Early Metabolomic Profiling as a Predictor of Renal Function Six Months After Kidney Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Metabolomic Analysis
2.4. Statistical Analysis
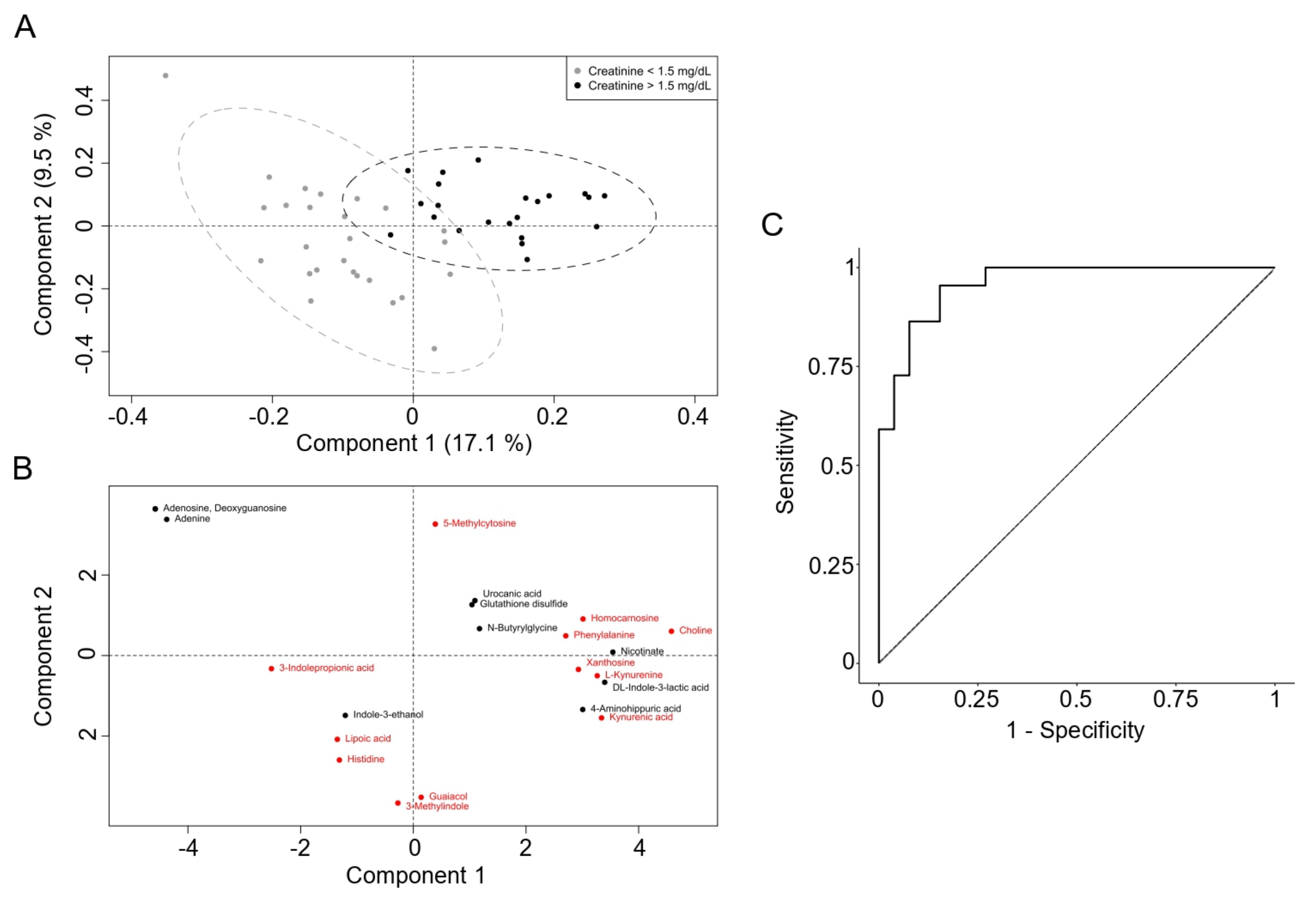
3. Results
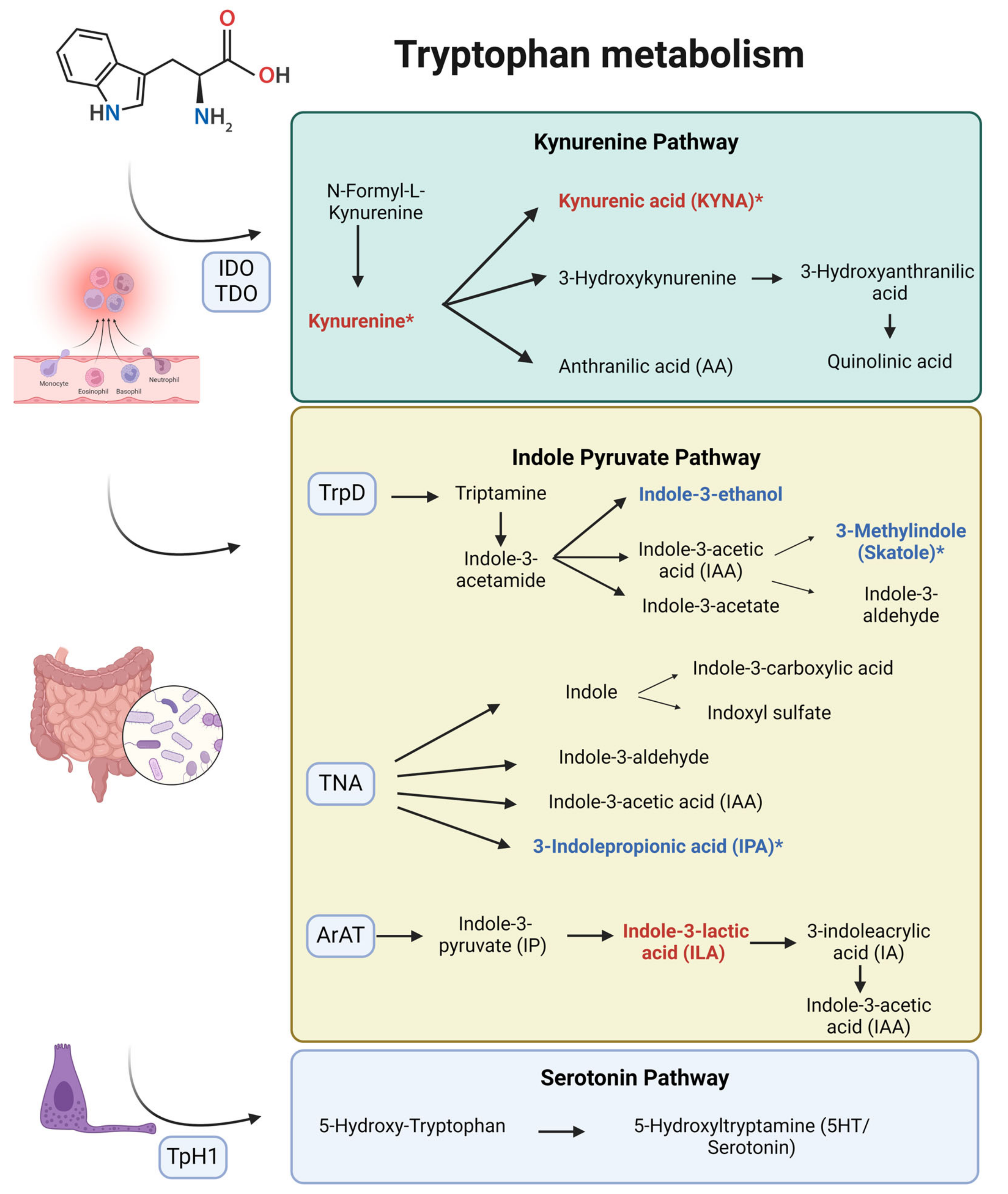
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Yozgat, I.; Cakır, U.; Serdar, M.A.; Sahin, S.; Sezerman, O.U.; Nemutlu, E.; Baykal, A.T.; Serteser, M. Longitudinal non-targeted metabolomic profiling of urine samples for monitoring of kidney transplantation patients. Ren. Fail. 2024, 46, 2300736. [Google Scholar] [CrossRef] [PubMed]
- Stanimirova, I.; Banasik, M.; Ząbek, A.; Dawiskiba, T.; Kościelska-Kasprzak, K.; Wojtowicz, W.; Krajewska, M.; Janczak, D.; Młynarz, P. Serum metabolomics approach to monitor the changes in metabolite profiles following renal transplantation. Sci. Rep. 2020, 10, 17223. [Google Scholar] [CrossRef] [PubMed]
- Peris-Fernández, M.; Roca-Marugán, M.; Amengual, J.L.; Balaguer-Timor, Á.; Viejo-Boyano, I.; Soldevila-Orient, A.; Devesa-Such, R.; Sánchez-Pérez, P.; Hernández-Jaras, J. Uremic Toxins and Inflammation: Metabolic Pathways Affected in Non-Dialysis-Dependent Stage 5 Chronic Kidney Disease. Biomedicines 2024, 12, 607. [Google Scholar] [CrossRef]
- Hariharan, S.; Mcbride, M.A.; Cherikh, W.S.; Tolleris, C.B.; Bresnahan, B.A.; Johnson, C.P. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002, 62, 311–318. [Google Scholar] [CrossRef]
- Legendre, C.; Canaud, G.; Martinez, F. Factors influencing long-term outcome after kidney transplantation. Transpl. Int. 2014, 27, 19–27. [Google Scholar] [CrossRef]
- Josephson, M.A. Monitoring and Managing Graft Health in the Kidney Transplant Recipient. Clin. J. Am. Soc. Nephrol. 2011, 6, 1774. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Ye, L.; Xu, G. Serum metabolomics study of the acute graft rejection in human renal transplantation based on liquid chromatography-mass spectrometry. J. Proteome Res. 2014, 13, 2659–2667. [Google Scholar] [CrossRef]
- Mao, Y.Y.; Bai, J.Q.; Chen, J.H.; Shou, Z.F.; He, Q.; Wu, J.Y.; Chen, Y.; Cheng, Y.Y. A pilot study of GC/MS-based serum metabolic profiling of acute rejection in renal transplantation. Transpl. Immunol. 2008, 19, 74–80. [Google Scholar] [CrossRef]
- Blydt-Hansen, T.D.; Sharma, A.; Gibson, I.W.; Wishart, D.S.; Mandal, R.; Ho, J.; Nickerson, P.; Rush, D. Urinary Metabolomics for Noninvasive Detection of Antibody-Mediated Rejection in Children After Kidney Transplantation. Transplantation 2017, 101, 2553–2561. [Google Scholar] [CrossRef]
