The Relevance of Spinal Muscular Atrophy Biomarkers in the Treatment Era
Abstract
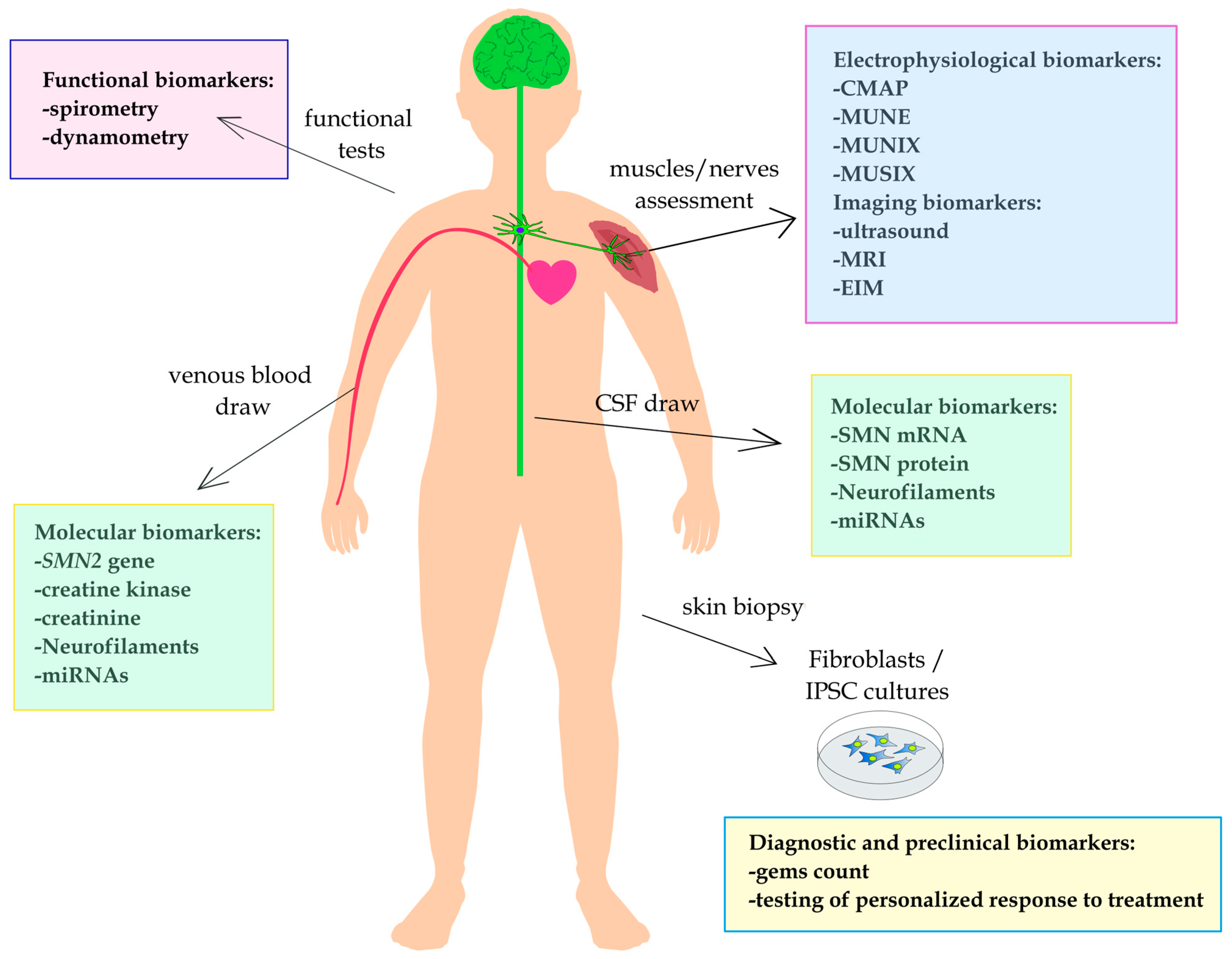
1. Introduction
2. Physiological Biomarkers
2.1. Electrophysiological Biomarkers
2.1.1. Compound Muscle Action Potential
2.1.2. Motor Unit Number Estimation
2.1.3. Motor Unit Number Index and Motor Unit Size Index
2.2. Functional Biomarkers
2.2.1. Spirometric Measurements
2.2.2. Dynamometric Measurements
3. Imaging Biomarkers
3.1. Ultrasound Measurements
3.2. Magnetic Resonance Imaging
3.3. Electrical Impedance Myography
4. Molecular Biomarkers
4.1. SMN2 Gene Copy Number
4.2. SMN Transcripts Level
4.3. SMN Protein
4.4. MicroRNAs
4.5. Neurofilaments
4.6. Creatine Kinase and Creatinine System
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- D’Amico, A.; Mercuri, E.; Tiziano, F.D.; Bertini, E. Spinal muscular atrophy. Orphanet J. Rare Dis. 2011, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Wilson, R.B. Genetic testing and risk assessment for spinal muscular atrophy (SMA). Hum. Genet. 2002, 111, 477–500. [Google Scholar] [CrossRef] [PubMed]
- Zerres, K.; Wirth, B.; Rudnik-Schöneborn, S. Spinal muscular atrophy—Clinical and genetic correlations. Neuromuscul. Disord. 1997, 7, 202–207. [Google Scholar] [CrossRef]
- Wijngaarde, C.A.; Veldhoen, E.S.; van Eijk, R.P.A.; Stam, M.; Otto, L.A.M.; Asselman, F.-L.; Wösten-van Asperen, R.M.; Hulzebos, E.H.J.; Verweij-van den Oudenrijn, L.P.; Bartels, B.; et al. Natural history of lung function in spinal muscular atrophy. Orphanet J. Rare Dis. 2020, 15, 88. [Google Scholar] [CrossRef]
- Talbot, K.; Tizzano, E.F. The clinical landscape for SMA in a new therapeutic era. Gene Ther. 2017, 24, 529–533. [Google Scholar] [CrossRef]
- Lorson, C.L.; Hahnen, E.; Androphy, E.J.; Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 1999, 96, 6307–6311. [Google Scholar] [CrossRef]
- Monani, U.R.; Lorson, C.L.; Parsons, D.W.; Prior, T.W.; Androphy, E.J.; Burghes, A.H.M.; McPherson, J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999, 8, 1177–1183. [Google Scholar] [CrossRef]
- Prior, T.W.; Swoboda, K.J.; Scott, H.D.; Hejmanowski, A.Q. Clinical Report Homozygous SMN1 Deletions in Unaffected Family Members and Modification of the Phenotype by SMN2. Am. J. Med. Genet. Part A 2004, 310, 307–310. [Google Scholar] [CrossRef]
- Harada, Y.; Sutomo, R.; Sadewa, A.H.; Akutsu, T.; Takeshima, Y.; Wada, H.; Matsuo, M.; Nishio, H. Correlation between SMN2 copy number and clinical phenotype of spinal muscular atrophy: Three SMN2 copies fail to rescue some patients from the disease severity. J. Neurol. 2002, 249, 1211–1219. [Google Scholar] [CrossRef]
- Audic, F.; Dubois, S.M.; Durigneux, J.; Barnerias, C.; Isapof, A.; Nougues, M.C.; Davion, J.B.; Richelme, C.; Vuillerot, C.; Legoff, L.; et al. Effect of nusinersen after 3 years of treatment in 57 young children with SMA in terms of SMN2 copy number or type. Arch. Pediatr. 2024, 31, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Dosi, C.; Masson, R. The impact of three SMN2 gene copies on clinical characteristics and effect of disease-modifying treatment in patients with spinal muscular atrophy: A systematic literature review. Front. Neurol. 2024, 15, 1308296. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, K.J.; Prior, T.W.; Scott, C.B.; McNaught, T.P.; Wride, M.C.; Reyna, S.P.; Bromberg, M.B. Natural history of denervation in SMA: Relation to age, SMN2 copy number, and function. Ann. Neurol. 2005, 57, 704–712. [Google Scholar] [CrossRef]
- Aartsma-Rus, A. FDA Approval of Nusinersen for Spinal Muscular Atrophy Makes 2016 the Year of Splice Modulating Oligonucleotides. Nucleic Acid Ther. 2017, 27, 67–69. [Google Scholar] [CrossRef]
- Hoy, S.M. Nusinersen: First Global Approval. Drugs 2017, 77, 473–479. [Google Scholar] [CrossRef]
- Hoy, S.M. Onasemnogene Abeparvovec: First Global Approval. Drugs 2019, 79, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Risdiplam: First Approval. Drugs 2020, 80, 1853–1858. [Google Scholar] [CrossRef]
- Glascock, J.; Darras, B.T.; Crawford, T.O.; Sumner, C.J.; Kolb, S.J.; Didonato, C.; Elsheikh, B.; Howell, K.; Farwell, W.; Valente, M.; et al. Identifying Biomarkers of Spinal Muscular Atrophy for Further Development. J. Neuromuscul. Dis. 2023, 10, 937–954. [Google Scholar] [CrossRef]
- Ribero, V.A.; Daigl, M.; Martí, Y.; Gorni, K.; Evans, R.; Scott, D.A.; Mahajan, A.; Abrams, K.R.; Hawkins, N. How does risdiplam compare with other treatments for Types 1-3 spinal muscular atrophy: A systematic literature review and indirect treatment comparison. J. Comp. Eff. Res. 2022, 11, 347–370. [Google Scholar] [CrossRef]
- Łusakowska, A.; Wójcik, A.; Frączek, A.; Aragon-Gawińska, K.; Potulska-Chromik, A.; Baranowski, P.; Nowak, R.; Rosiak, G.; Milczarek, K.; Konecki, D.; et al. Long-term nusinersen treatment across a wide spectrum of spinal muscular atrophy severity: A real-world experience. Orphanet J. Rare Dis. 2023, 18, 230. [Google Scholar] [CrossRef]
- Lapp, H.S.; Freigang, M.; Hagenacker, T.; Weiler, M.; Wurster, C.D.; Günther, R. Biomarkers in 5q-associated spinal muscular atrophy—A narrative review. J. Neurol. 2023, 270, 4157–4178. [Google Scholar] [CrossRef] [PubMed]
- Cuddon, P.A. Electrophysiology in neuromuscular disease. Vet. Clin. Small Anim. Pract. 2002, 32, 31–62. [Google Scholar] [CrossRef]
- Alves, C.R.R.; Petrillo, M.; Spellman, R.; Garner, R.; Zhang, R.; Kiefer, M.; Simeone, S.; Sohn, J.; Eichelberger, E.J.; Rodrigues, E.; et al. Implications of circulating neurofilamentsfor spinal muscular atrophytreatment early in life: A case series. Mol. Ther. Methods Clin. Dev. 2021, 23, 524–538. [Google Scholar] [CrossRef]
- Lewelt, A.; Krosschell, K.J.; Scott, C.; Sakonju, A.; Kissel, J.T.; Crawford, T.O.; Acsadi, G.; D’Anjou, G.; Elsheikh, B.; Reyna, S.P.; et al. Compound muscle action potential and motor function in children with spinal muscular atrophy. Muscle Nerve 2010, 42, 703–708. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, D.; D’silva, A.; Howells, J.; Herbert, K.; Geelan-Small, P.; Lin, C.S.Y.; Farrar, M.A. Motor unit changes in children with symptomatic spinal muscular atrophy treated with nusinersen. J. Neurol. Neurosurg. Psychiatry 2021, 92, 78–85. [Google Scholar] [CrossRef]
- Barrois, R.; Barnerias, C.; Deladrière, E.; Leloup-Germa, V.; Tervil, B.; Audic, F.; Boulay, C.; Cances, C.; Cintas, P.; Davion, J.B.; et al. A new score combining compound muscle action potential (CMAP) amplitudes and motor score is predictive of motor outcome after AVXS-101 (Onasemnogene Abeparvovec) SMA therapy. Neuromuscul. Disord. 2023, 33, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.C.; Hsu, Y.K.; Chang, F.M.; Lin, C.Y.; Hwu, W.L.; Lee, W.T.; Lee, N.C.; Chien, Y.H. CMAP changes upon symptom onset and during treatment in spinal muscular atrophy patients: Lessons learned from newborn screening. Genet. Med. 2021, 23, 415–420. [Google Scholar] [CrossRef]
- David Arnold, W.; Porensky, P.N.; Mcgovern, V.L.; Iyer, C.C.; Duque, S.; Li, X.; Meyer, K.; Schmelzer, L.; Kaspar, B.K.; Kolb, S.J.; et al. Electrophysiological biomarkers in spinal muscular atrophy: Proof of concept. Ann. Clin. Transl. Neurol. 2014, 1, 34–44. [Google Scholar] [CrossRef]
- Daube, J.R.; Gooch, C.; Shefner, J.; Olney, R.; Felice, K.; Bromberg, M. Chapter 14 Motor Unit Number Estimation (MUNE) with Nerve Conduction Studies; Elsevier B.V.: Amsterdam, The Netherlands, 2000; Volume 53. [Google Scholar]
- Darras, B.T.; Chiriboga, C.A.; Iannaccone, S.T.; Swoboda, K.J.; Montes, J.; Mignon, L.; Xia, S.; Bennett, C.F.; Bishop, K.M.; Shefner, J.M.; et al. Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies. Neurology 2019, 92, e2492–e2506. [Google Scholar] [CrossRef]
- Pino, M.G.; Rich, K.A.; Kolb, S.J. Update on Biomarkers in Spinal Muscular Atrophy. Biomark. Insights 2021, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gooch, C.L.; Doherty, T.J.; Chan, K.M.; Bromberg, M.B.; Lewis, R.A.; Stashuk, D.W.; Berger, M.J.; Andary, M.T.; Daube, J.R. Motor unit number estimation: A technology and literature review. Muscle Nerve 2014, 50, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Boulay, C.; Delmont, E.; Audic, F.; Chabrol, B.; Attarian, S. Motor unit number index: A potential electrophysiological biomarker for pediatric spinal muscular atrophy. Muscle Nerve 2021, 64, 445–453. [Google Scholar] [CrossRef]
