Mitochondrial Creatine Kinase 2 (Ckmt2) as a Plasma-Based Biomarker for Evaluating Reperfusion Injury in Acute Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Induction of Reperfused AMI
2.3. Induction of Non-Reperfused AMI
2.4. Plasma Protein Analysis via ELISA
2.5. Echocardiography
2.6. Determination of Infarct and Scar Size with TTC
2.7. Mass Spectrometry
2.8. Statistics
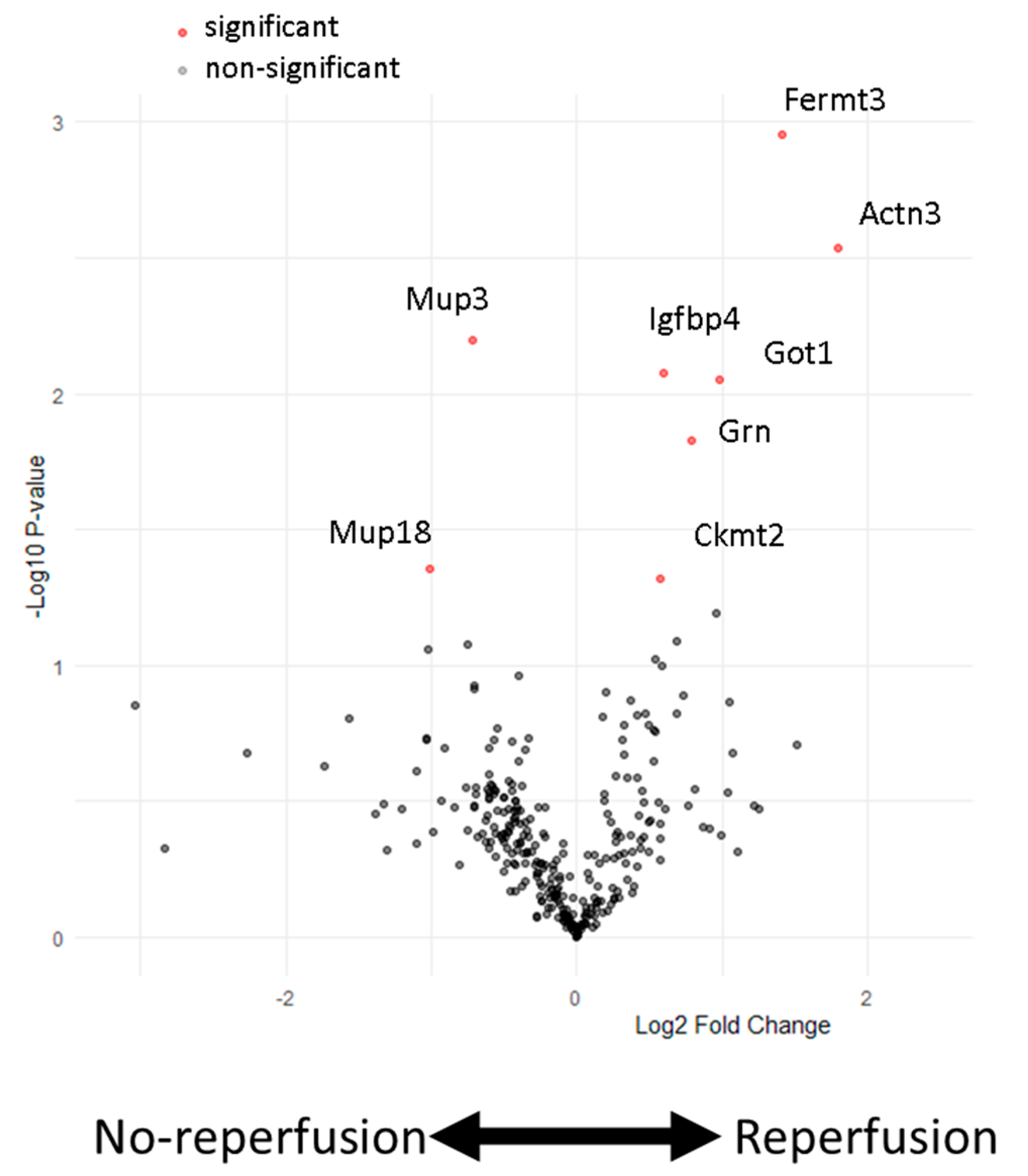
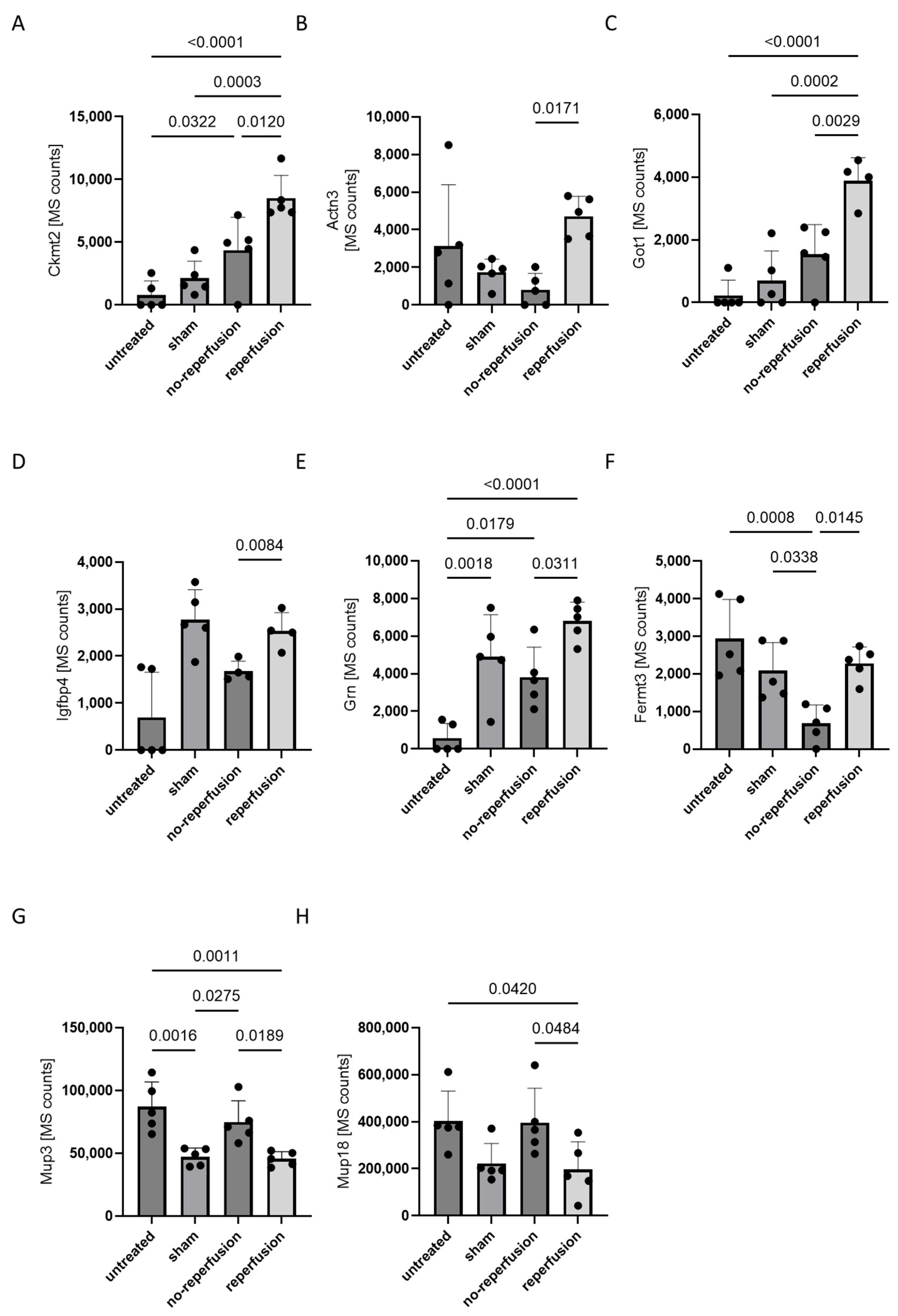
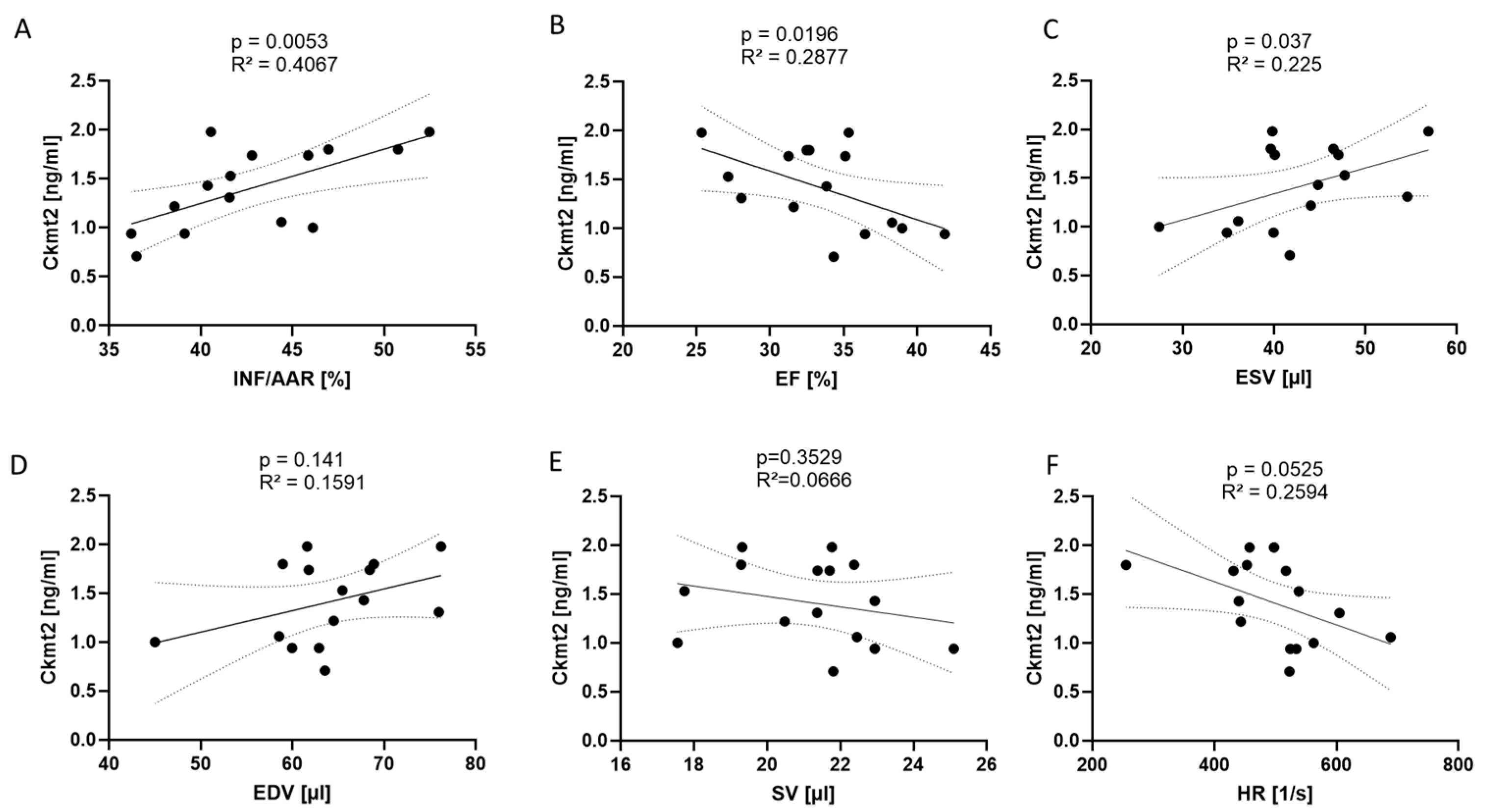
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Mechanic, O.J.; Gavin, M.; Grossman, S.A.; Ziegler, K. Acute Myocardial Infarction (Nursing); StatPearls: Saint Petersburg, FL, USA, 2021. [Google Scholar]
- Di Lisa, F.; Bernardi, P. Mitochondria and ischemia-reperfusion injury of the heart: Fixing a hole. Cardiovasc. Res. 2006, 70, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Di Lisa, F.; Canton, M.; Carpi, A.; Kaludercic, N.; Menabo, R.; Menazza, S.; Semenzato, M. Mitochondrial injury and protection in ischemic pre- and postconditioning. Antioxid. Redox. Signal. 2011, 14, 881–891. [Google Scholar] [CrossRef]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management (Review). Exp. Ther. Med. 2022, 23, 430. [Google Scholar] [CrossRef]
- Li, L.; Lin, L.; Lei, S.; Shi, S.; Chen, C.; Xia, Z. Maslinic Acid Inhibits Myocardial Ischemia-Reperfusion Injury-Induced Apoptosis and Necroptosis via Promoting Autophagic Flux. DNA Cell Biol. 2022, 41, 487–497. [Google Scholar] [CrossRef]
- Lu, D.; Xia, Y.; Chen, Z.; Chen, A.; Wu, Y.; Jia, J.; Sun, A.; Zou, Y.; Qian, J.; Ge, J. Cardiac Proteome Profiling in Ischemic and Dilated Cardiomyopathy Mouse Models. Front. Physiol. 2019, 10, 750. [Google Scholar] [CrossRef]