- Kostidis, S.; Bank, J.R.; Soonawala, D.; Nevedomskaya, E.; Van Kooten, C.; Mayboroda, O.A.; De Fijter, J.W. Urinary metabolites predict prolonged duration of delayed graft function in DCD kidney transplant recipients. Am. J. Transplant. 2019, 19, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Paeslack, N.; Mimmler, M.; Becker, S.; Gao, Z.; Khuu, M.P.; Mann, A.; Malinarich, F.; Regen, T.; Reinhardt, C. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids 2022, 54, 1339–1356. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Hong, Y.; Lee, M.; Keum, B.R.; Kim, G.H. Natural Product Skatole Ameliorates Lipotoxicity-Induced Multiple Hepatic Damage under Hyperlipidemic Conditions in Hepatocytes. Nutrients 2023, 15, 1490. [Google Scholar] [CrossRef] [PubMed]
- Madella, A.M.; Van Bergenhenegouwen, J.; Garssen, J.; Masereeuw, R.; Overbeek, S.A. Microbial-Derived Tryptophan Catabolites, Kidney Disease and Gut Inflammation. Toxins 2022, 14, 645. [Google Scholar] [CrossRef]
- Zgarbová, E.; Vrzal, R. Skatole: A thin red line between its benefits and toxicity. Biochimie 2023, 208, 1–12. [Google Scholar] [CrossRef]
- Galano, A.; León-Carmona, J.R.; Alvarez-Idaboy, J.R. Influence of the Environment on the Protective Effects of Guaiacol Derivatives against Oxidative Stress: Mechanisms, Kinetics, and Relative Antioxidant Activity. J. Phys. Chem. B 2012, 116, 7129–7137. [Google Scholar] [CrossRef]
- Premkumar, J.; Sampath, P.; Sanjay, R.; Chandrakala, A.; Rajagopal, D. Synthetic Guaiacol Derivatives as Promising Myeloperoxidase Inhibitors Targeting Atherosclerotic Cardiovascular Disease. ChemMedChem 2020, 15, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, M.T.; Rahman, N.U.; Khan, A.U.; Khan, M.T.; Ashraf, Z.; ul Hassan, S.S.; Bungau, S.G.; Majid, M. Cardioprotective effect of 2-methoxy phenol derivatives against oxidative stress-induced vascular complications: An integrated in vitro, in silico, and in vivo investigation. Biomed. Pharmacother. 2023, 165, 115240. [Google Scholar] [CrossRef]
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef]
- Watanabe, M.; Suliman, M.E.; Qureshi, A.R.; Garcia-Lopez, E.; Bárány, P.; Heimbürger, O.; Stenvinkel, P.; Lindholm, B. Consequences of low plasma histidine in chronic kidney disease patients: Associations with inflammation, oxidative stress, and mortality1. Am. J. Clin. Nutr. 2008, 87, 1860–1866. [Google Scholar] [CrossRef]