- Nandedkar, S.D.; Barkhaus, P.E.; Stålberg, E.V. Motor unit number index (MUNIX): Principle, method, and findings in healthy subjects and in patients with motor neuron disease. Muscle Nerve 2010, 42, 798–807. [Google Scholar] [CrossRef]
- Günther, R.; Neuwirth, C.; Koch, J.C.; Lingor, P.; Braun, N.; Untucht, R.; Petzold, D.; Weber, M.; Hermann, A. Motor Unit Number Index (MUNIX) of hand muscles is a disease biomarker for adult spinal muscular atrophy. Clin. Neurophysiol. 2019, 130, 315–319. [Google Scholar] [CrossRef]
- Querin, G.; Lenglet, T.; Debs, R.; Stojkovic, T.; Behin, A.; Salachas, F.; Le Forestier, N.; del Mar Amador, M.; Lacomblez, L.; Meininger, V.; et al. The motor unit number index (MUNIX) profile of patients with adult spinal muscular atrophy. Clin. Neurophysiol. 2018, 129, 2333–2340. [Google Scholar] [CrossRef]
- Verma, S.; Forte, J.; Ritchey, M.; Shah, D. Motor unit number index in children with later-onset spinal muscular atrophy. Muscle Nerve 2020, 62, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Moore, V.C. Spirometry: Step by step. Breathe 2012, 8, 232–240. [Google Scholar] [CrossRef]
- Ward, N.S.; Hill, N.S. Pulmonary function testing in neuromuscular disease. Clin. Chest Med. 2001, 22, 769–781. [Google Scholar] [CrossRef]
- Phillips, M.F.; Quinlivan, R.C.M.; Edwards, R.H.T.; Calverley, P.M.A. Changes in spirometry over time as a prognostic marker in patients with duchenne muscular dystrophy. Am. J. Respir. Crit. Care Med. 2002, 164, 2191–2194. [Google Scholar] [CrossRef]
- Hermann, W.; Langner, S.; Freigang, M.; Fischer, S.; Storch, A.; Günther, R.; Hermann, A. Affection of Respiratory Muscles in ALS and SMA. J. Clin. Med. 2022, 11, 1163. [Google Scholar] [CrossRef] [PubMed]
- Pirola, A.; De Mattia, E.; Lizio, A.; Sannicolò, G.; Carraro, E.; Rao, F.; Sansone, V.; Lunetta, C. The prognostic value of spirometric tests in Amyotrophic Lateral Sclerosis patients. Clin. Neurol. Neurosurg. 2019, 184, 105456. [Google Scholar] [CrossRef] [PubMed]
- Baranello, G.; Gorni, K.; Daigl, M.; Kotzeva, A.; Evans, R.; Hawkins, N.; Scott, D.A.; Mahajan, A.; Muntoni, F.; Servais, L. Prognostic Factors and Treatment-Effect Modifiers in Spinal Muscular Atrophy. Clin. Pharmacol. Ther. 2021, 110, 1435–1454. [Google Scholar] [CrossRef]
- Bach, J.R.; Tuccio, M.C.; Khan, U.; Saporito, L.R. Vital capacity in spinal muscular atrophy. Am. J. Phys. Med. Rehabil. 2012, 91, 487–493. [Google Scholar] [CrossRef]
- Gómez-García de la Banda, M.; Amaddeo, A.; Khirani, S.; Pruvost, S.; Barnerias, C.; Dabaj, I.; Bénézit, A.; Durigneux, J.; Carlier, R.Y.; Desguerre, I.; et al. Assessment of respiratory muscles and motor function in children with SMA treated by nusinersen. Pediatr. Pulmonol. 2021, 56, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.; Sly, P.D.; Ware, R.S.; Begum, N.; Deegan, S.; Thomas, N.; Gauld, L.M. Effect of nusinersen on respiratory function in paediatric spinal muscular atrophy types 1–3. Thorax 2022, 77, 40–46. [Google Scholar] [CrossRef]
- Trucco, F.; Ridout, D.; Weststrate, H.; Scoto, M.; Rohwer, A.; Coratti, G.; Main, M.L.; Mayhew, A.G.; Montes, J.; De Sanctis, R.; et al. Therapeutic Role of Nusinersen on Respiratory Progression in Pediatric Patients With Spinal Muscular Atrophy Type 2 and Nonambulant Type 3. Neurol. Clin. Pract. 2024, 14, e200298. [Google Scholar] [CrossRef]
- LoMauro, A.; Mastella, C.; Alberti, K.; Masson, R.; Aliverti, A.; Baranello, G. Effect of nusinersen on respiratory muscle function in different subtypes of type 1 spinal muscular atrophy. Am. J. Respir. Crit. Care Med. 2019, 200, 1547–1550. [Google Scholar] [CrossRef]
- Pechmann, A.; Behrens, M.; Dörnbrack, K.; Tassoni, A.; Stein, S.; Vogt, S.; Zöller, D.; Bernert, G.; Hagenacker, T.; Schara-Schmidt, U.; et al. Effect of nusinersen on motor, respiratory and bulbar function in early-onset spinal muscular atrophy. Brain 2023, 146, 668–677. [Google Scholar] [CrossRef]
- Nicot, F.; Hart, N.; Forin, V.; Le Boulé, M.; Clément, A.; Polkey, M.I.; Lofaso, F.; Fauroux, B. Respiratory muscle testing: A valuable tool for children with neuromuscular disorders. Am. J. Respir. Crit. Care Med. 2006, 174, 67–74. [Google Scholar] [CrossRef]
- Bohannon, R.W. Muscle strength: Clinical and prognostic value of hand-grip dynamometry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Barker, L.; Smith, C.; Perkins, B.; Burhans, K.; Newman, R.; Zimmerman, L.; Cook, J.; Russman, B.S.; Samaha, F.; Iannaccone, S.; et al. Clinical Trials in Spinal Muscular Atrophy: Protocol Development and Reliability of Quantitative Strength Assessment Method. Neurorehabil. Neural Repair. 1992, 6, 175–183. [Google Scholar] [CrossRef]
- Merlini, L.; Bertini, E.; Minetti, C.; Mongini, T.; Morandi, L.; Angelini, C.; Vita, G. Motor function-muscle strength relationship in spinal muscular atrophy. Muscle Nerve 2004, 29, 548–552. [Google Scholar] [CrossRef] [PubMed]
- De Wel, B.; Goosens, V.; Sobota, A.; Van Camp, E.; Geukens, E.; Van Kerschaver, G.; Jagut, M.; Claes, K.; Claeys, K.G. Nusinersen treatment significantly improves hand grip strength, hand motor function and MRC sum scores in adult patients with spinal muscular atrophy types 3 and 4. J. Neurol. 2021, 268, 923–935. [Google Scholar] [CrossRef]
- Febrer, A.; Rodriguez, N.; Alias, L.; Tizzano, E. Measurement of muscle strength with a handheld dynamometer in patients with chronic spinal muscular atrophy. J. Rehabil. Med. 2010, 42, 228–231. [Google Scholar] [CrossRef]
- Milev, E.; Selby, V.; Wolfe, A.; Rohwer, A.; Tillmann, R.; Ramsey, D.; Iodice, M.; Hogrel, J.Y.; Baranello, G.; Scoto, M.; et al. Assessment of the upper limb function, strength, and mobility in treatment-naive children with spinal muscular atrophy Types 2 and 3. Muscle Nerve 2024, 69, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Merlini, L.; Mazzone, E.S.; Solari, A.; Morandi, L. Reliability of hand-held dynamometry in spinal muscular atrophy. Muscle Nerve 2002, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Werlauff, U.; Steffensen, B.F. The applicability of four clinical methods to evaluate arm and hand function in all stages of spinal muscular atrophy type II. Disabil. Rehabil. 2014, 36, 2120–2126. [Google Scholar] [CrossRef]
- Wu, J.S.; Darras, B.T.; Rutkove, S.B. Assessing spinal muscular atrophy with quantitative ultrasound. Neurology 2010, 75, 526–531. [Google Scholar] [CrossRef]
- Heckmatt, J.Z.; Leeman, S.; Dubowitz, V. Ultrasound imaging in the diagnosis of muscle disease. J. Pediatr. 1982, 101, 656–660. [Google Scholar] [CrossRef]
- Maurits, N.M.; Beenakker, E.A.C.; Van Schaik, D.E.C.; Fock, J.M.; Van Der Hoeven, J.H. Muscle ultrasound in children: Normal values and application to neuromuscular disorders. Ultrasound Med. Biol. 2004, 30, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Hobson-Webb, L.D. Neuromuscular ultrasound in polyneuropathies and motor neuron disease. Muscle Nerve 2013, 47, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Pillen, S.; van Keimpema, M.; Nievelstein, R.A.J.; Verrips, A.; van Kruijsbergen-Raijmann, W.; Zwarts, M.J. Skeletal muscle ultrasonography: Visual versus quantitative evaluation. Ultrasound Med. Biol. 2006, 32, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Shklyar, I. Electrical Impedance Myography and Quantitative Ultrasound As Biomarkers in Duchenne Muscular Dystrophy. Yale Med. Thesis Digit. Libr. 2014, 1922, 1–50. [Google Scholar]
- Zaidman, C.M.; Wu, J.S.; Kapur, K.; Pasternak, A.; Madabusi, L.; Yim, S.; Pacheck, A.; Szelag, H.; Harrington, T.; Darras, B.T.; et al. Quantitative muscle ultrasound detects disease progression in Duchenne muscular dystrophy. Ann. Neurol. 2017, 81, 633–640. [Google Scholar] [CrossRef]
- Shahrizaila, N.; Noto, Y.; Simon, N.G.; Huynh, W.; Shibuya, K.; Matamala, J.M.; Dharmadasa, T.; Devenney, E.; Kennerson, M.L.; Nicholson, G.A.; et al. Quantitative muscle ultrasound as a biomarker in Charcot-Marie-Tooth neuropathy. Clin. Neurophysiol. 2017, 128, 227–232. [Google Scholar] [CrossRef]
- Pathak, S.; Caress, J.B.; Wosiski-Kuhn, M.; Milligan, C.; Williams, D.; Cartwright, M.S. A pilot study of neuromuscular ultrasound as a biomarker for amyotrophic lateral sclerosis. Muscle Nerve 2019, 59, 181–186. [Google Scholar] [CrossRef]
- Jansen, M.; van Alfen, N.; Nijhuis van der Sanden, M.W.G.; van Dijk, J.P.; Pillen, S.; de Groot, I.J.M. Quantitative muscle ultrasound is a promising longitudinal follow-up tool in Duchenne muscular dystrophy. Neuromuscul. Disord. 2012, 22, 306–317. [Google Scholar] [CrossRef]