- Geyer, P.E.; Holdt, L.M.; Teupser, D.; Mann, M. Revisiting biomarker discovery by plasma proteomics. Mol. Syst. Biol. 2017, 13, 942. [Google Scholar] [CrossRef]
- Lagging, C.; Klasson, S.; Pedersen, A.; Nilsson, S.; Jood, K.; Stanne, T.M.; Jern, C. Investigation of 91 proteins implicated in neurobiological processes identifies multiple candidate plasma biomarkers of stroke outcome. Sci. Rep. 2022, 12, 20080. [Google Scholar] [CrossRef]
- Xiao, J.; Howard, L.; Wan, J.; Wiggins, E.; Vidal, A.; Cohen, P.; Freedland, S.J. Low circulating levels of the mitochondrial-peptide hormone SHLP2: Novel biomarker for prostate cancer risk. Oncotarget 2017, 8, 94900–94909. [Google Scholar] [CrossRef]
- Woodhead, J.S.T.; D’Souza, R.F.; Hedges, C.P.; Wan, J.; Berridge, M.V.; Cameron-Smith, D.; Cohen, P.; Hickey, A.J.R.; Mitchell, C.J.; Merry, T.L. High-intensity interval exercise increases humanin, a mitochondrial encoded peptide, in the plasma and muscle of men. J. Appl. Physiol. 2020, 128, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Marenzi, G.; Cosentino, N.; Boeddinghaus, J.; Trinei, M.; Giorgio, M.; Milazzo, V.; Moltrasio, M.; Cardinale, D.; Sandri, M.T.; Veglia, F.; et al. Diagnostic and Prognostic Utility of Circulating Cytochrome c in Acute Myocardial Infarction. Circ. Res. 2016, 119, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Dolder, M.; Wendt, S.; Wallimann, T. Mitochondrial creatine kinase in contact sites: Interaction with porin and adenine nucleotide translocase, role in permeability transition and sensitivity to oxidative damage. Biol. Signals Recept. 