- Vera-Aviles, M.; Vantana, E.; Kardinasari, E.; Koh, N.L.; Latunde-Dada, G.O. Protective Role of Histidine Supplementation Against Oxidative Stress Damage in the Management of Anemia of Chronic Kidney Disease. Pharmaceuticals 2018, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, C.; Gao, J. Extensive Summary of the Important Roles of Indole Propionic Acid, a Gut Microbial Metabolite in Host Health and Disease. Nutrients 2022, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Yisireyili, M.; Takeshita, K.; Saito, S.; Murohara, T.; Niwa, T. Indole-3-propionic acid suppresses indoxyl sulfate-induced expression of fibrotic and inflammatory genes in proximal tubular cells. Nagoya J. Med. Sci. 2017, 79, 477–486. [Google Scholar] [PubMed]
- Sun, C.Y.; Lin, C.J.; Pan, H.C.; Lee, C.C.; Lu, S.C.; Hsieh, Y.T.; Huang, S.Y.; Huang, H.Y. Clinical association between the metabolite of healthy gut microbiota, 3-indolepropionic acid and chronic kidney disease. Clin. Nutr. 2019, 38, 2945–2948. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, N.; Guerrieri, D.; Caro, F.; Sanchez, F.; Haeublein, G.; Casadei, D.; Incardona, C.; Chuluyan, E. Alpha Lipoic Acid: A Therapeutic Strategy that Tend to Limit the Action of Free Radicals in Transplantation. Int. J. Mol. Sci. 2018, 19, 102. [Google Scholar] [CrossRef]
- Kamt, S.F.; Liu, J.; Yan, L.J. Renal-Protective Roles of Lipoic Acid in Kidney Disease. Nutrients 2023, 15, 1732. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Y.; Zhang, W.; Shang, J.; Xie, Z.; Chen, C. Effects of Lipoic Acid on Ischemia-Reperfusion Injury. Oxidative Med. Cell Longev. 2021, 2021, 5093216. [Google Scholar]
- Wang, J.; Zhou, C.; Zhang, Q.; Liu, Z. Metabolomic profiling of amino acids study reveals a distinct diagnostic model for diabetic kidney disease. Amino Acids 2023, 55, 1563–1572. [Google Scholar] [CrossRef]
- Fox, B.M.; Gil, H.W.; Kirkbride-Romeo, L.; Bagchi, R.A.; Wennersten, S.A.; Haefner, K.R.; Skrypnyk, N.I.; Brown, C.N.; Soranno, D.E.; Gist, K.M.; et al. Metabolomics assessment reveals oxidative stress and altered energy production in the heart after ischemic acute kidney injury in mice. Kidney Int. 2019, 95, 590–610. [Google Scholar]
- Xiang, X.; Zhu, J.; Dong, G.; Dong, Z. Epigenetic Regulation in Kidney Transplantation. Front. Immunol. 2022, 13, 861498. [Google Scholar] [CrossRef]
- Heylen, L.; Thienpont, B.; Naesens, M.; Busschaert, P.; Depreeuw, J.; Smeets, D.; Jochmans, I.; Monbaliu, D.; Pirenne, J.; Lerut, E.; et al. Ischemia-Induced DNA Hypermethylation during Kidney Transplant Predicts Chronic Allograft Injury. J. Am. Soc. Nephrol. 2018, 29, 1566. [Google Scholar] [PubMed]
