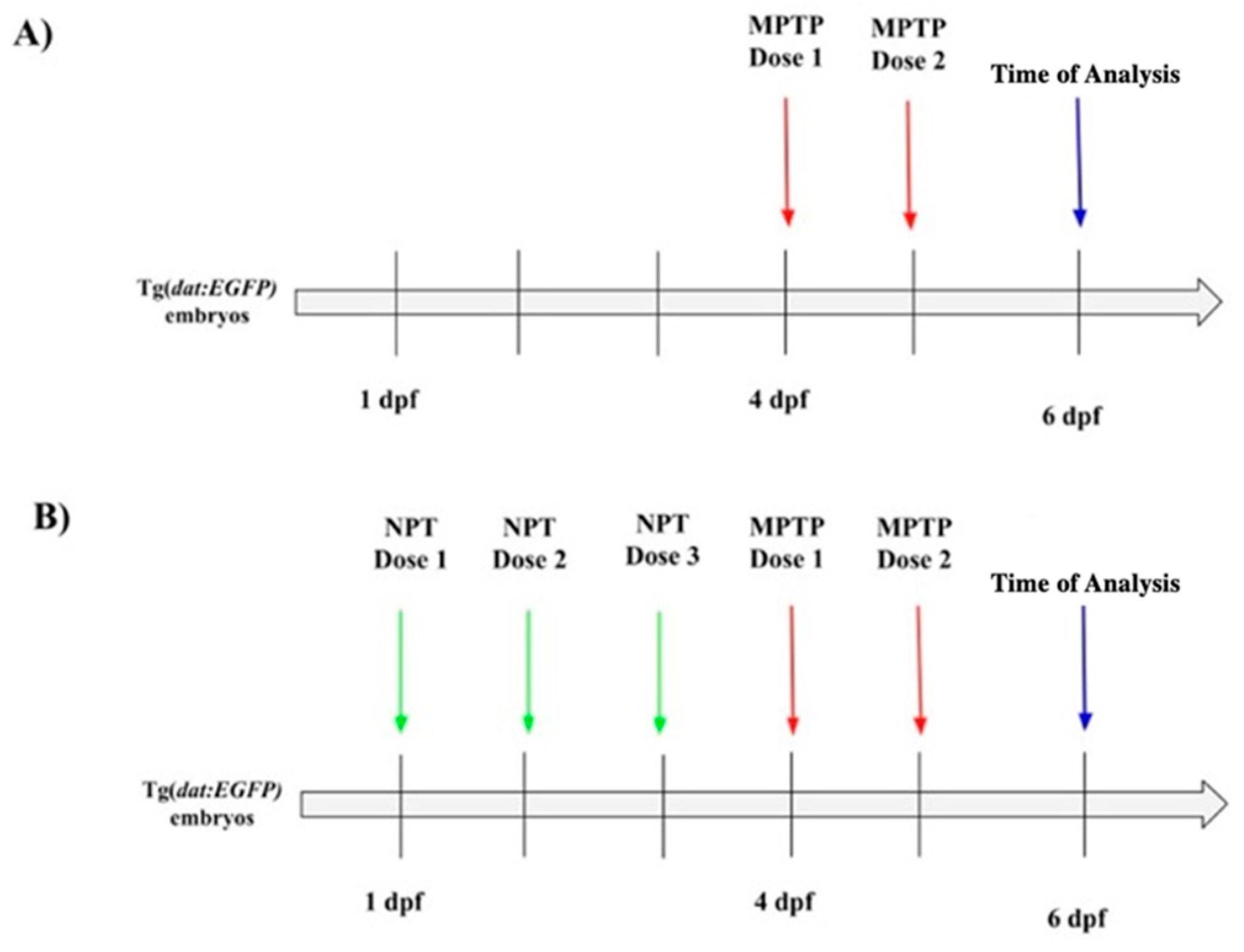
2.3. Neuroprotective Treatment and MPTP Exposure
2.4. Swimming Activity
2.5. RNA Extraction, cDNA Synthesis, and qRT-PCR
2.6. Immunohistochemistry on Whole-Mount Zebrafish Larvae
2.7. Confocal Microscopy and Image Analysis
2.8. Statistical Analysis
3. Results
3.1. Dose Determination for Neuroprotection Treatment
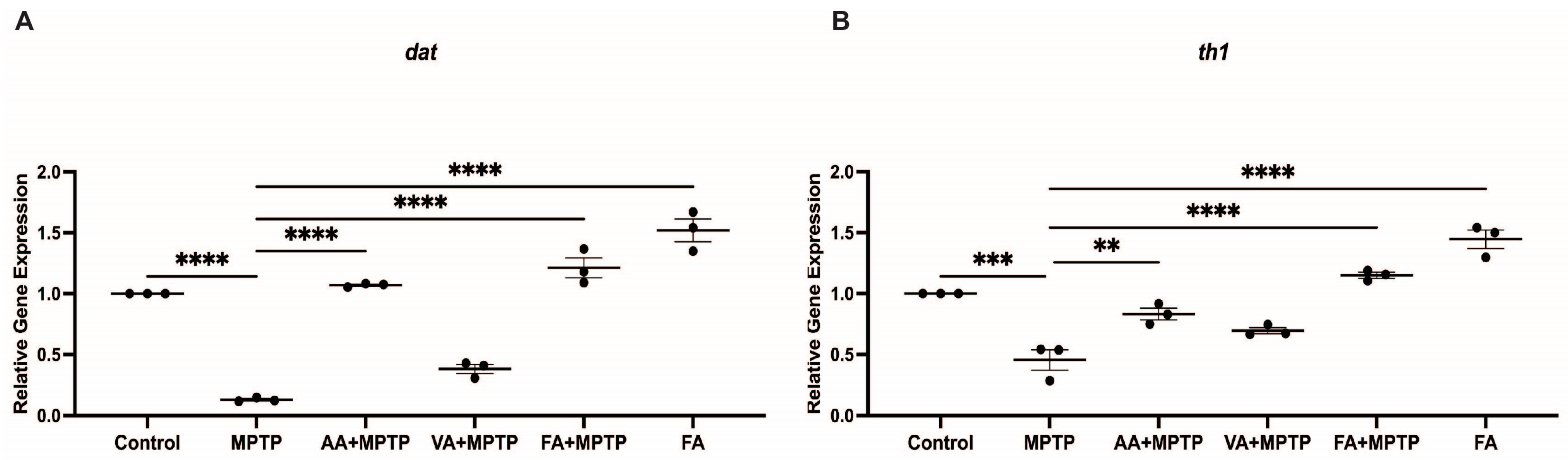
3.2. Protective Effect of Natural Phenolic Compounds on DA Biosynthesis Genes
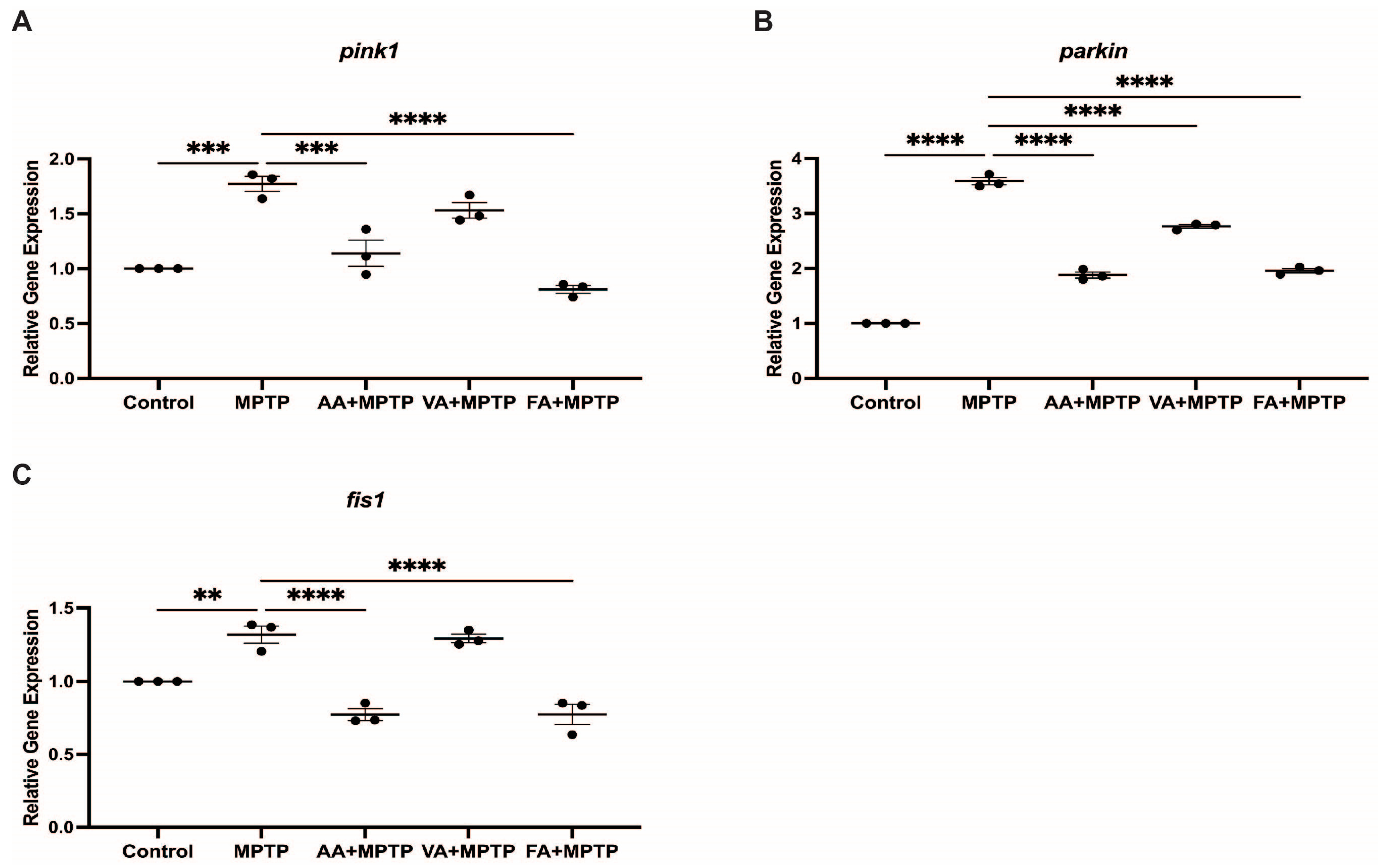
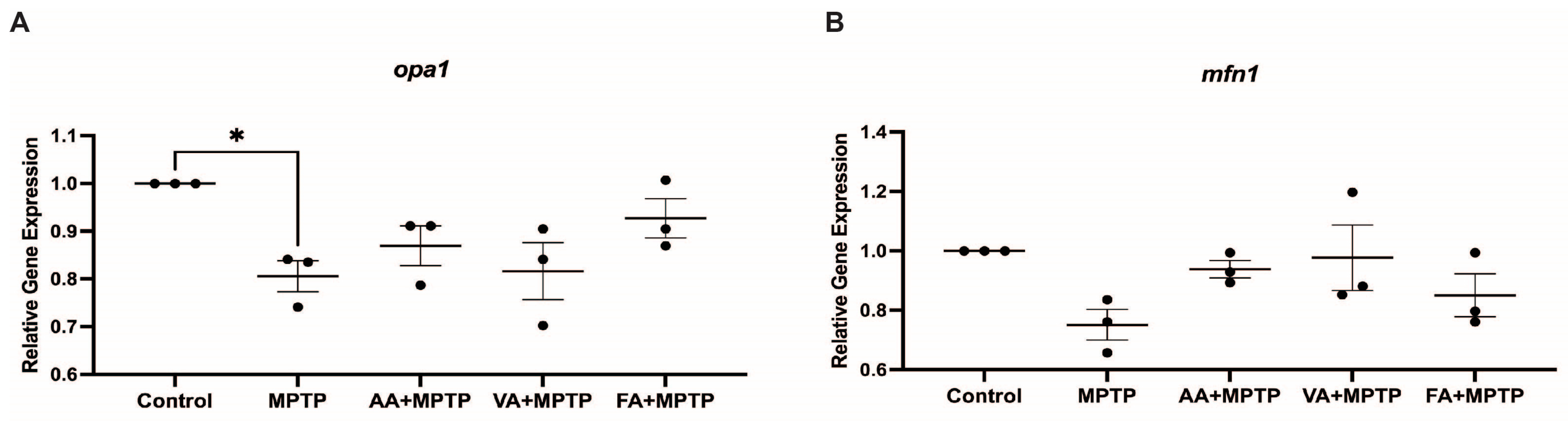
3.3. Effect on Mitochondrial Fission Genes
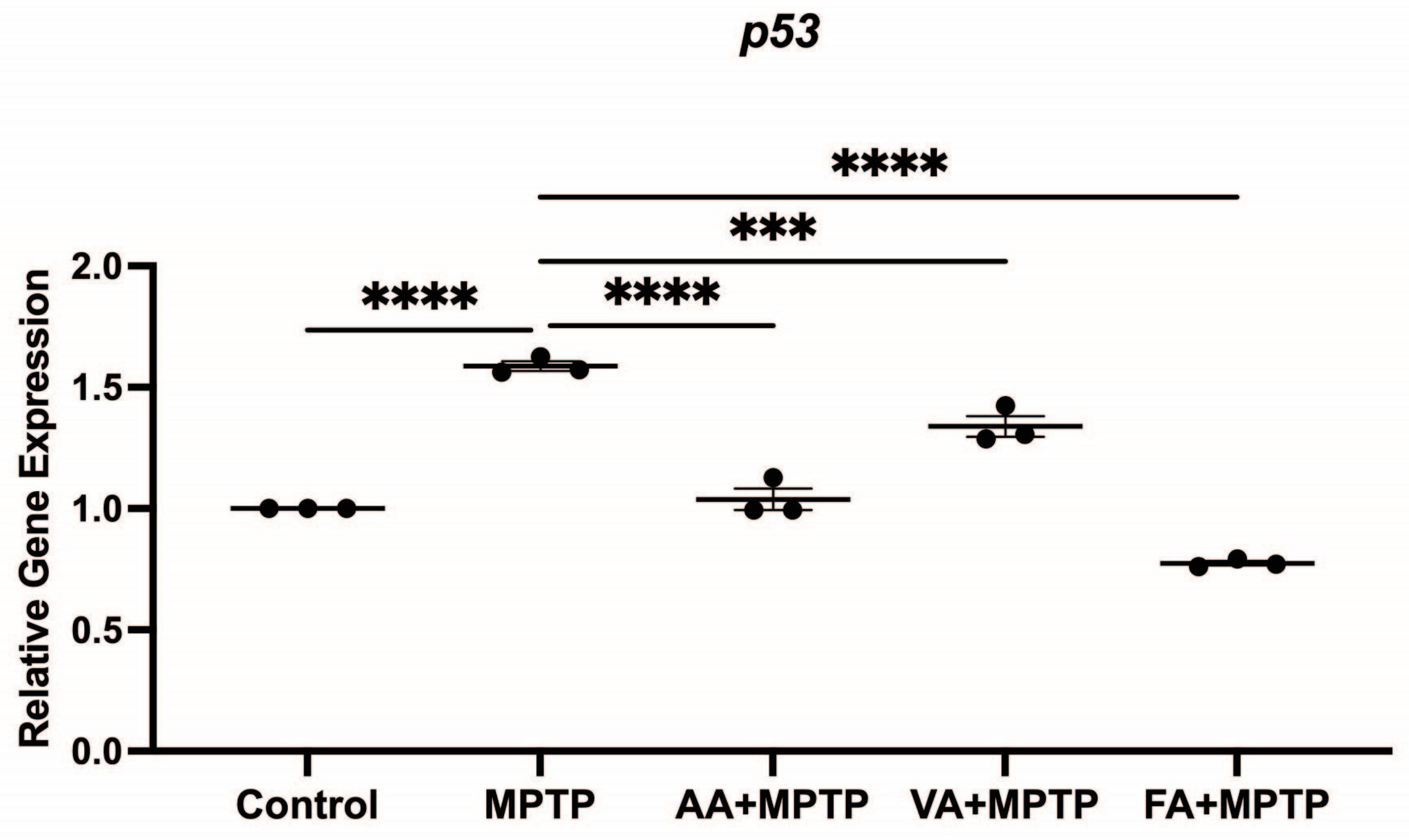
3.4. Effect on p53 Gene Expression
3.5. Neuroprotective Effects of Ascorbic Acid, Vanillic Acid, and Ferulic Acid
3.6. Ferulic Acid Alone Increases the Number of DAnergic Neurons
3.7. Effects on Locomotion
4. Discussion
4.1. Ascorbic Acid
4.2. Ferulic Acid
4.3. Vanillic Acid
4.4. Correlative and Comparative Effects of the Three Neuroprotectants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.; Bench, C.; Howard, R. Ageing and the nigrostriatal dopaminergic system. Int. J. Geriatr. Psychiatry 2002, 17, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.W.; Nemeroff, C.B. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 2007, 64, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.M.; Bristot, I.J.; da Motta, L.L.; Parsons, R.B.; Klamt, F. Mimicking Parkinson’s disease in a dish: Merits and pitfalls of the most commonly used dopaminergic in vitro models. NeuroMolecular Med. 2017, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Bartel, W.P.; Van Laar, V.S.; Burton, E.A. Chapter 23—Parkinson’s Disease. In Behavioral and Neural Genetics of Zebrafish; Gerlai, R.T., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 377–412. [Google Scholar]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888. [Google Scholar] [CrossRef]