2001, 10, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.G.; Kane, D.A.; Herbst, E.A.; Mukai, K.; Lark, D.S.; Wright, D.C.; Heigenhauser, G.J.; Neufer, P.D.; Spriet, L.L.; Holloway, G.P. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. J. Physiol. 2012, 590, 5475–5486. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Wischmann, P.; Kuhn, V.; Suvorava, T.; Muessig, J.M.; Fischer, J.W.; Isakson, B.E.; Haberkorn, S.M.; Flögel, U.; Schrader, J.; Jung, C.; et al. Anaemia is associated with severe RBC dysfunction and a reduced circulating NO pool: Vascular and cardiac eNOS are crucial for the adaptation to anaemia. Basic Res. Cardiol. 2020, 115, 43. [Google Scholar] [CrossRef]
- Polzin, A.; Dannenberg, L.; Benkhoff, M.; Barcik, M.; Keul, P.; Ayhan, A.; Weske, S.; Ahlbrecht, S.; Trojovsky, K.; Helten, C.; et al. Sphingosine-1-phosphate improves outcome of no-reflow acute myocardial infarction via sphingosine-1-phosphate receptor 1. ESC Heart Fail. 2023, 10, 334–341. [Google Scholar] [CrossRef]
- Maslov, L.N.; Popov, S.V.; Mukhomedzyanov, A.V.; Naryzhnaya, N.V.; Voronkov, N.S.; Ryabov, V.V.; Boshchenko, A.A.; Khaliulin, I.; Prasad, N.R.; Fu, F.; et al. Reperfusion Cardiac Injury: Receptors and the Signaling Mechanisms. Curr. Cardiol. Rev. 2022, 18, 63–79. [Google Scholar] [CrossRef]
- Ong, S.B.; Samangouei, P.; Kalkhoran, S.B.; Hausenloy, D.J. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell Cardiol. 2015, 78, 23–34. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Wang, Q.; Zuurbier, C.J.; Huhn, R.; Torregroza, C.; Hollmann, M.W.; Preckel, B.; van den Brom, C.E.; Weber, N.C. Pharmacological Cardioprotection against Ischemia Reperfusion Injury-The Search for a Clinical Effective Therapy. Cells 2023, 12, 1432. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, İ.; Yardim, M. Biomarkers in acute myocardial infarction: Current perspectives. Vasc. Health Risk Manag. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Halladin, N.L. Oxidative and inflammatory biomarkers of ischemia and reperfusion injuries. Dan. Med. J. 2015, 62, B5054. [Google Scholar]
- Zhao, W.K.; Zhou, Y.; Xu, T.T.; Wu, Q. Ferroptosis: Opportunities and Challenges in Myocardial Ischemia-Reperfusion Injury. Oxid. Med. Cell Longev. 2021, 2021, 9929687. [Google Scholar] [CrossRef]
- Daiber, A.; Andreadou, I.; Oelze, M.; Davidson, S.M.; Hausenloy, D.J. Discovery of new therapeutic redox targets for cardioprotection against ischemia/reperfusion injury and heart failure. Free Radic. Biol. Med. 2021, 163, 325–343. [Google Scholar] [CrossRef]
- Robichaux, D.J.; Harata, M.; Murphy, E.; Karch, J. Mitochondrial permeability transition pore-dependent necrosis. J. Mol. Cell Cardiol. 2023, 174, 47–55. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, D.; Li, Z.; Zhou, L.; Cen, T.; Wei, B.; Wei, L.; Wu, H.; Su, L.; Sooranna, S.R.; et al. A plasma proteomic approach in patients with heart failure after acute myocardial infarction: Insights into the pathogenesis and progression of the disease. Front. Cardiovasc. Med. 2023, 10, 1153625. [Google Scholar] [CrossRef]
- Song, Z.; Yang, Y.; Wu, Y.; Zheng, M.; Sun, D.; Li, H.; Chen, L. Glutamic oxaloacetic transaminase 1 as a potential target in human cancer. Eur. J. Pharmacol. 2022, 917, 174754. [Google Scholar] [CrossRef]