- Chen, Y.; Zelnick, L.R.; Wang, K.; Hoofnagle, A.N.; Becker, J.O.; Hsu, C.Y.; Feldman, H.I.; Mehta, R.C.; Lash, J.P.; Waikar, S.S.; et al. Kidney Clearance of Secretory Solutes Is Associated with Progression of CKD: The CRIC Study. J. Am. Soc. Nephrol. 2020, 31, 817–827. [Google Scholar] [CrossRef]
- Granda, M.L.; Zelnick, L.R.; Prince, D.K.; Hoofnagle, A.; Young, B.A.; Kestenbaum, B.R. Tubular Secretion and Estimated GFR Decline in the Jackson Heart Study. Kidney Int. Rep. 2022, 7, 2668–2675. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Clish, C.B.; Ghorbani, A.; Larson, M.G.; Elmariah, S.; McCabe, E.; Yang, Q.; Cheng, S.; Pierce, K.; Deik, A.; et al. A Combined Epidemiologic and Metabolomic Approach Improves CKD Prediction. J. Am. Soc. Nephrol. 2013, 24, 1330–1338. [Google Scholar]
- Saeb-Parsy, K.; Martin, J.L.; Summers, D.M.; Watson, C.J.E.; Krieg, T.; Murphy, M.P. Mitochondria as Therapeutic Targets in Transplantation. Trends Mol. Med. 2021, 27, 185–198. [Google Scholar] [PubMed]
- Farthing, D.E.; Farthing, C.A.; Xi, L. Inosine and hypoxanthine as novel biomarkers for cardiac ischemia: From bench to point-of-care. Exp. Biol. Med. 2015, 240, 821–831. [Google Scholar] [CrossRef]
- Rivera-Pérez, C.; Gaxiola-Robles, R.; Olguín-Monroy, N.O.; Lugo-Lugo, O.; López-Cruz, R.I.; Zenteno-Savín, T. Effect of hypoxia on purine metabolism in human skeletal muscle cells. Biotecnia 2021, 23, 141–148. [Google Scholar]
- Zhang, W.; Miikeda, A.; Zuckerman, J.; Jia, X.; Charugundla, S.; Zhou, Z.; Kaczor-Urbanowicz, K.E.; Magyar, C.; Guo, F.; Wang, Z.; et al. Inhibition of microbiota-dependent TMAO production attenuates chronic kidney disease in mice. Sci. Rep. 2021, 11, 518. [Google Scholar]
- Brühl, A.; Hafner, G.; Löffelholz, K. Release of choline in the isolated heart, an indicator of ischemic phospholipid degradation and its protection by ischemic preconditioning: No evidence for a role of phospholipase D. Life Sci. 2004, 75, 1609–1620. [Google Scholar] [CrossRef]
- Lindeman, J.H.; Wijermars, L.G.; Kostidis, S.; Mayboroda, O.A.; Harms, A.C.; Hankemeier, T.; Bierau, J.; Gupta, K.B.S.S.; Giera, M.; Reinders, M.E.; et al. Results of an explorative clinical evaluation suggest immediate and persistent post-reperfusion metabolic paralysis drives kidney ischemia reperfusion injury. Kidney Int. 2020, 98, 1476–1488. [Google Scholar]