- Przedborski, S.; Jackson-Lewis, V.; Djaldetti, R.; Liberatore, G.; Vila, M.; Vukosavic, S.; Almer, G. The parkinsonian toxin MPTP: Action and mechanism. Restor. Neurol Neurosci. 2000, 16, 135–142. [Google Scholar]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Seo, B.J.; Yoon, S.H.; Do, J.T. Mitochondrial Dynamics in Stem Cells and Differentiation. Int. J. Mol. Sci. 2018, 19, 3893. [Google Scholar] [CrossRef]
- Ban, T.; Ishihara, T.; Kohno, H.; Saita, S.; Ichimura, A.; Maenaka, K.; Oka, T.; Mihara, K.; Ishihara, N. Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat. Cell Biol. 2017, 19, 856–863. [Google Scholar] [CrossRef]
- Howe, K.M.D.; Clark, C.F.; Torroja, J.; Torrance, C.; Berthelot, M.; Muffato, J.E.; Collins, S.; Humphray, K.; McLaren, L.; Matthews, S.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Gemberling, M.; Bailey, T.J.; Hyde, D.R.; Poss, K.D. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013, 29, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Noble, S.; Ekker, M. Modeling Neurodegeneration in Zebrafish. Curr. Neurol. Neurosci. Rep. 2011, 11, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Salamon, A.; Zádori, D.; Szpisjak, L.; Klivényi, P.; Vécsei, L. Neuroprotection in Parkinson’s disease: Facts and hopes. J. Neural Transm. 2019, 127, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Koh, P. Ferulic acid prevents cerebral ischemic injury-induced reduction of hippocalcin expression. Synapse 2013, 67, 390–398. [Google Scholar] [CrossRef]