- Ng, K.W.; Connolly, A.M.; Zaidman, C.M. Quantitative muscle ultrasound measures rapid declines over time in children with SMA type 1. J. Neurol. Sci. 2015, 358, 178–182. [Google Scholar] [CrossRef]
- Pelosi, L.; Rodrigues, M.; Zhong, C.; Patel, S.; Roxburgh, R. Quantitative muscle ultrasound in adult spinal muscular atrophy. A pilot study. Muscle Nerve 2024, 69, 349–353. [Google Scholar] [CrossRef]
- Harlaar, L.; Ciet, P.; van der Ploeg, A.T.; Brusse, E.; van der Beek, N.A.M.E.; Wielopolski, P.A.; de Bruijne, M.; Tiddens, H.A.W.M.; van Doorn, P.A. Imaging of respiratory muscles in neuromuscular disease: A review. Neuromuscul. Disord. 2018, 28, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Berti, B.; Palermo, C.; Leone, D.; Ferrantini, G.; De Sanctis, R.; Onesimo, R.; Curatola, A.; Fanelli, L.; Forcina, N.; et al. Ultrasound assessment of diaphragmatic function in type 1 spinal muscular atrophy. Pediatr. Pulmonol. 2020, 55, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Freigang, M.; Langner, S.; Hermann, A.; Günther, R. Impaired diaphragmatic motility in treatment-naive adult patients with spinal muscular atrophy improved during nusinersen treatment. Muscle Nerve 2023, 68, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Pillen, S.; Scholten, R.R.; Zwarts, M.J.; Verrips, A. Quantitative skeletal muscle ultrasonography in children with suspected neuromuscular disease. Muscle Nerve 2003, 27, 699–705. [Google Scholar] [CrossRef]
- Brockmann, K.; Becker, P.; Schreiber, G.; Neubert, K.; Brunner, E.; Bönnemann, C. Sensitivity and specificity of qualitative muscle ultrasound in assessment of suspected neuromuscular disease in childhood. Neuromuscul. Disord. 2007, 17, 517–523. [Google Scholar] [CrossRef]
- Nakamura, R.; Kitamura, A.; Tsukamoto, T.; Otowa, Y.; Okamoto, N.; Ogawa, N.; Yamakawa, I.; Kim, H.; Sanada, M.; Urushitani, M. Spinal muscular atrophy type 3 showing a specific pattern of selective vulnerability on muscle ultrasound. Intern. Med. 2021, 60, 1935–1939. [Google Scholar] [CrossRef]
- Wijntjes, J.; van Alfen, N. Muscle ultrasound: Present state and future opportunities. Muscle Nerve 2021, 63, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Misawa, S.; Noto, Y.; Shibuya, K.; Isose, S.; Sekiguchi, Y.; Nasu, S.; Kuwabara, S. Ultrasonographic detection of fasciculations markedly increases diagnostic sensitivity of ALS. Neurology 2011, 77, 1532–1537. [Google Scholar] [CrossRef]
- Walker, F.O.; Cartwright, M.S.; Wiesler, E.R.; Caress, J. Ultrasound of nerve and muscle. Clin. Neurophysiol. 2004, 115, 495–507. [Google Scholar] [CrossRef]
- Mayans, D.; Cartwright, M.S.; Walker, F.O. Neuromuscular Ultrasonography: Quantifying Muscle and Nerve Measurements. Phys. Med. Rehabil. Clin. N. Am. 2012, 23, 133–148. [Google Scholar] [CrossRef]
- Regensburger, A.P.; Wagner, A.L.; Hanslik, G.; Schüssler, S.C.; Fahlbusch, F.B.; Woelfle, J.; Jüngert, J.; Trollmann, R.; Knieling, F. Ultra–high-frequency ultrasound in patients with spinal muscular atrophy: A retrospective feasibility study. Muscle Nerve 2020, 61, E18–E21. [Google Scholar] [CrossRef] [PubMed]
- Leroy-Willig, A.; Willig, T.N.; Henry-Feugeas, M.C.; Frouin, V.; Marinier, E.; Boulier, A.; Barzic, F.; Schouman-Claeys, E.; Syrota, A. Body composition determined with MR in patients with Duchenne muscular dystrophy, spinal muscular atrophy, and normal subjects. Magn. Reson. Imaging 1997, 15, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.M.; Sinclair, C.D.J.; Fischmann, A.; Machado, P.M.; Reilly, M.M.; Yousry, T.A.; Thornton, J.S.; Hanna, M.G. MRI biomarker assessment of neuromuscular disease progression: A prospective observational cohort study. Lancet Neurol. 2016, 15, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.C.; Jong, Y.J.; Chiang, C.H.; Yang, C.W. Spinal muscular atrophy: MR evaluation. Pediatr. Radiol. 1992, 22, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Yoshioka, H.; Iwasaki, N.; Tanaka, R.; Saida, Y. MR findings of spinal muscular atrophy type II: Sibling cases. Magn. Reson. Med. Sci. 2003, 2, 195–198. [Google Scholar] [CrossRef]
- Mercuri, E.; Pichiecchio, A.; Allsop, J.; Messina, S.; Pane, M.; Muntoni, F. Muscle MRI in inherited neuromuscular disorders: Past, present, and future. J. Magn. Reson. Imaging 2007, 25, 433–440. [Google Scholar] [CrossRef]
- Sproule, D.M.; Punyanitya, M.; Shen, W.; Dashnaw, S.; Martens, B.; Montgomery, M.; Montes, J.; Battista, V.; Finkel, R.; Darras, B.; et al. Muscle volume estimation by magnetic resonance imaging in spinal muscular atrophy. J. Child Neurol. 2011, 26, 309–317. [Google Scholar] [CrossRef]
- Sproule, D.M.; Montgomery, M.J.; Punyanitya, M.; Shen, W.; Dashnaw, S.; Montes, J.; Dunaway, S.; Finkel, R.; Darras, B.; De Vivo, D.C.; et al. Thigh muscle volume measured by magnetic resonance imaging is stable over a 6-month interval in spinal muscular atrophy. J. Child Neurol. 2011, 26, 1252–1259. [Google Scholar] [CrossRef]
- Durmus, H.; Yilmaz, R.; Gulsen-Parman, Y.; Oflazer-Serdaroglu, P.; Cuttini, M.; Dursun, M.; Deymeer, F. Muscle magnetic resonance imaging in spinal muscular atrophy type 3: Selective and progressive involvement. Muscle Nerve 2017, 55, 651–656. [Google Scholar] [CrossRef]
- Brogna, C.; Cristiano, L.; Verdolotti, T.; Pichiecchio, A.; Cinnante, C.; Sansone, V.; Sconfienza, L.M.; Berardinelli, A.; Garibaldi, M.; Antonini, G.; et al. MRI patterns of muscle involvement in type 2 and 3 spinal muscular atrophy patients. J. Neurol. 2020, 267, 898–912. [Google Scholar] [CrossRef]
- Otto, L.A.M.; van der Pol, W.L.; Schlaffke, L.; Wijngaarde, C.A.; Stam, M.; Wadman, R.I.; Cuppen, I.; van Eijk, R.P.A.; Asselman, F.L.; Bartels, B.; et al. Quantitative MRI of skeletal muscle in a cross-sectional cohort of patients with spinal muscular atrophy types 2 and 3. NMR Biomed. 2020, 33, e4357. [Google Scholar] [CrossRef] [PubMed]
- Shimizu-Motohashi, Y.; Chiba, E.; Mizuno, K.; Yajima, H.; Ishiyama, A.; Takeshita, E.; Sato, N.; Oba, M.; Sasaki, M.; Ito, S.; et al. Muscle impairment in MRI affect variability in treatment response to nusinersen in patients with spinal muscular atrophy type 2 and 3: A retrospective cohort study. Brain Dev. 2023, 45, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Otto, L.A.M.; Froeling, M.; van Eijk, R.P.A.; Asselman, F.L.; Wadman, R.; Cuppen, I.; Hendrikse, J.; van der Pol, W.L. Quantification of disease progression in spinal muscular atrophy with muscle MRI—A pilot study. NMR Biomed. 2021, 34, e4473. [Google Scholar] [CrossRef] [PubMed]
- Kollmer, J.; Bendszus, M.; Pham, M. MR Neurography: Diagnostic Imaging in the PNS. Clin. Neuroradiol. 2015, 25, 283–289. [Google Scholar] [CrossRef]
- Kollmer, J.; Hilgenfeld, T.; Ziegler, A.; Saffari, A.; Sam, G.; Hayes, J.M.; Pietsch, A.; Jost, M.; Heiland, S.; Bendszus, M.; et al. Quantitative MR neurography biomarkers in 5q-linked spinal muscular atrophy. Neurology 2019, 93, E653–E664. [Google Scholar] [CrossRef]
- Mercuri, E.; Pichiecchio, A.; Counsell, S.; Allsop, J.; Cini, C.; Jungbluth, H.; Uggetti, C.; Bydder, G. A short protocol for muscle MRI in children with muscular dystrophies. Eur. J. Paediatr. Neurol. 2002, 6, 305–307. [Google Scholar] [CrossRef]
- Rutkove, S.B. Electrical impedance myography: Background, current state, and future directions. Muscle Nerve 2009, 40, 936–946. [Google Scholar] [CrossRef]
- Rutkove, S.B.; Caress, J.B.; Cartwright, M.S.; Burns, T.M.; Warder, J.; David, W.S.; Goyal, N.; Maragakis, N.J.; Clawson, L.; Benatar, M.; et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotroph. Lateral Scler. 2012, 13, 439–445. [Google Scholar] [CrossRef]
- Sanchez, B.; Rutkove, S.B. Electrical Impedance Myography and Its Applications in Neuromuscular Disorders. Neurotherapeutics 2017, 14, 107–118. [Google Scholar] [CrossRef]
- Leitner, M.L.; Kapur, K.; Darras, B.T.; Yang, M.; Wong, B.; Dalle Pazze, L.; Florence, J.; Buck, M.; Freedman, L.; Bohorquez, J.; et al. Electrical impedance myography for reducing sample size in Duchenne muscular dystrophy trials. Ann. Clin. Transl. Neurol. 2020, 7, 4–14. [Google Scholar] [CrossRef]
- Rutkove, S.B.; Shefner, J.M.; Gregas, M.; Butler, H.; Caracciolo, J.; Lin, C.; Fogerson, P.M.; Mongiovi, P.; Darras, B.T. Characterizing spinal muscular atrophy with electrical impedance myography. Muscle Nerve 2010, 42, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Rutkove, S.B.; Gregas, M.C.; Darras, B.T. Electrical impedance myography in spinal muscular atrophy: A longitudinal study. Muscle Nerve 2012, 45, 642–647. [Google Scholar] [CrossRef]
