Evaluating the Efficacy of Pre-Emptive Peribulbar Blocks with Different Local Anesthetics or Paracetamol Using the Adequacy of Anesthesia Guidance for Vitreoretinal Surgeries: A Preliminary Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Anesthesia Technique
2.1.1. Stage 1
2.1.2. Stage 2
2.1.3. Stage 3—Intraoperatively
2.1.4. Stage 4—Postoperatively
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sallam, A.A.B.; Donachie, P.H.J.; Williamson, T.H.; Sparrow, J.M.; Johnston, R.L. The Royal College of Ophthalmologists’ National Ophthalmology Database Study of Vitreoretinal Surgery: Report 5, Anaesthetic Techniques. Br. J. Ophthalmol. 2016, 100, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Licina, A.; Sidhu, S.; Xie, J.; Wan, C. Local versus General Anaesthesia for Adults Undergoing Pars Plana Vitrectomy Surgery. Cochrane Database Syst. Rev. 2016, 9, CD009936. [Google Scholar] [CrossRef] [PubMed]
- Huebert, I.; Heinicke, N.; Kook, D.; Boost, K.A.; Miller, C.V.; Mayer, W.J.; Haritoglou, C.; Kampik, A.; Gandorfer, A.; Hintschich, C.; et al. Dual Platelet Inhibition in Cases of Severe Retrobulbar Hemorrhage Following Retrobulbar and Peribulbar Anesthesia. J. Cataract. Refract. Surg. 2015, 41, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Loeser, J.; Schwemmer, J.; Gostian, A.-O.; Gostian, M.; Bachmann, B.; Cursiefen, C.; Heindl, L.M. Postoperative Pain Following Descemet Membrane Endothelial Keratoplasty (DMEK): A Prospective Study. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2203–2211. [Google Scholar] [CrossRef] [PubMed]
- Ghali, A.M.; El Btarny, A.M. The Effect on Outcome of Peribulbar Anaesthesia in Conjunction with General Anesthesia for Vitreoretinal Surgery. Anaesthesia 2010, 65, 249–253. [Google Scholar] [CrossRef]
- Misiołek, H.; Cettler, M.; Woroń, J.; Wordliczek, J.; Dobrogowski, J.; Mayzner-Zawadzka, E. The 2014 Guidelines for Post-Operative Pain Management. Anaesthesiol. Intensive Ther. 2014, 46, 221–244. [Google Scholar] [CrossRef]
- Woolf, C.J.; Chong, M.S. Preemptive Analgesia--Treating Postoperative Pain by Preventing the Establishment of Central Sensitization. Anesth. Analg. 1993, 77, 362–379. [Google Scholar] [CrossRef]
- Fekrat, S.; Elsing, S.H.; Raja, S.C.; Campochiaro, P.A.; de Juan, E.; Haller, J.A. Eye Pain after Vitreoretinal Surgery: A Prospective Study of 185 Patients. Retina 2001, 21, 627–632. [Google Scholar] [CrossRef]
- Bayerl, K.; Boost, K.A.; Wolf, A.; Kampik, A.; Schaumberger, M.; Haritoglou, C. A 23-gauge pars plana vitrectomy after induction of general anesthesia: Effect of additional retrobulbar anesthesia on postoperative pain. Ophthalmologe 2014, 111, 1194–1200. [Google Scholar] [CrossRef]
- Schönfeld, C.-L.; Hierneis, S.; Kampik, A. Preemptive Analgesia with Ropivacaine for Pars Plana Vitrectomy: Randomized Controlled Trial on Efficacy and Required Dose. Retina 2012, 32, 912–917. [Google Scholar] [CrossRef]