- Wu, C.; Lee, H.; Liu, C.; Korivi, M.; Chen, H.; Chan, M. Protective role of L-ascorbic acid, N-acetylcysteine and apocynin on neomycin-induced hair cell loss in zebrafish. J. Appl. Toxicol. 2015, 35, 273–279. [Google Scholar] [CrossRef]
- Kumar, S.; Prahalathan, P.; Raja, B. Antihypertensive and antioxidant potential of vanillic acid, a phenolic compound in l-NAME-induced hypertensive rats: A dose-dependence study. Redox Rep. 2011, 16, 208–215. [Google Scholar] [CrossRef]
- Nualart, F.; Mack, L.; García, A.; Cisternas, P.; Bongarzone, E.R.; Heitzer, M.; Jara, N.; Martinez, F.; Ferrada LEspinoza, F.; Baeza, V.; et al. Vitamin C transporters, recycling and the bystander effect in the nervous system: SVCT2 versus gluts. J. Stem Cell Res. Ther. 2014, 4, 209. [Google Scholar] [CrossRef]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Ginter, E. ASCORBIC ACID IN CHOLESTEROL AND BILE ACID METABOLISM. Ann. New York Acad. Sci. 1975, 258, 410–421. [Google Scholar] [CrossRef]
- Fraga, D.B.; Costa, A.P.; Olescowicz, G.; Camargo, A.; Pazini, F.L.; Freitas, A.E.; Moretti, M.; Brocardo, P.S.; Rodrigues, A.L.S. Ascorbic acid presents rapid behavioral and hippocampal synaptic plasticity effects. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2020, 96, 109757. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Kodrík, D.; Kłudkiewicz, B.; Sehnal, F. Glutathione–ascorbic acid redox cycle and thioredoxin reductase activity in the digestive tract of Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2009, 39, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Xu, B.; Ding, Y.; Liu, X.; Zhou, Y.; Ahmad, F. Oxidative Stress Response Induced by Butachlor in Zebrafish Embryo/Larvae: The Protective Effect of Vitamin C. Bull. Environ. Contam. Toxicol. 