- Kolb, S.J.; Kissel, J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015, 33, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Geisbush, T.R.; Arnold, W.D.; Rosen, G.D.; Zaworski, P.G.; Rutkove, S.B. A comparison of three electrophysiological methods for the assessment of disease status in a mild spinal muscular atrophy mouse model. PLoS ONE 2014, 9, e111428. [Google Scholar] [CrossRef]
- Arnold, W.D.; McGovern, V.L.; Sanchez, B.; Li, J.; Corlett, K.M.; Kolb, S.J.; Rutkove, S.B.; Burghes, A.H. The neuromuscular impact of symptomatic SMN restoration in a mouse model of spinal muscular atrophy. Neurobiol. Dis. 2016, 87, 116–123. [Google Scholar] [CrossRef]
- Simic, G. Pathogenesis of proximal autosomal recessive spinal muscular atrophy. Acta Neuropathol. 2008, 116, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Calucho, M.; Bernal, S.; Alías, L.; March, F.; Venceslá, A.; Rodríguez-Álvarez, F.J.; Aller, E.; Fernández, R.M.; Borrego, S.; Millán, J.M.; et al. Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul. Disord. 2018, 28, 208–215. [Google Scholar] [CrossRef]
- Wirth, B.; Brichta, L.; Schrank, B.; Lochmüller, H.; Blick, S.; Baasner, A.; Heller, R. Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum. Genet. 2006, 119, 422–428. [Google Scholar] [CrossRef]
- Feldkötter, M.; Schwarzer, V.; Wirth, R.; Wienker, T.F.; Wirth, B. Quantitative Analyses of SMN1 and SMN2 Based on Real-Time LightCycler PCR: Fast and Highly Reliable Carrier Testing and Prediction of Severity of Spinal Muscular Atrophy. Am. J. Hum. Genet. 2002, 70, 358–368. [Google Scholar] [CrossRef]
- Wadman, R.I.; Stam, M.; Gijzen, M.; Lemmink, H.H.; Snoeck, I.N.; Wijngaarde, C.A.; Braun, K.P.J.; Schoenmakers, M.A.G.C.; Van Den Berg, L.H.; Dooijes, D.; et al. Association of motor milestones, SMN2 copy and outcome in spinal muscular atrophy types 0-4. J. Neurol. Neurosurg. Psychiatry 2017, 88, 364–367. [Google Scholar] [CrossRef]
- Jędrzejowska, M.; Borkowska, J.; Zimowski, J.; Kostera-Pruszczyk, A.; Milewski, M.; Jurek, M.; Sielska, D.; Kostyk, E.; Nyka, W.; Zaremba, J.; et al. Unaffected patients with a homozygous absence of the SMN1 gene. Eur. J. Hum. Genet. 2008, 16, 930–934. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheleznyakova, G.Y.; Kiselev, A.V.; Vakharlovsky, V.G.; Rask-Andersen, M.; Chavan, R.; Egorova, A.A.; Schiöth, H.B.; Baranov, V.S. Genetic and expression studies of SMN2 gene in Russian patients with spinal muscular atrophy type II and III. BMC Med. Genet. 2011, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Cicala, G.; Capasso, A.; Coratti, G.; Fiori, S.; Cutrona, C.; D’Amico, A.; Sansone, V.A.; Bruno, C.; Messina, S.; et al. Clinical Phenotype of Pediatric and Adult Patients with Spinal Muscular Atrophy with Four SMN2 Copies: Are They Really All Stable? Ann. Neurol. 2023, 94, 1126–1135. [Google Scholar] [CrossRef]
- Monani, U.R.; Sendtner, M.; Coovert, D.D.; Parsons, D.W.; Andreassi, C.; Le, T.T.; Jablonka, S.; Schrank, B.; Rossol, W.; Prior, T.W.; et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn−/− mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 2000, 9, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Hsieh-Li, H.M.; Chang, J.G.; Jong, Y.J.; Wu, M.H.; Wang, N.M.; Tsai, C.H.; Li, H. A mouse model for spinal muscular atrophy. Nat. Genet. 2000, 24, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, B.; Prior, T.; Zhang, X.; Miller, R.; Kolb, S.J.; Moore, D.; Bradley, W.; Barohn, R.; Bryan, W.; Gelinas, D.; et al. An analysis of disease severity based on SMN2 copy number in adults with spinal muscular atrophy. Muscle Nerve 2009, 40, 652–656. [Google Scholar] [CrossRef]
- Qu, Y.J.; Ge, X.S.; Bai, J.L.; Wang, L.W.; Cao, Y.Y.; Lu, Y.Y.; Jin, Y.W.; Wang, H.; Song, F. Association of copy numbers of survival motor neuron gene 2 and neuronal apoptosis inhibitory protein gene with the natural history in a Chinese spinal muscular atrophy cohort. J. Child Neurol. 2015, 30, 429–436. [Google Scholar] [CrossRef]
- Petit, F.; Cuisset, J.M.; Rouaix-Emery, N.; Cancés, C.; Sablonnière, B.; Bieth, E.; Moerman, A.; Sukno, S.; Hardy, N.; Holder-Espinasse, M.; et al. Insights into genotype-phenotype correlations in spinal muscular atrophy: A retrospective study of 103 patients. Muscle Nerve 2011, 43, 26–30. [Google Scholar] [CrossRef]
- Farrar, M.A.; Vucic, S.; Johnston, H.M.; Du Sart, D.; Kiernan, M.C. Pathophysiological insights derived by natural history and motor function of spinal muscular atrophy. J. Pediatr. 2013, 162, 155–159. [Google Scholar] [CrossRef]
- Glanzman, A.M.; O’Hagen, J.M.; McDermott, M.P.; Martens, W.B.; Flickinger, J.; Riley, S.; Quigley, J.; Montes, J.; Dunaway, S.; Deng, L.; et al. Validation of the Expanded Hammersmith Functional Motor Scale in Spinal Muscular Atrophy Type II and III. J. Child Neurol. 2011, 26, 1499–1507. [Google Scholar] [CrossRef]
- Brichta, L.; Hofmann, Y.; Hahnen, E.; Siebzehnrubi, F.A.; Raschke, H.; Blumcke, I.; Eyupoglu, I.Y.; Wirth, B. Valproic acid increases the SMN2 protein level: A well-known drug as a potential therapy for spinal muscular atrophy. Hum. Mol. Genet. 2003, 12, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Baranov, V.S.; Kiselev, A.V.; Vakharlovsky, V.G.; Zheleznjakova, G.J.; Komantzev, V.N.; Malisheva, O.V.; Glotov, A.S.; Ivashchenko, T.E.; Baranov, A.N. Molecular genetic basis of proximal spinal muscular atrophy and experience in its pharmaceutical treatment. Russ. J. Genet. 2008, 44, 1148–1159. [Google Scholar] [CrossRef]
- Tiziano, F.D.; Pinto, A.M.; Fiori, S.; Lomastro, R.; Messina, S.; Bruno, C.; Pini, A.; Pane, M.; D’Amico, A.; Ghezzo, A.; et al. SMN transcript levels in leukocytes of SMA patients determined by absolute real-time PCR. Eur. J. Hum. Genet. 2010, 18, 52–58. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.-L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef]
- Pane, M.; Coratti, G.; Sansone, V.A.; Messina, S.; Bruno, C.; Catteruccia, M.; Sframeli, M.; Albamonte, E.; Pedemonte, M.; D’Amico, A.; et al. Nusinersen in type 1 spinal muscular atrophy: Twelve-month real-world data. Ann. Neurol. 2019, 86, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1390–1397. [Google Scholar] [CrossRef]
- Helmken, C.; Hofmann, Y.; Schoenen, F.; Oprea, G.; Raschke, H.; Rudnik-Schöneborn, S.; Zerres, K.; Wirth, B. Evidence for a modifying pathway in SMA discordant families: Reduced SMN level decreases the amount of its interacting partners and Htra2-beta1. Hum. Genet. 2003, 114, 11–21. [Google Scholar] [CrossRef]
- Cuscó, I.; Barceló, M.J.; Rojas–García, R.; Illa, I.; Gámez, J.; Cervera, C.; Pou, A.; Izquierdo, G.; Baiget, M.; Tizzano, E.F. SMN2 copy number predicts acute or chronic spinal muscular atrophy but does not account for intrafamilial variability in siblings. J. Neurol. 2006, 253, 21–25. [Google Scholar] [CrossRef]
- McAndrew, P.E.; Parsons, D.W.; Simard, L.R.; Rochette, C.; Ray, P.N.; Mendell, J.R.; Prior, T.W.; Burghes, A.H.M. Identification of Proximal Spinal Muscular Atrophy Carriers and Patients by Analysis of SMNT and SMNC Gene Copy Number. Am. J. Hum. Genet. 1997, 60, 1411–1422. [Google Scholar] [CrossRef]
- Maretina, M.A.; Zheleznyakova, G.Y.; Lanko, K.M.; Egorova, A.A.; Baranov, V.S.; Kiselev, A.V. Molecular Factors Involved in Spinal Muscular Atrophy Pathways as Possible Disease-modifying Candidates. Curr. Genom. 2018, 19, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Ruhno, C.; McGovern, V.L.; Avenarius, M.R.; Snyder, P.J.; Prior, T.W.; Nery, F.C.; Muhtaseb, A.; Roggenbuck, J.S.; Kissel, J.T.; Sansone, V.A.; et al. Complete sequencing of the SMN2 gene in SMA patients detects SMN gene deletion junctions and variants in SMN2 that modify the SMA phenotype. Hum. Genet. 2019, 138, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Pérez, L.; Paramonov, I.; Leno, J.; Bernal, S.; Alias, L.; Fuentes-Prior, P.; Cuscó, I.; Tizzano, E.F. Beyond copy number: A new, rapid, and versatile method for sequencing the entire SMN2 gene in SMA patients. Hum. Mutat. 2021, 42, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Bernal, S.; Alias, L.; Barcelo, M.J.; Also-Rallo, E.; Martinez-Hernandez, R.; Gamez, J.; Guillen-Navarro, E.; Rosell, J.; Hernando, I.; Rodriguez-Alvarez, F.J.; et al. The c.859G>C variant in the SMN2 gene is associated with types II and III SMA and originates from a common ancestor. J. Med. Genet. 2010, 47, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.W.; Krainer, A.R.; Hua, Y.; Swoboda, K.J.; Snyder, P.C.; Bridgeman, S.J.; Burghes, A.H.M.; Kissel, J.T. A Positive Modifier of Spinal Muscular Atrophy in the SMN2 Gene. Am. J. Hum. Genet. 2009, 85, 408–413. [Google Scholar] [CrossRef]