- Møller, N.; Meek, S.; Bigelow, M.; Andrews, J.; Nair, K.S. The kidney is an important site for in vivo phenylalanine-to-tyrosine conversion in adult humans: A metabolic role of the kidney. Proc. Natl. Acad. Sci. USA 2000, 97, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D. Phenylalanine and tyrosine metabolism in chronic kidney failure. J. Nutr. 2007, 137, 1586S–1590S, discussion 1597S–1598S. [Google Scholar] [CrossRef] [PubMed]
- Supavekin, S.; Zhang, W.; Kucherlapati, R.; Kaskel, F.J.; Moore, L.C.; Devarajan, P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003, 63, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Wee, H.N.; Liu, J.J.; Ching, J.; Kovalik, J.P.; Lim, S.C. The Kynurenine Pathway in Acute Kidney Injury and Chronic Kidney Disease. Am. J. Nephrol. 2021, 52, 771–787. [Google Scholar] [CrossRef]
- Zulpaite, R.; Miknevicius, P.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Tryptophan Metabolism via Kynurenine Pathway: Role in Solid Organ Transplantation. Int. J. Mol. Sci. 2021, 22, 1921. [Google Scholar] [CrossRef]
- Goek, O.N.; Prehn, C.; Sekula, P.; Römisch-Margl, W.; Döring, A.; Gieger, C.; Heier, M.; Koenig, W.; Wang-Sattler, R.; Illig, T.; et al. Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol. Dial. Transplant. 2013, 28, 2131–2138. [Google Scholar] [CrossRef]
- Silva, R.E.; Baldim, J.L.; Chagas-Paula, D.A.; Soares, M.G.; Lago, J.H.; Gonçalves, R.V.; Novaes, R.D. Predictive metabolomic signatures of end-stage renal disease: A multivariate analysis of population-based data. Biochimie 2018, 152, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Vavrincova-Yaghi, D.; Seelen, M.A.; Kema, I.P.; Deelman, L.E.; van der Heuvel, M.C.; Breukelman, H.; Van den Eynde, B.J.; Henning, R.H.; van Goor, H.; Sandovici, M. Early Posttransplant Tryptophan Metabolism Predicts Long-term Outcome of Human Kidney Transplantation. Transplantation 2015, 99, e97–e104. [Google Scholar] [CrossRef]
- Lahdou, I.; Sadeghi, M.; Daniel, V.; Schenk, M.; Renner, F.; Weimer, R.; Löb, S.; Schmidt, J.; Mehrabi, A.; Schnitzler, P.; et al. Increased pretransplantation plasma kynurenine levels do not protect from but predict acute kidney allograft rejection. Hum. Immunol. 2010, 71, 1067–1072. [Google Scholar] [CrossRef]
- Dharnidharka, V.R.; Al Khasawneh, E.; Gupta, S.; Shuster, J.J.; Theriaque, D.W.; Shahlaee, A.H.; Garrett, T.J. Verification of association of elevated serum IDO enzyme activity with acute rejection and low CD4-ATP levels with infection. Transplantation 2013, 96, 567–572. [Google Scholar] [CrossRef]