2018, 100, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Paduraru, E.; Flocea, E.-I.; Lazado, C.C.; Simionov, I.-A.; Nicoara, M.; Ciobica, A.; Faggio, C.; Jijie, R. Vitamin C Mitigates Oxidative Stress and Behavioral Impairments Induced by Deltamethrin and Lead Toxicity in Zebrafish. Int. J. Mol. Sci. 2021, 22, 12714. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, T.; Satoh, K.; Kadoma, Y.; Murakami, Y.; Unten, S.; Atsumi, T.; Sakagami, H.; Fujisawa, S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2003, 22, 2711–2717. [Google Scholar]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Influence of Oryzanol and Ferulic Acid on the Lipid Metabolism and Antioxidative Status in High Fat-Fed Mice. J. Clin. Biochem. Nutr. 2010, 46, 150–156. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Ho, T.-Y.; Lee, E.-J.; Su, S.-Y.; Tang, N.-Y.; Hsieh, C.-L. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am. J. Chin. Med. 2008, 36, 1105–1119. [Google Scholar] [CrossRef]
- Kawabata, K.; Yamamoto, T.; Hara, A.; Shimizu, M.; Yamada, Y.; Matsunaga, K.; Tanaka, T.; Mori, H. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000, 157, 15–21. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Sarkaki, A.; Rashno, M.; Farbood, Y. Memory deficits and hippocampal inflammation in cerebral hypoperfusion and reperfusion in male rats: Neuroprotective role of vanillic acid. Life Sci. 2018, 211, 126–132. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Olasehinde, T.A.; Koorbanally, N.A.; Islam, S. Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe2+-induced brain tissues damage. Metab. Brain Dis. 2020, 35, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.S.M.; Rajakumar, S.; Dhanasekar, K. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur. J. Pharmacol. 2011, 668, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Yu, M.; Godoy, R.; Hatch, G.; Poitras, L.; Ekker, M. Transgenic zebrafish expressing green fluorescent protein in dopaminergic neurons of the ventral diencephalon. Dev. Dyn. 2011, 240, 2539–2547. [Google Scholar] [CrossRef]
- Chang-Chien, J.; Yen, Y.-C.; Li, S.-Y.; Hsu, T.-C.; Yang, J.-J. Ferulic acid-mediated protection against neomycin-induced hair cell loss in transgenic zebrafish. J. Funct. Foods 2017, 28, 157–167. [Google Scholar] [CrossRef]
- Dickinson, A.J.; Turner, S.D.; Wahl, S.; Kennedy, A.E.; Wyatt, B.H.; Howton, D.A. E-liquids and vanillin flavoring disrupts retinoic acid signaling and causes craniofacial defects in Xenopus embryos. Dev. Biol. 2022, 481, 14–29. [Google Scholar] [CrossRef]
- Du, Y.; Guo, Q.; Shan, M.; Wu, Y.; Huang, S.; Zhao, H.; Hong, H.; Yang, M.; Yang, X.; Ren, L.; et al. Spatial and Temporal Distribution of Dopaminergic Neurons during Development in Zebrafish. Front. Neuroanat. 2016, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, V.; Torkko, V.; Sundvik, M.; Reenilä, I.; Khrustalyov, D.; Kaslin, J.; Panula, P. MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. J. Neurochem. 2009, 108, 719–731. [Google Scholar] [CrossRef]
- Sershen, H.; Reith, M.; Hashim, A.; Lajtha, A. Protection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity by the antioxidant ascorbic acid. Neuropharmacology 1985, 24, 1257–1259. [Google Scholar] [CrossRef]