- Blasco-Pérez, L.; Costa-Roger, M.; Leno-Colorado, J.; Bernal, S.; Alias, L.; Codina-Solà, M.; Martínez-Cruz, D.; Castiglioni, C.; Bertini, E.; Travaglini, L.; et al. Deep Molecular Characterization of Milder Spinal Muscular Atrophy Patients Carrying the c.859G>C Variant in SMN2. Int. J. Mol. Sci. 2022, 23, 8289. [Google Scholar] [CrossRef]
- Vezain, M.; Saugier-Veber, P.; Goina, E.; Touraine, R.; Manel, V.; Toutain, A.; Fehrenbach, S.; Frébourg, T.; Pagani, F.; Tosi, M.; et al. A rare SMN2 variant in a previously unrecognized composite splicing regulatory element induces exon 7 inclusion and reduces the clinical severity of spinal muscular atrophy. Hum. Mutat. 2010, 31, E1110–E1125. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.H.; Sun, J.; Krainer, A.R.; Hua, Y.; Prior, T.W. A-44G transition in SMN2 intron 6 protects patients with spinal muscular atrophy. Hum. Mol. Genet. 2017, 26, 2768–2780. [Google Scholar] [CrossRef]
- Didonato, C.J.; Ingraham, S.E.; Mendell, J.R.; Prior, T.W.; Lenard, S.; Moxley, R.T.; Florence, J.; Burghes, A.H.M. Deletion and conversion in spinal muscular atrophy patients: Is there a relationship to severity? Ann. Neurol. 1997, 41, 230–237. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, K.; Chen, D.; Wang, C.; Abdalla, M.; Zhang, H.; Tian, R.; Liu, Y.; Song, L.; Zhang, X.; et al. Real-world evidence: Risdiplam in a patient with spinal muscular atrophy type I with a novel splicing mutation and one SMN2 copy. Hum. Mol. Genet. 2024, 33, 1120–1130. [Google Scholar] [CrossRef]
- Hauke, J.; Riessland, M.; Lunke, S.; Eyüpoglu, I.Y.; Blümcke, I.; El-Osta, A.; Wirth, B.; Hahnen, E. Survival motor neuron gene 2 silencing by DNA methylation correlates with spinal muscular atrophy disease severity and can be bypassed by histone deacetylase inhibition. Hum. Mol. Genet. 2009, 18, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qu, Y.; He, S.; Li, Y.; Bai, J.; Jin, Y.; Wang, H.; Song, F. Association between SMN2 methylation and disease severity in Chinese children with spinal muscular atrophy SMN2. J. Zhejiang Univ. B 2016, 17, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sumner, C.J.; Kolb, S.J.; Harmison, G.G.; Jeffries, N.O.; Schadt, K.; Finkel, R.S.; Dreyfuss, G.; Fischbeck, K.H. SMN mRNA and protein levels in peripheral blood: Biomarkers for SMA clinical trials. Neurology 2006, 66, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Brichta, L.; Holker, I.; Haug, K.; Klockgether, T.; Wirth, B. In vivo activation of SMN in spinal muscular atrophy carriers and patients treated with valproate. Ann. Neurol. 2006, 59, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Simard, L.R.; Bélanger, M.C.; Morissette, S.; Wride, M.; Prior, T.W.; Swoboda, K.J. Preclinical validation of a multiplex real-time assay to quantify SMN mRNA in patients with SMA. Neurology 2007, 68, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Vezain, M.; Saugier-Veber, P.; Melki, J.; Toutain, A.; Bieth, E.; Husson, M.; Pedespan, J.M.; Viollet, L.; Pénisson-Besnier, I.; Fehrenbach, S.; et al. A sensitive assay for measuring SMN mRNA levels in peripheral blood and in muscle samples of patients affected with spinal muscular atrophy. Eur. J. Hum. Genet. 2007, 15, 1054–1062. [Google Scholar] [CrossRef]
- Crawford, T.O.; Paushkin, S.V.; Kobayashi, D.T.; Forrest, S.J.; Joyce, C.L.; Finkel, R.S.; Kaufmann, P.; Swoboda, K.J.; Tiziano, D.; Lomastro, R.; et al. Evaluation of SMN Protein, Transcript, and Copy Number in the Biomarkers for Spinal Muscular Atrophy (BforSMA) Clinical Study. PLoS ONE 2012, 7, e33572. [Google Scholar] [CrossRef]
- Kolb, S.J.; Coffey, C.S.; Yankey, J.W.; Krosschell, K.; Arnold, W.D.; Rutkove, S.B.; Swoboda, K.J.; Reyna, S.P.; Sakonju, A.; Darras, B.T.; et al. Baseline results of the NeuroNEXT spinal muscular atrophy infant biomarker study. Ann. Clin. Transl. Neurol. 2016, 3, 132–145. [Google Scholar] [CrossRef]
- Maretina, M.; Egorova, A.; Lanko, K.; Baranov, V.; Kiselev, A. Evaluation of Mean Percentage of Full-Length SMN Transcripts as a Molecular Biomarker of Spinal Muscular Atrophy. Genes 2022, 13, 1911. [Google Scholar] [CrossRef]
- Maretina, M.; Il’ina, A.; Egorova, A.; Glotov, A.; Kiselev, A. Development of 2′-O-Methyl and LNA Antisense Oligonucleotides for SMN2 Splicing Correction in SMA Cells. Biomedicines 2023, 11, 3071. [Google Scholar] [CrossRef]
- Liu, Q.; Dreyfuss, G. A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 1996, 15, 3555–3565. [Google Scholar] [CrossRef] [PubMed]
- Al-Hilal, H.; Maretina, M.; Egorova, A.; Glotov, A.; Kiselev, A. Assessment of Nuclear Gem Quantity for Evaluating the Efficacy of Antisense Oligonucleotides in Spinal Muscular Atrophy Cells. Methods Protoc. 2024, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.T.; Decker, D.; Zaworski, P.; Klott, K.; McGonigal, J.; Ghazal, N.; Sly, L.; Chung, B.; Vanderlugt, J.; Chen, K.S. Evaluation of Peripheral Blood Mononuclear Cell Processing and Analysis for Survival Motor Neuron Protein. PLoS ONE 2012, 7, e50763. [Google Scholar] [CrossRef][Green Version]
- Piepers, S.; Cobben, J.M.; Sodaar, P.; Jansen, M.D.; Wadman, R.I.; Meester-Delver, A.; Poll-The, B.T.; Lemmink, H.H.; Wokke, J.H.J.; Van Der Pol, W.L.; et al. Quantification of SMN protein in leucocytes from spinal muscular atrophy patients: Effects of treatment with valproic acid. J. Neurol. Neurosurg. Psychiatry 2011, 82, 850–852. [Google Scholar] [CrossRef][Green Version]
- Man, N.T.; Humphrey, E.; Lam, L.T.; Fuller, H.R.; Lynch, T.A.; Sewry, C.A.; Goodwin, P.R.; MacKenzie, A.E.; Morris, G.E. A two-site ELISA can quantify upregulation of SMN protein by drugs for spinal muscular atrophy. Neurology 2008, 71, 1757–1763. [Google Scholar] [CrossRef]
- Tiziano, F.D.; Lomastro, R.; Abiusi, E.; Pasanisi, M.B.; Di Pietro, L.; Fiori, S.; Baranello, G.; Angelini, C.; Sorarù, G.; Gaiani, A.; et al. Longitudinal evaluation of SMN levels as biomarker for spinal muscular atrophy: Results of a phase IIb double-blind study of salbutamol. J. Med. Genet. 2019, 56, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Czech, C.; Tang, W.; Bugawan, T.; Mano, C.; Horn, C.; Iglesias, V.A.; Fröhner, S.; Zaworski, P.G.; Paushkin, S.; Chen, K.; et al. Biomarker for Spinal Muscular Atrophy: Expression of SMN in Peripheral Blood of SMA Patients and Healthy Controls. PLoS ONE 2015, 10, e0139950. [Google Scholar] [CrossRef]
- Zaworski, P.; Von Herrmann, K.M.; Taylor, S.; Sunshine, S.S.; Mccarthy, K.; Risher, N.; Newcomb, T.; Weetall, M.; Prior, T.W.; Swoboda, K.J.; et al. SMN Protein can be reliably measured in whole blood with an electrochemiluminescence (ECL) Immunoassay: Implications for clinical trials. PLoS ONE 2016, 11, e0150640. [Google Scholar] [CrossRef]
- Alves, C.R.R.; Zhang, R.; Johnstone, A.J.; Garner, R.; Eichelberger, E.J.; Lepez, S.D.S.D.; Yi, V.; Stevens, V.; Poxson, R.; Schwartz, R.; et al. Whole blood survival motor neuron protein levels correlate with severity of denervation in spinal muscular atrophy. Muscle Nerve 2020, 62, 351–357. [Google Scholar] [CrossRef]
- Coovert, D.D.; Le, T.T.; McAndrew, P.E.; Strasswimmer, J.; Crawford, T.O.; Mendell, J.R.; Coulson, S.E.; Androphy, E.J.; Prior, T.W.; Burghes, A.H.M. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997, 6, 1205–1214. [Google Scholar] [CrossRef]
- Patrizi, A.L.; Tiziano, F.; Zappata, S.; Donati, M.A.; Neri, G.; Brahe, C. SMN protein analysis in fibroblast, amniocyte and CVS cultures from spinal muscular atrophy patients and its relevance for diagnosis. Eur. J. Hum. Genet. 1999, 7, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Burlet, P.; Liu, Q.; Bertrandy, S.; Clermont, O.; Munnich, A.; Dreyfuss, G.; Melki, J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 1997, 16, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.L.; Stout, C.; Kirkeby, L.; Vidal-Folch, N.; Oglesbee, D.; Hasadsri, L.; Selcen, D.; Milone, M.; Anderson, D.; Staff, N.P. SMN1 c.5C>G (p.Ala2Gly) missense variant, a challenging molecular SMA diagnosis associated with mild disease, preserves SMN nuclear gems in patient-specific fibroblasts. Front. Genet. 2024, 15, 1406819. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, C.; Angelozzi, C.; Tiziano, F.D.; Vitali, T.; De Vincenzi, E.; Boninsegna, A.; Villanova, M.; Bertini, E.; Pini, A.; Neri, G.; et al. Phenylbutyrate increases SMN expression in vitro: Relevance for treatment of spinal muscular atrophy. Eur. J. Hum. Genet. 2004, 12, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Wolstencroft, E.C. A non-sequence-specific requirement for SMN protein activity: The role of aminoglycosides in inducing elevated SMN protein levels. Hum. Mol. Genet. 2005, 14, 1199–1210. [Google Scholar] [CrossRef][Green Version]