| Kidney Transplant Recipient | |
|---|---|
| Variable | Mean/Frequency |
| Age (years) (mean ± SD) | 53.98 ± 10.94 |
| Gender, n (%) | Male: 35 (70%) Female: 15 (30%) |
| Race, n (%) | Caucasian: 47 (94%) |
| African American: 3 (6%) | |
| Blood type, n (%) | 0+: 18 (36%) |
| 0−: 1 (2%) | |
| A+: 19 (38%) | |
| A−: 6 (12%) | |
| B+: 5 (10%) | |
| B− 0 (0%). | |
| AB+: 1 (2%) | |
| AB−: 0 (0%) | |
| Hypertension, n (%) | 41 (82%) |
| Type 2 diabetes, n (%) | 8 (16%) |
| Dyslipidemia, n (%) | 35 (70%) |
| BMI (mean ± SD) | 26.58 (3.89) |
| Obesity, n (%) | Underweight: 0 (0%) |
| Normal weight: 18 (36%) | |
| Overweight: 18 (36%) | |
| Obesity: 14 (28%) | |
| Hyperuricemia, n (%) | 17 (34%) |
| Smoking status, n (%) | Non-smoker: 21 (42%) |
| Former smoker: 20 (40%) | |
| Current smoker: 9 (18%) | |
| Physical activity, n (%) | Sedentary: 39 (78%) |
| Moderately active: 5 (10%) | |
| Very active: 6 (12%) | |
| Etiology of chronic kidney disease, n (%) | Glomerulonephritis: 5 (10%) |
| Chronic pyelonephritis/tubulointerstitial: 7 (14%) | |
| Diabetes mellitus: 6 (12%) | |
| Hypertension/vascular diseases: 3 (6%) | |
| Hereditary/familial: 13 (26%) | |
| Systemic diseases: 4 (8%) | |
| Unclassified: 12 (24%) | |
| Renal replacement therapy, n (%) | Hemodialysis: 37 (74%) |
| Peritoneal dialysis: 13 (26%) | |
| Time on dialysis (years) (mean ± SD) | 3.44 ± 2.32 |
| Residual diuresis, n (%) | <500 mL: 32 (64%) |
| 500–1000 mL: 7 (14%) | |
| >1000 mL: 11 (22%) | |
| Heart failure, n (%) | 2 (4%) |
| Coronary artery disease, n (%) | 7 (14%) |
| Vascular disease, n (%) | 4 (18%) |
| Previous transplant, n (%) | 6 (12%) |
| Transfusions history, n (%) | 17 (34%) |
| Pregnancy history, n (%) | 12 (24%) |
| Sensitization, n (%) | No: 41 (82%) |
| <98% PRAc: 6 (12%) | |
| >98% PRAc (PATHI): 3 (6%) | |
| EPTS, (mean ± SD) | 41.96 ± 26.94 |
| Kidney Donor | |
| Variable | Mean/Frequency |
| Age (mean ± SD) | 50.58 ± 16.30 |
| Gender, n (%) | Male: 15 (30%) |
| Female: 35 (70%) | |
| Donor type, n (%) | DBD: 29 (58%) |
| DCD: 21 (42%) | |
| Hypertension, n (%) | 14 (28%) |
| Diabetes mellitus, n (%) | 9 (18%) |
| BMI (mean ± SD) | 26.29 ± 5.85 |
| Donor AKI, n (%) | 2 (4%) |
| Expanded criteria donor (EC), n (%) | 16 (32%) |
| KDPI (mean ± SD) | 52.66 ± 28.72 |
| Transplant Process | |
| Variable | Mean/Frequency |
| Cold ischemia time (mean ± SD) | 17.30 ± 4.58 |
| Mismatch 6/6 (mean ± SD) | 4.3 ± 1.18 |
| Mismatch 10/10 (mean ± SD) | 7.2 ± 1.82 |
| One-Week Events | |
| Variable | Mean/Frequency |