- Pardo, B.; Mena, M.A.; Fahn, S.; de Yébenes, J.G. Ascorbic acid protects against levodopa-induced neurotoxicity on a catecholamine-rich human neuroblastoma cell line. Mov. Disord. 1993, 8, 278–284. [Google Scholar] [CrossRef]
- Seitz, G.; Gebhardt, S.; Beck, J.F.; Böhm, W.; Lode, H.N.; Niethammer, D.; Bruchelt, G. Ascorbic acid stimulates DOPA synthesis and tyrosine hydroxylase gene expression in the human neuroblastoma cell line SK-N-SH. Neurosci. Lett. 1998, 244, 33–36. [Google Scholar] [CrossRef]
- Nagayama, H.; Hamamoto, M.; Ueda, M.; Nito, C.; Yamaguchi, H.; Katayama, Y. The Effect of Ascorbic Acid on the Pharmacokinetics of Levodopa in Elderly Patients with Parkinson Disease. Clin. Neuropharmacol. 2004, 27, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Jin, S.; Lendahl, U.; Nistér, M.; Zhao, J. Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery. EMBO J. 2019, 38, e99748. [Google Scholar] [CrossRef]
- Kalyn, M.; Ekker, M. Cerebroventricular Microinjections of MPTP on Adult Zebrafish Induces Dopaminergic Neuronal Death, Mitochondrial Fragmentation, and Sensorimotor Impairments. Front. Neurosci. 2021, 15, 718244. [Google Scholar] [CrossRef]
- Covarrubias-Pinto, A.; Acuña, A.I.; Beltrán, F.A.; Torres-Díaz, L.; Castro, M.A. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int. J. Mol. Sci. 2015, 16, 28194–28217. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Kim, H.-S.; Kim, D.-H.; Yan, J.-J.; Suh, H.-W.; Song, D.-K. Inhibitory effects of long-term administration of ferulic acid on astrocyte activation induced by intracerebroventricular injection of β-amyloid peptide (1–42) in mice. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2005, 29, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Deng, H.-M.; Zhu, M.-M.; Xiao, F.; Yang, L.; Zhang, Z.-J.; Xiao, Y.; Nie, H. [Inhibitory effect of ferulic acid on inflammatory response in microglia induced by lipopolysaccharides]. Zoöl. Res. 2011, 32, 311–316. [Google Scholar] [CrossRef]
- Kikugawa, M.; Tsutsuki, H.; Ida, T.; Nakajima, H.; Ihara, H.; Sakamoto, T. Water-soluble ferulic acid derivatives improve amyloid-β-induced neuronal cell death and dysmnesia through inhibition of amyloid-β aggregation. Biosci. Biotechnol. Biochem. 2016, 80, 547–553. [Google Scholar] [CrossRef]
- Anis, E.; Zafeer, M.F.; Firdaus, F.; Islam, S.N.; Anees Khan, A.; Ali, A.; Hossain, M.M. Ferulic acid reinstates mitochondrial dynamics through PGC1α expression modulation in 6-hydroxydopamine lesioned rats. Phytother. Res. 2020, 34, 214–226. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Rong, H.; Zhang, X.; Dong, M. Ferulic Acid Ameliorates MPP+/MPTP-Induced Oxidative Stress via ERK1/2-Dependent Nrf2 Activation: Translational Implications for Parkinson Disease Treatment. Mol. Neurobiol. 2020, 57, 2981–2995. [Google Scholar] [CrossRef]
- Ay, M. Vanillic acid induces mitochondrial biogenesis in SH-SY5Y cells. Mol. Biol. Rep. 2022, 49, 4443–4449. [Google Scholar] [CrossRef]
- Sharma, N.; Khurana, N.; Muthuraman, A.; Utreja, P. Pharmacological evaluation of vanillic acid in rotenone-induced Parkinson’s disease rat model. Eur. J. Pharmacol. 2021, 903, 174112. [Google Scholar] [CrossRef] [PubMed]




| Primer | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) |
|---|---|---|
| th1 | GACGGAAGATGATCGGAGACA | CCGCCATGTTCCGATTTCT |