- Mattis, V.B.; Rai, R.; Wang, J.; Chang, C.W.T.; Coady, T.; Lorson, C.L. Novel aminoglycosides increase SMN levels in spinal muscular atrophy fibroblasts. Hum. Genet. 2006, 120, 589–601. [Google Scholar] [CrossRef]
- Riessland, M.; Brichta, L.; Hahnen, E.; Wirth, B. The benzamide M344, a novel histone deacetylase inhibitor, significantly increases SMN2 RNA/protein levels in spinal muscular atrophy cells. Hum. Genet. 2006, 120, 101–110. [Google Scholar] [CrossRef]
- Ebert, A.D.; Yu, J.; Rose, F.F.; Mattis, V.B.; Lorson, C.L.; Thomson, J.A.; Svendsen, C.N. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 2009, 457, 277–280. [Google Scholar] [CrossRef]
- Lumpkin, C.J.; Harris, A.W.; Connell, A.J.; Kirk, R.W.; Whiting, J.A.; Saieva, L.; Pellizzoni, L.; Burghes, A.H.M.; Butchbach, M.E.R. Evaluation of the orally bioavailable 4-phenylbutyrate-tethered trichostatin A analogue AR42 in models of spinal muscular atrophy. Sci. Rep. 2023, 13, 10374. [Google Scholar] [CrossRef]
- Coady, T.H.; Baughan, T.D.; Shababi, M.; Passini, M.A.; Lorson, C.L. Development of a single vector system that enhances Trans-splicing of SMN2 transcripts. PLoS ONE 2008, 3, e3468. [Google Scholar] [CrossRef]
- Afonso-Grunz, F.; Müller, S. Principles of miRNA-mRNA interactions: Beyond sequence complementarity. Cell. Mol. Life Sci. 2015, 72, 3127–3141. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Marin-Muller, C.; Bharadwaj, U.; Chow, K.H.; Yao, Q.; Chen, C. MicroRNAs: Control and loss of control in human physiology and disease. World J. Surg. 2009, 33, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Kye, M.J.; Gonçalves, I.d.C.G. The role of miRNA in motor neuron disease. Front. Cell. Neurosci. 2014, 8, 15. [Google Scholar] [CrossRef]
- De Paola, E.; Verdile, V.; Paronetto, M.P. Dysregulation of microRNA metabolism in motor neuron diseases: Novel biomarkers and potential therapeutics. Non-Coding RNA Res. 2019, 4, 15–22. [Google Scholar] [CrossRef]
- Yedigaryan, L.; Sampaolesi, M. Therapeutic implications of miRNAs for muscle-wasting conditions. Cells 2021, 10, 3035. [Google Scholar] [CrossRef]
- Haramati, S.; Chapnik, E.; Sztainberg, Y.; Eilam, R.; Zwang, R.; Gershoni, N.; McGlinn, E.; Heiser, P.W.; Wills, A.M.; Wirguin, I.; et al. miRNA malfunction causes spinal motor neuron disease. Proc. Natl. Acad. Sci. USA 2010, 107, 13111–13116. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, A.; Ciafrè, S.A.; Murdocca, M.; Malgieri, A.; Masotti, A.; Sanchez, M.; Farace, M.G.; Novelli, G.; Sangiuolo, F. A perturbed MicroRNA expression pattern characterizes embryonic neural stem cells derived from a severe mouse model of spinal muscular atrophy (SMA). Int. J. Mol. Sci. 2015, 16, 18312–18327. [Google Scholar] [CrossRef]
- Catapano, F.; Zaharieva, I.; Scoto, M.; Marrosu, E.; Morgan, J.; Muntoni, F.; Zhou, H. Altered Levels of MicroRNA-9,-206, and-132 in Spinal Muscular Atrophy and Their Response to Antisense Oligonucleotide Therapy. Mol. Ther. Nucleic Acids 2016, 5, e331. [Google Scholar] [CrossRef]
- Yoo, A.S.; Staahl, B.T.; Chen, L.; Crabtree, G.R. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature 2009, 460, 642–646. [Google Scholar] [CrossRef]
- Magill, S.T.; Cambronne, X.A.; Luikart, B.W.; Lioy, D.T.; Leighton, B.H.; Westbrook, G.L.; Mandel, G.; Goodman, R.H. MicroRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 20382–20387. [Google Scholar] [CrossRef]
- Williams, A.H.; Valdez, G.; Moresi, V.; Qi, X.; McAnally, J.; Elliott, J.L.; Bassel-Duby, R.; Sanes, J.R.; Olson, E.N. MicroRNA-206 Delays ALS Progression and Promotes Regeneration of Neuromuscular Synapses in Mice. Science 2009, 326, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, V.; Boido, M.; De Amicis, E.; Piras, A.; Vercelli, A. Expression of muscle-specific MiRNA 206 in the progression of disease in a murine SMA model. PLoS ONE 2015, 10, e0128560. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, J.P.; Ward, J.; Taylor, I.A.; Waters, M.; Shi, Y.; Cannon, B.; Kelnar, K.; Kemppainen, J.; Brown, D.; Chen, C.; et al. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimer’s Dis. 2008, 14, 27–41. [Google Scholar] [CrossRef]
- Cacchiarelli, D.; Legnini, I.; Martone, J.; Cazzella, V.; D’Amico, A.; Bertini, E.; Bozzoni, I. miRNAs as serum biomarkers for Duchenne muscular dystrophy. EMBO Mol. Med. 2011, 3, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Waller, R.; Goodall, E.F.; Milo, M.; Cooper-Knock, J.; Da Costa, M.; Hobson, E.; Kazoka, M.; Wollff, H.; Heath, P.R.; Shaw, P.J.; et al. Serum miRNAs miR-206, 143–3p and 374b-5p as potential biomarkers for amyotrophic lateral sclerosis (ALS). Neurobiol. Aging 2017, 55, 123–131. [Google Scholar] [CrossRef]
- Carrico, A.; Cumba, L.R.; Medina, M.; Engel, T.; Forster, R.J. Ultrasensitive, label-free, electrochemical detection of miRNA-206 in human plasma: A potential biomarker associated with Alzheimer’s disease. Electrochem. Commun. 2024, 162, 107704. [Google Scholar] [CrossRef]
- Roberts, T.C.; Godfrey, C.; McClorey, G.; Vader, P.; Briggs, D.; Gardiner, C.; Aoki, Y.; Sargent, I.; Morgan, J.E.; Wood, M.J.A. Extracellular microRNAs are dynamic non-vesicular biomarkers of muscle turnover. Nucleic Acids Res. 2013, 41, 9500–9513. [Google Scholar] [CrossRef]
- Dobrowolny, G.; Martone, J.; Lepore, E.; Casola, I.; Petrucci, A.; Inghilleri, M.; Morlando, M.; Colantoni, A.; Scicchitano, B.M.; Calvo, A.; et al. A longitudinal study defined circulating microRNAs as reliable biomarkers for disease prognosis and progression in ALS human patients. Cell Death Discov. 2021, 7, 4. [Google Scholar] [CrossRef]
- Malacarne, C.; Galbiati, M.; Giagnorio, E.; Cavalcante, P.; Salerno, F.; Andreetta, F.; Cagnoli, C.; Taiana, M.; Nizzardo, M.; Corti, S.; et al. Dysregulation of muscle-specific micrornas as common pathogenic feature associated with muscle atrophy in als, sma and sbma: Evidence from animal models and human patients. Int. J. Mol. Sci. 2021, 22, 5673. [Google Scholar] [CrossRef]
- Welby, E.; Rehborg, R.J.; Harmelink, M.; Ebert, A.D. Assessment of cerebral spinal fluid biomarkers and microRNA-mediated disease mechanisms in spinal muscular atrophy patient samples. Hum. Mol. Genet. 2022, 31, 1830–1843. [Google Scholar] [CrossRef]
- Amin, N.D.; Bai, G.; Klug, J.R.; Bonanomi, D.; Pankratz, M.T.; Gifford, W.D.; Hinckley, C.A.; Sternfeld, M.J.; Driscoll, S.P.; Dominguez, B.; et al. Loss of motoneuron-specific microRNA-218 causes systemic neuromuscular failure. Science 2015, 350, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Kaifer, K.A.; Villalón, E.; O’Brien, B.S.; Sison, S.L.; Smith, C.E.; Simon, M.E.; Marquez, J.; O’Day, S.; Hopkins, A.E.; Neff, R.; et al. AAV9-mediated delivery of miR-23a reduces disease severity in Smn2B/− SMA model mice. Hum. Mol. Genet. 2019, 28, 3199–3210. [Google Scholar] [CrossRef] [PubMed]
- Sison, S.L.; Patitucci, T.N.; Seminary, E.R.; Villalon, E.; Lorson, C.L.; Ebert, A.D. Astrocyte-produced miR-146a as a mediator of motor neuron loss in spinal muscular atrophy. Hum. Mol. Genet. 2017, 26, 3409–3420. [Google Scholar] [CrossRef] [PubMed]
- Abiusi, E.; Infante, P.; Cagnoli, C.; Severini, L.L.; Pane, M.; Coratti, G.; Pera, M.C.; D’amico, A.; Diano, F.; Novelli, A.; et al. SMA-miRs (MiR-181a- 5p, -324-5p, and -451a) are overexpressed in spinal muscular atrophy skeletal muscle and serum samples. eLife 2021, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, S.; Marcuzzo, S.; Malacarne, C.; Giagnorio, E.; Masson, R.; Zanin, R.; Arnoldi, M.T.; Andreetta, F.; Simoncini, O.; Venerando, A.; et al. Circulating myomirs as potential biomarkers to monitor response to nusinersen in pediatric SMA patients. Biomedicines 2020, 8, 21. [Google Scholar] [CrossRef]
- Tasca, E.; Pegoraro, V.; Merico, A.; Angelini, C. Circulating microRNAs as biomarkers of muscle differentiation and atrophy in ALS. Clin. Neuropathol. 2016, 35, 22–30. [Google Scholar] [CrossRef]