| Overdose of calcineurin inhibitor, n (%) | 30 (60%) |
| Urinary infection, n (%) | 11 (22%) |
| Graft rejection, n (%) | 3 (6%) |
| Graft function, n (%) | Immediate graft function: 10 (20%) |
| Slow graft function: 13 (26%) | |
| Delayed graft function: 27 (54%) | |
| Six-Month Post-Transplant Events (Excluding Week 1) | |
| Variable | Mean/Frequency |
| Graft rejection, n (%) | 1 (2%) |
| Urinary infection, n (%) | 18 (36%) |
| CMV infection, n (%) | 3 (6%) |
| BK infection, n (%) | Yes (viremia): 5 (10%) |
| Nephropathy: 0 (0%) | |
| MACE | 6 (12%) |
| Compound | Formula | Properties | Standardized Coefficient | p-Value | Confidence Interval |
|---|---|---|---|---|---|
| 3-Methylindole (Skatole) | C9H9N | m/z: 132,080,765 Rt: 3.46 Adduction: [M+H]+ | −0.16695299 | 0.012 | −0.29594 to −0.03795 |
| Guaiacol | C7H8O2 | m/z: 125,059,898 Rt: 2.811 Adduction: [M+H]+ | −0.15992134 | 0.040 | −0.31206 to −0.00777 |
| Homocarnosine | C10H16N4O3 | m/z: 241,129,028 Rt: 13.951 Adduction: [M+H]+ | 0.15774800 | 0.010 | 0.03892 to 0.27657 |
| 5-Methylcytosine | C5H7N3O | m/z: 126,066,383 Rt: 8.141 Adduction: [M+H]+ | 0.15492272 | 0.023 | 0.02254 to 0.28729 |
| Histidine | C6H9N3O2 | m/z: 156,076,614 Rt: 13.985 Adduction: [M+H]+ | −0.14781219 | 0.002 | −0.23791 to −0.05770 |
| Xanthosine | C10H12N4O6 | m/z: 283,068,512 Rt: 8.486 Adduction: [M-H]- | 0.14141625 | 0.029 | 0.01564 to 0.26718 |
| Choline | C5H13NO | m/z: 104,107,384 Rt: 15.016 Adduction: [M+H]+ | 0.13956783 | 0.028 | 0.01596 to 0.26317 |
| Glutathione disulfide | C20H32N6O12S2 | m/z: 613,161,499 Rt: 13.655 Adduction: [M+H]+ | 0.12867136 | 0.065 | −0.00778 to 0.26512 |
| 3-Indolepropionic acid | C11H11NO2 | m/z: 188,071,854 Rt: 2.661 Adduction: [M-H]- | −0.12850942 | 0.001 | −0.19942 to −0.05759 |
| Nicotinate | C6H5NO2 | m/z: 124,039,261 Rt: 8.114 Adduction: [M+H]+ | 0.12825902 | 0.074 | −0.01247 to 0.26899 |
| α-Lipoic acid | C8H14O2S2 | m/z: 207,051,132 Rt: 2.506 Adduction: [M+H]+ | −0.12588711 | 0.047 | −0.25017 to −0.00160 |
| Phenylalanine | C9H11NO2 | m/z: 166,086,014 Rt: 8.604 Adduction: [M+H]+ | 0.11587927 | 0.041 | 0.00471 to 0.22704 |
| Kynurenic acid | C10H7NO3 | m/z: 188,035,202 Rt: 4.198 Adduction: [M-H]- | 0.11255399 | 0.039 | 0.00591 to 0.21918 |
| L-Kynurenine | C10H12N2O3 | m/z: 209,091,873 Rt: 8.843 Adduction: [M+H]+ | 0.11071323 | 0.020 | 0.01812 to 0.20330 |
| Urocanic acid | C6H6N2O2 | m/z: 139,050,247 Rt: 7.991 Adduction: [M+H]+ | 0.10565113 | 0.233 | −0.06970 to 0.28100 |