- Bernardez-Pereira, S.; Santos, P.C.; Krieger, J.E.; Mansur, A.J.; Pereira, A.C. ACTN3 R577X polymorphism and long-term survival in patients with chronic heart failure. BMC Cardiovasc. Disord. 2014, 14, 90. [Google Scholar] [CrossRef]
- Putra, M.A.; Alwi, I.; Soetisna, T.W.; Gunanti; Sandora, N.; Busro, P.W.; Supomo; Fitria, N.A.; Kusuma, T.R. Remodeling in early myocardial infarction: Alteration of extracellular matrix; Collagen-1, Collagen-3, α-SMA, and α-Actinin in Porcine heart model. Bali Med. J. 2023, 12, 2721–2728. [Google Scholar] [CrossRef]
- Nguyen, B.Y.; Ruiz-Velasco, A.; Bui, T.; Collins, L.; Wang, X.; Liu, W. Mitochondrial function in the heart: The insight into mechanisms and therapeutic potentials. Br. J. Pharmacol. 2019, 176, 4302–4318. [Google Scholar] [CrossRef]
- Zhang, W.; Lyu, P.; Andreev, D.; Jia, Y.; Zhang, F.; Bozec, A. Hypoxia-immune-related microenvironment prognostic signature for osteosarcoma. Front. Cell Dev. Biol. 2022, 10, 974851. [Google Scholar] [CrossRef]
- Whittington, H.J.; Ostrowski, P.J.; McAndrew, D.J.; Cao, F.; Shaw, A.; Eykyn, T.R.; Lake, H.A.; Tyler, J.; Schneider, J.E.; Neubauer, S.; et al. Over-expression of mitochondrial creatine kinase in the murine heart improves functional recovery and protects against injury following ischaemia–reperfusion. Cardiovasc. Res. 2018, 114, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Zervou, S.; Whittington, H.J.; Ostrowski, P.J.; Cao, F.; Tyler, J.; Lake, H.A.; Neubauer, S.; Lygate, C.A. Increasing creatine kinase activity protects against hypoxia/reoxygenation injury but not against anthracycline toxicity in vitro. PLoS ONE 2017, 12, e0182994. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, A.; Oehler, D.; Benkhoff, M.; Reinders, Y.; Barcik, M.; Shahrjerdi, K.; Kaldirim, M.; Sickmann, A.; Dannenberg, L.; Polzin, A.; et al. Mitochondrial Creatine Kinase 2 (Ckmt2) as a Plasma-Based Biomarker for Evaluating Reperfusion Injury in Acute Myocardial Infarction. Biomedicines 2024, 12, 2368. https://doi.org/10.3390/biomedicines12102368
Lang A, Oehler D, Benkhoff M, Reinders Y, Barcik M, Shahrjerdi K, Kaldirim M, Sickmann A, Dannenberg L, Polzin A, et al. Mitochondrial Creatine Kinase 2 (Ckmt2) as a Plasma-Based Biomarker for Evaluating Reperfusion Injury in Acute Myocardial Infarction. Biomedicines. 2024; 12(10):2368. https://doi.org/10.3390/biomedicines12102368
Chicago/Turabian StyleLang, Alexander, Daniel Oehler, Marcel Benkhoff, Yvonne Reinders, Maike Barcik, Khatereh Shahrjerdi, Madlen Kaldirim, Albert Sickmann, Lisa Dannenberg, Amin Polzin, and et al. 2024. "Mitochondrial Creatine Kinase 2 (Ckmt2) as a Plasma-Based Biomarker for Evaluating Reperfusion Injury in Acute Myocardial Infarction" Biomedicines 12, no. 10: 2368. https://doi.org/10.3390/biomedicines12102368
APA StyleLang, A., Oehler, D., Benkhoff, M., Reinders, Y., Barcik, M., Shahrjerdi, K., Kaldirim, M., Sickmann, A., Dannenberg, L., Polzin, A., Pfeiler, S., Kelm, M., Grandoch, M., Jung, C., & Gerdes, N. (2024). Mitochondrial Creatine Kinase 2 (Ckmt2) as a Plasma-Based Biomarker for Evaluating Reperfusion Injury in Acute Myocardial Infarction. Biomedicines, 12(10), 2368. https://doi.org/10.3390/biomedicines12102368