| dat | AGACATCTGGGAAGGTGGTG | ACCTGAGCATCATACAGGCG |
| pink1 | GGCAATGAAGATGATGTGGAAC | GGTCGGCAGGACATCAGGA |
| parkin | GCGAGTGTGTCTGAGCTGAA | CACACTGGAACACCAGCACT |
| fis1 | CCCTGAACCTTCCAGTGTTT | GTCTCTGGAAACGGGTCCTT |
| mfn1 | CTGGGTCCCGTCAACGCCAA | ACTGAACCACCGCTGGGGCT |
| opa1 | GCTTGAGCGCTTGGAAAAGGAA | TGGCAGGTGATCTTGAGTGTTGT |
| p53 | ATATCCTGGCGAACATTTGG | ACGTCCACCACCACCATTTGAAC |
| rpl13a | TCTGGAGGACTGTAAGAGGTATGC | AGACGCACAATCTTGAGAGCAG |
| ef1a | CTGGAGGCCAGCTCAAACAT | ATCAAGAAGAGTAGTACCGCTAGCATTAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hedayatikatouli, F.; Kalyn, M.; Elsaid, D.; Mbesha, H.A.; Ekker, M. Neuroprotective Effects of Ascorbic Acid, Vanillic Acid, and Ferulic Acid in Dopaminergic Neurons of Zebrafish. Biomedicines 2024, 12, 2497. https://doi.org/10.3390/biomedicines12112497
Hedayatikatouli F, Kalyn M, Elsaid D, Mbesha HA, Ekker M. Neuroprotective Effects of Ascorbic Acid, Vanillic Acid, and Ferulic Acid in Dopaminergic Neurons of Zebrafish. Biomedicines. 2024; 12(11):2497. https://doi.org/10.3390/biomedicines12112497
Chicago/Turabian StyleHedayatikatouli, Fatemeh, Michael Kalyn, Dana Elsaid, Herman Aishi Mbesha, and Marc Ekker. 2024. "Neuroprotective Effects of Ascorbic Acid, Vanillic Acid, and Ferulic Acid in Dopaminergic Neurons of Zebrafish" Biomedicines 12, no. 11: 2497. https://doi.org/10.3390/biomedicines12112497
APA StyleHedayatikatouli, F., Kalyn, M., Elsaid, D., Mbesha, H. A., & Ekker, M. (2024). Neuroprotective Effects of Ascorbic Acid, Vanillic Acid, and Ferulic Acid in Dopaminergic Neurons of Zebrafish. Biomedicines, 12(11), 2497. https://doi.org/10.3390/biomedicines12112497