| N-Butyrylglycine | C6H11NO3 | m/z: 146,081,177 Rt: 7.097 Adduction: [M+H]+ | 0.10469998 | 0.061 | −0.00492 to 0.21432 |
| Indole-3-lactic acid | C11H11NO3 | m/z: 204,066,437 Rt: 2.615 Adduction: [M-H]- | 0.10158428 | 0.097 | −0.01908 to 0.22225 |
| 4-Aminohippuric acid | C9H10N2O3 | m/z: 195,076,187 Rt: 5.703 Adduction: [M+H]+ | 0.10137408 | 0.081 | −0.01293 to 0.21568 |
| Índole-3-ethanol | C10H11NO | m/z: 162,091,446 Rt: 2.668 Adduction: [M+H]+ | −0.09839586 | 0.137 | −0.22901 to 0.03222 |
| Adenine | C5H5N5 | m/z: 134,047,058 Rt: 7.545 Adduction: [M-H]- | −0.03343797 | 0.339 | −0.10300 to 0.03612 |
| Adenosine | C10H13N5O4 | m/z: 268,104,065 Rt: 7.39 Adduction: [M+H]+ | −0.02616427 | 0.308 | −0.07729 to 0.02496 |
| Deoxyguanosine | C10H13N5O4 | m/z: 268,104,065 Rt: 7.39 Adduction: [M+H]+ | −0.02616427 | 0.308 | −0.07729 to 0.02496 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viejo-Boyano, I.; Roca-Marugán, M.I.; Peris-Fernández, M.; Amengual, J.L.; Balaguer-Timor, Á.; Moreno-Espinosa, M.; Felipe-Barrera, M.; González-Calero, P.; Espí-Reig, J.; Ventura-Galiano, A.; et al. Early Metabolomic Profiling as a Predictor of Renal Function Six Months After Kidney Transplantation. Biomedicines 2024, 12, 2424. https://doi.org/10.3390/biomedicines12112424
Viejo-Boyano I, Roca-Marugán MI, Peris-Fernández M, Amengual JL, Balaguer-Timor Á, Moreno-Espinosa M, Felipe-Barrera M, González-Calero P, Espí-Reig J, Ventura-Galiano A, et al. Early Metabolomic Profiling as a Predictor of Renal Function Six Months After Kidney Transplantation. Biomedicines. 2024; 12(11):2424. https://doi.org/10.3390/biomedicines12112424
Chicago/Turabian StyleViejo-Boyano, Iris, Marta Isabel Roca-Marugán, María Peris-Fernández, Julián Luis Amengual, Ángel Balaguer-Timor, Marta Moreno-Espinosa, María Felipe-Barrera, Pablo González-Calero, Jordi Espí-Reig, Ana Ventura-Galiano, and et al. 2024. "Early Metabolomic Profiling as a Predictor of Renal Function Six Months After Kidney Transplantation" Biomedicines 12, no. 11: 2424. https://doi.org/10.3390/biomedicines12112424
APA StyleViejo-Boyano, I., Roca-Marugán, M. I., Peris-Fernández, M., Amengual, J. L., Balaguer-Timor, Á., Moreno-Espinosa, M., Felipe-Barrera, M., González-Calero, P., Espí-Reig, J., Ventura-Galiano, A., Rodríguez-Ortega, D., Ramos-Cebrián, M., Beneyto-Castelló, I., & Hernández-Jaras, J. (2024). Early Metabolomic Profiling as a Predictor of Renal Function Six Months After Kidney Transplantation. Biomedicines, 12(11), 2424. https://doi.org/10.3390/biomedicines12112424