- Magen, I.; Aharoni, S.; Yacovzada, N.S.; Tokatly Latzer, I.; Alves, C.R.R.; Sagi, L.; Fattal-Valevski, A.; Swoboda, K.J.; Katz, J.; Bruckheimer, E.; et al. Muscle microRNAs in the cerebrospinal fluid predict clinical response to nusinersen therapy in type II and type III spinal muscular atrophy patients. Eur. J. Neurol. 2022, 29, 2420–2430. [Google Scholar] [CrossRef]
- Chen, T.H.; Chang, S.H.; Wu, Y.F.; Yen, Y.P.; Hsu, F.Y.; Chen, Y.C.; Ming, Y.; Hsu, H.C.; Su, Y.C.; Wong, S.T.; et al. MiR34 contributes to spinal muscular atrophy and AAV9-mediated delivery of MiR34a ameliorates the motor deficits in SMA mice. Mol. Ther. Nucleic Acids 2023, 32, 144–160. [Google Scholar] [CrossRef]
- Zaharieva, I.T.; Scoto, M.; Aragon-Gawinska, K.; Ridout, D.; Doreste, B.; Servais, L.; Muntoni, F.; Zhou, H. Response of plasma microRNAs to nusinersen treatment in patients with SMA. Ann. Clin. Transl. Neurol. 2022, 9, 1011–1026. [Google Scholar] [CrossRef]
- D’Silva, A.M.; Kariyawasam, D.; Venkat, P.; Mayoh, C.; Farrar, M.A. Identification of Novel CSF-Derived miRNAs in Treated Paediatric Onset Spinal Muscular Atrophy: An Exploratory Study. Pharmaceutics 2023, 15, 170. [Google Scholar] [CrossRef]
- Yuan, A.; Nixon, R.A. Neurofilament Proteins as Biomarkers to Monitor Neurological Diseases and the Efficacy of Therapies. Front. Neurosci. 2021, 15, 689938. [Google Scholar] [CrossRef]
- Gentil, B.J.; Tibshirani, M.; Durham, H.D. Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res. 2015, 360, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xie, F.; Siedlak, S.L.; Nunomura, A.; Honda, K.; Moreira, P.I.; Zhua, X.; Smith, M.A.; Perry, G. Neurofilament proteins in neurodegenerative diseases. Cell. Mol. Life Sci. 2004, 61, 3057–3075. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef] [PubMed]
- Perrot, R.; Berges, R.; Bocquet, A.; Eyer, J. Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol. Neurobiol. 2008, 38, 27–65. [Google Scholar] [CrossRef]
- Gafson, A.R.; Barthélemy, N.R.; Bomont, P.; Carare, R.O.; Durham, H.D.; Julien, J.P.; Kuhle, J.; Leppert, D.; Nixon, R.A.; Weller, R.O.; et al. Neurofilaments: Neurobiological foundations for biomarker applications. Brain 2020, 143, 1975–1998. [Google Scholar] [CrossRef]
- Lu, C.H.; Macdonald-Wallis, C.; Gray, E.; Pearce, N.; Petzold, A.; Norgren, N.; Giovannoni, G.; Fratta, P.; Sidle, K.; Fish, M.; et al. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015, 84, 2247–2257. [Google Scholar] [CrossRef]
- Benatar, M.; Wuu, J.; Andersen, P.M.; Lombardi, V.; Malaspina, A. Neurofilament light: A candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann. Neurol. 2018, 84, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Poesen, K.; De Schaepdryver, M.; Stubendorff, B.; Gille, B.; Muckova, P.; Wendler, S.; Prell, T.; Ringer, T.M.; Rhode, H.; Stevens, O.; et al. Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 2017, 88, 2302–2309. [Google Scholar] [CrossRef]
- Darras, B.T.; Crawford, T.O.; Finkel, R.S.; Mercuri, E.; De Vivo, D.C.; Oskoui, M.; Tizzano, E.F.; Ryan, M.M.; Muntoni, F.; Zhao, G.; et al. Neurofilament as a potential biomarker for spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2019, 6, 932–944. [Google Scholar] [CrossRef]
- Olsson, B.; Alberg, L.; Cullen, N.C.; Michael, E.; Wahlgren, L.; Kroksmark, A.K.; Rostasy, K.; Blennow, K.; Zetterberg, H.; Tulinius, M. NFL is a marker of treatment response in children with SMA treated with nusinersen. J. Neurol. 2019, 266, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Nitz, E.; Smitka, M.; Schallner, J.; Akgün, K.; Ziemssen, T.; von der Hagen, M.; Tüngler, V. Serum neurofilament light chain in pediatric spinal muscular atrophy patients and healthy children. Ann. Clin. Transl. Neurol. 2021, 8, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, J.; Weiss, D.; Daubmann, A.; Schmitz, L.; Denecke, J. Evaluation of putative CSF biomarkers in paediatric spinal muscular atrophy (SMA) patients before and during treatment with nusinersen. J. Cell. Mol. Med. 2021, 25, 8419–8431. [Google Scholar] [CrossRef] [PubMed]
- Badina, M.; Sporea, C.; Bejan, G.C.; Mirea, A.; Ion, D.A. Impact of Nusinersen on Neurofilament, Creatinine Levels, and Motor Function in Pediatric Spinal Muscular Atrophy Rehabilitation: A Biomarker Analysis. Balneo PRM Res. J. 2024, 15, 681. [Google Scholar] [CrossRef]
- Wurster, C.D.; Günther, R.; Steinacker, P.; Dreyhaupt, J.; Wollinsky, K.; Uzelac, Z.; Witzel, S.; Kocak, T.; Winter, B.; Koch, J.C.; et al. Neurochemical markers in CSF of adolescent and adult SMA patients undergoing nusinersen treatment. Ther. Adv. Neurol. Disord. 2019, 12, 1–8. [Google Scholar] [CrossRef]
- Totzeck, A.; Stolte, B.; Kizina, K.; Bolz, S.; Schlag, M.; Thimm, A.; Kleinschnitz, C.; Hagenacker, T. Neurofilament heavy chain and tau protein are not elevated in cerebrospinal fluid of adult patients with spinal muscular atrophy during loading with nusinersen. Int. J. Mol. Sci. 2019, 20, 5397. [Google Scholar] [CrossRef]
- Milella, G.; Introna, A.; D’Errico, E.; Fraddosio, A.; Scaglione, G.; Morea, A.; Ucci, M.; Ruggieri, M.; Mastrapasqua, M.; Megna, M.; et al. Cerebrospinal Fluid and Clinical Profiles in Adult Type 2–3 Spinal Muscular Atrophy Patients Treated with Nusinersen: An 18-Month Single-Centre Experience. Clin. Drug Investig. 2021, 41, 775–784. [Google Scholar] [CrossRef]
- Musso, G.; Bello, L.; Capece, G.; Bozzoni, V.; Caumo, L.; Sabbatini, D.; Zangaro, V.; Sogus, E.; Cosma, C.; Petrosino, A.; et al. Neurofilament light chain and profilin-1 dynamics in 30 spinal muscular atrophy type 3 patients treated with nusinersen. Eur. J. Neurol. 2024, 31, e16393. [Google Scholar] [CrossRef]
- Faravelli, I.; Meneri, M.; Saccomanno, D.; Velardo, D.; Abati, E.; Gagliardi, D.; Parente, V.; Petrozzi, L.; Ronchi, D.; Stocchetti, N.; et al. Nusinersen treatment and cerebrospinal fluid neurofilaments: An explorative study on Spinal Muscular Atrophy type 3 patients. J. Cell. Mol. Med. 2020, 24, 3034–3039. [Google Scholar] [CrossRef]
- De Wel, B.; De Schaepdryver, M.; Poesen, K.; Claeys, K.G. Biochemical and clinical biomarkers in adult SMA 3–4 patients treated with nusinersen for 22 months. Ann. Clin. Transl. Neurol. 2022, 9, 1241–1251. [Google Scholar] [CrossRef]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Hosten, A.O. Clinical Methods: The History, Physical, and Laboratory Examinations. Ann. Intern. Med. 1990, 113, 563. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, H.W.; Kim, C.H.; Kim, K.I.; Chin, H.J.; Lee, H. A new equation to estimate muscle mass from creatinine and cystatin C. PLoS ONE 2016, 11, 0148495. [Google Scholar] [CrossRef] [PubMed]
- Van De Velde, N.M.; Koeks, Z.; Signorelli, M.; Verwey, N.; Overzier, M.; Bakker, J.A.; Sajeev, G.; Signorovitch, J.; Ricotti, V.; Verschuuren, J.; et al. Longitudinal Assessment of Creatine Kinase, Creatine/Creatinineratio, and Myostatin as Monitoring Biomarkers in Becker Muscular Dystrophy. Neurology 2023, 100, E975–E984. [Google Scholar] [CrossRef] [PubMed]
- Spitali, P.; Hettne, K.; Tsonaka, R.; Sabir, E.; Seyer, A.; Hemerik, J.B.A.; Goeman, J.J.; Picillo, E.; Ergoli, M.; Politano, L.; et al. Cross-sectional serum metabolomic study of multiple forms of muscular dystrophy. J. Cell. Mol. Med. 2018, 22, 2442–2448. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Lee, E.; Bradburn, M.; McDermott, C.J.; Shaw, P.J. Creatine kinase enzyme level correlates positively with serum creatinine and lean body mass, and is a prognostic factor for survival in amyotrophic lateral sclerosis. Eur. J. Neurol. 2016, 23, 1071–1078. [Google Scholar] [CrossRef]
- Tai, H.; Cui, L.; Guan, Y.; Liu, M.; Li, X.; Shen, D.; Li, D.; Cui, B.; Fang, J.; Ding, Q.; et al. Correlation of creatine kinase levels with clinical features and survival in amyotrophic lateral sclerosis. Front. Neurol. 2017, 8, 322. [Google Scholar] [CrossRef]
- Chen, X.P.; Wei, Q.Q.; Ou, R.W.; Hou, Y.B.; Zhang, L.Y.; Yuan, X.Q.; Yao, Y.Q.; Jia, D.S.; Zhang, Q.; Li, W.X.; et al. Creatine kinase in the diagnosis and prognostic prediction of amyotrophic lateral sclerosis: A retrospective case-control study. Neural Regen. Res. 2021, 16, 591–595. [Google Scholar] [CrossRef]
- Ceccanti, M.; Pozzilli, V.; Cambieri, C.; Libonati, L.; Onesti, E.; Frasca, V.; Fiorini, I.; Petrucci, A.; Garibaldi, M.; Palma, E.; et al. Creatine Kinase and Progression Rate in Amyotrophic Lateral Sclerosis. Cells 2020, 9, 1174. [Google Scholar] [CrossRef]
- Kittipeerapat, N.; Fabian, R.; Bernsen, S.; Weydt, P.; Castro-Gomez, S. Creatine Kinase MB Isoenzyme Is a Complementary Biomarker in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2023, 24, 11682. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Calvo, A.; Bovio, G.; Canosa, A.; Bertuzzo, D.; Galmozzi, F.; Cugnasco, P.; Clerico, M.; De Mercanti, S.; Bersano, E.; et al. Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: A population-based study. JAMA Neurol. 2014, 71, 1134–1142. [Google Scholar] [CrossRef]
- Patin, F.; Corcia, P.; Madji Hounoum, B.; Veyrat-Durebex, C.; Respaud, E.; Piver, E.; Benz-de Bretagne, I.; Vourc’h, P.; Andres, C.R.; Blasco, H. Biological follow-up in amyotrophic lateral sclerosis: Decrease in creatinine levels and increase in ferritin levels predict poor prognosis. Eur. J. Neurol. 2015, 22, 1385–1390. [Google Scholar] [CrossRef]
- Van Eijk, R.P.A.; Eijkemans, M.J.C.; Ferguson, T.A.; Nikolakopoulos, S.; Veldink, J.H.; Van Den Berg, L.H. Monitoring disease progression with plasma creatinine in amyotrophic lateral sclerosis clinical trials. J. Neurol. Neurosurg. Psychiatry 2018, 89, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, H.; Garofalo, D.C.; Santella, R.M.; Sorenson, E.J.; Oskarsson, B.; Fernandes, J.A.M., Jr.; Andrews, H.; Hupf, J.; Gilmore, M.; Heitzman, D.; et al. Plasma creatinine and oxidative stress biomarkers in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 263–272. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Sun, Y.; Liang, Y.; Li, Y.; Zhang, Y.; Deng, L.; Wen, X.; Zhang, C. Serum creatinine level: A supplemental index to distinguish Duchenne muscular dystrophy from Becker muscular dystrophy. Dis. Markers 2015, 2015, 10–14. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.; Liu, D.; Liang, Y.; Feng, P.; Li, H.; Zhu, Y.; He, R.; Lin, J.; Zhang, H.; et al. Serum creatinine as a biomarker for dystrophinopathy: A cross-sectional and longitudinal study. BMC Neurol. 2021, 21, 372. [Google Scholar] [CrossRef] [PubMed]
- Hijikata, Y.; Hashizume, A.; Yamada, S.; Inagaki, T.; Ito, D.; Hirakawa, A.; Suzuki, K.; Atsuta, N.; Tsuboi, T.; Hattori, M.; et al. Biomarker-based analysis of preclinical progression in spinal and bulbar muscular atrophy. Neurology 2018, 90, E1501–E1509. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, V.; Querin, G.; Ziff, O.J.; Zampedri, L.; Martinelli, I.; Heller, C.; Foiani, M.; Bertolin, C.; Lu, C.H.; Malik, B.; et al. Muscle and not neuronal biomarkers correlate with severity in spinal and bulbar muscular atrophy. Neurology 2019, 92, E1205–E1211. [Google Scholar] [CrossRef]
- Rudnik-Schöneborn, S.; Lützenrath, S.; Borkowska, J.; Karwanska, A.; Hausmanowa-Petrusewicz, I.; Zerres, K. Analysis of creatine kinase activity in 504 patients with proximal spinal muscular atrophy types I-III from the point of view of progression and severity. Eur. Neurol. 1998, 39, 154–162. [Google Scholar] [CrossRef]
- Alves, C.R.R.; Zhang, R.; Johnstone, A.J.; Garner, R.; Nwe, P.H.; Siranosian, J.J.; Swoboda, K.J. Serum creatinine is a biomarker of progressive denervation in spinal muscular atrophy. Neurology 2020, 94, e921–e931. [Google Scholar] [CrossRef] [PubMed]
- Stolte, B.; Nonnemacher, M.; Kizina, K.; Bolz, S.; Totzeck, A.; Thimm, A.; Wagner, B.; Deuschl, C.; Kleinschnitz, C.; Hagenacker, T. Nusinersen treatment in adult patients with spinal muscular atrophy: A safety analysis of laboratory parameters. J. Neurol. 2021, 268, 4667–4679. [Google Scholar] [CrossRef]
- Freigang, M.; Wurster, C.D.; Hagenacker, T.; Stolte, B.; Weiler, M.; Kamm, C.; Schreiber-Katz, O.; Osmanovic, A.; Petri, S.; Kowski, A.; et al. Serum creatine kinase and creatinine in adult spinal muscular atrophy under nusinersen treatment. Ann. Clin. Transl. Neurol. 2021, 8, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Luo, H.; Li, Z.; Gao, R.; Yang, K.; Deng, W.; Peng, S.; Ba, L.; Liu, Y.; Zhang, M. A Cross-sectional and Longitudinal Evaluation of Serum Creatinine as a Biomarker in Spinal Muscular Atrophy. Res. Sq. 2024, 1–11. [Google Scholar] [CrossRef]
- Deutsch, L.; Osredkar, D.; Plavec, J.; Stres, B. Spinal Muscular Atrophy after Nusinersen Therapy: Improved Physiology in Pediatric Patients with No Significant Change in Urine, Serum, and Liquor 1H-NMR Metabolomes in Comparison to an Age-Matched, Healthy Cohort. Metabolites 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Hijikata, Y.; Katsuno, M.; Suzuki, K.; Hashizume, A.; Araki, A.; Yamada, S.; Inagaki, T.; Iida, M.; Noda, S.; Nakanishi, H.; et al. Impaired muscle uptake of creatine in spinal and bulbar muscular atrophy. Ann. Clin. Transl. Neurol. 2016, 3, 537–546. [Google Scholar] [CrossRef]
- Meneri, M.; Abati, E.; Gagliardi, D.; Faravelli, I.; Parente, V.; Ratti, A.; Verde, F.; Ticozzi, N.; Comi, G.P.; Ottoboni, L.; et al. Identification of Novel Biomarkers of Spinal Muscular Atrophy and Therapeutic Response by Proteomic and Metabolomic Profiling of Human Biological Fluid Samples. Biomedicines 2023, 11, 1254. [Google Scholar] [CrossRef]
| Biomarker | Findings on Biomarkers in SMA Patients | Functions as an SMA Biomarker |
|---|---|---|
| Electrophysiological biomarkers | ||
| CMAP and MUNE | - correlate with age, SMA type, SMN2 gene copy number, and motor function | - prognosis of the functional outcome |
| - monitoring the disease progression and the effect of therapy (=monitoring) | ||
| - prediction of the clinical improvement after therapy | ||
| MUNIX | - correlates with muscle strength and SMA severity | - monitoring |
| MUSIX | - is increased in SMA patients compared to healthy controls | - monitoring |
| Functional biomarkers | ||
| Spirometric measures | - differ between SMA types | - prognosis for autonomous breathing |
| Dynamometric measures | - correlate with motor function | - monitoring |
| Imaging biomarkers | ||
| Ultrasound measures | - correlate with SMA type and motor function | - monitoring |
| MRI measures | - correlate with SMA type, duration of the disease, and motor function | - monitoring |
| - prediction of the clinical improvement after therapy | ||
| EIM measures | - correlate with SMA type and motor function | - monitoring |
| Molecular biomarkers | ||
| SMN2 gene copy number | - correlates with SMA type, motor function, and duration of survival | - prognosis of the disease progression - prediction of the clinical improvement after therapy |
| SMN2 gene mutations and polymorphisms | - impact SMA severity | - prognosis of the disease progression |
| SMN transcripts level | - differs between SMA types | - monitoring - preclinical studies |
| SMN protein | - is associated with SMA type and the severity of denervation | - monitoring |
| Gems number | - correlates with SMA type | - monitoring - preclinical studies |
| microRNAs | - differ between SMA and healthy - correlate with motor function | - disclosure of new pathological pathways - prediction of the clinical improvement after therapy - prognosis of the disease progression - monitoring |
| Neurofilaments | - correlate with the SMN2 copy number and motor function | - monitoring |
| Creatine kinase | - is associated with SMA severity and motor function | - monitoring - prediction of the clinical improvement after therapy |
| Creatinine | - correlates with the SMN2 copy number, SMA type, and motor function | - monitoring - prediction of the clinical improvement after therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maretina, M.; Koroleva, V.; Shchugareva, L.; Glotov, A.; Kiselev, A. The Relevance of Spinal Muscular Atrophy Biomarkers in the Treatment Era. Biomedicines 2024, 12, 2486. https://doi.org/10.3390/biomedicines12112486
Maretina M, Koroleva V, Shchugareva L, Glotov A, Kiselev A. The Relevance of Spinal Muscular Atrophy Biomarkers in the Treatment Era. Biomedicines. 2024; 12(11):2486. https://doi.org/10.3390/biomedicines12112486
Chicago/Turabian StyleMaretina, Marianna, Valeria Koroleva, Lyudmila Shchugareva, Andrey Glotov, and Anton Kiselev. 2024. "The Relevance of Spinal Muscular Atrophy Biomarkers in the Treatment Era" Biomedicines 12, no. 11: 2486. https://doi.org/10.3390/biomedicines12112486
APA StyleMaretina, M., Koroleva, V., Shchugareva, L., Glotov, A., & Kiselev, A. (2024). The Relevance of Spinal Muscular Atrophy Biomarkers in the Treatment Era. Biomedicines, 12(11), 2486. https://doi.org/10.3390/biomedicines12112486