- Henzler, D.; Müller-Kaulen, B.; Steinhorst, U.H.; Broermann, H.; Piepenbrock, S. The combination of retrobulbar block with general anaesthesia may lead to pre-emptive analgesia in patients undergoing pars plana vitrectomy. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2002, 37, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Chari, P.; Kumar, P. Effect of Sevoflurane versus Propofol-Based Anesthesia on the Hemodynamic Response and Recovery Characteristics in Patients Undergoing Microlaryngeal Surgery. Saudi J. Anaesth. 2012, 6, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Thee, C.; Gruenewald, M.; Wnent, J.; Illies, C.; Hoecker, J.; Hanss, R.; Steinfath, M.; Bein, B. Comparison of Surgical Stress Index-Guided Analgesia with Standard Clinical Practice during Routine General Anesthesia: A Pilot Study. Anesthesiology 2010, 112, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jwa, E.K.; Choung, Y.; Yeon, H.J.; Kim, S.Y.; Kim, E. Comparison of Pupillometry with Surgical Pleth Index Monitoring on Perioperative Opioid Consumption and Nociception during Propofol-Remifentanil Anesthesia: A Prospective Randomized Controlled Trial. Anesth. Analg. 2020, 131, 1589–1598. [Google Scholar] [CrossRef]
- Gruenewald, M.; Harju, J.; Preckel, B.; Molnár, Z.; Yli-Hankala, A.; Rosskopf, F.; Koers, L.; Orban, A.; Bein, B.; AoA Study Group. Comparison of Adequacy of Anaesthesia Monitoring with Standard Clinical Practice Monitoring during Routine General Anaesthesia: An International, Multicentre, Single-Blinded Randomised Controlled Trial. Eur. J. Anaesthesiol. 2021, 38, 73–81. [Google Scholar] [CrossRef]
- Bergmann, I.; Göhner, A.; Crozier, T.A.; Hesjedal, B.; Wiese, C.H.; Popov, A.F.; Bauer, M.; Hinz, J.M. Surgical Pleth Index-Guided Remifentanil Administration Reduces Remifentanil and Propofol Consumption and Shortens Recovery Times in Outpatient Anaesthesia. Br. J. Anaesth. 2013, 110, 622–628. [Google Scholar] [CrossRef]
- Ilies, C.; Gruenewald, M.; Ludwigs, J.; Thee, C.; Höcker, J.; Hanss, R.; Steinfath, M.; Bein, B. Evaluation of the Surgical Stress Index during Spinal and General Anaesthesia. Br. J. Anaesth. 2010, 105, 533–537. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Kawka, M.; Krawczyk, L.; Niewiadomska, E.; Dobrowolski, D.; Rejdak, R.; Król, S.; Żak, J.; et al. Preventive Analgesia, Hemodynamic Stability, and Pain in Vitreoretinal Surgery. Medicina 2021, 57, 262. [Google Scholar] [CrossRef]
- Pluta, A.; Stasiowski, M.J.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during Vitrectomy under Adequacy of Anesthesia-An Additional Report. J. Clin. Med. 2021, 10, 4172. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during Vitreoretinal Surgery under Adequacy of Anesthesia Guidance-Risk Factor Analysis. Pharmaceuticals 2022, 15, 237. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Niewiadomska, E.; Krawczyk, L.; Dobrowolski, D.; Grabarek, B.O.; Kawka, M.; Rejdak, R.; Szumera, I.; et al. Adequacy of Anaesthesia for Nociception Detection during Vitreoretinal Surgery. Life 2023, 13, 505. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Lyssek-Boroń, A.; Zmarzły, N.; Marczak, K.; Grabarek, B.O. The Adequacy of Anesthesia Guidance for Vitreoretinal Surgeries with Preemptive Paracetamol/Metamizole. Pharmaceuticals 2024, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Owczuk, R. Guidelines for General Anaesthesia in the Elderly of the Committee on Quality and Safety in Anaesthesia, Polish Society of Anaesthesiology and Intensive Therapy. Anaesthesiol. Intensive Ther. 2013, 45, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.C. Techniques of Orbital Regional Anaesthesia. Br. J. Anaesth. 1995, 75, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.; Duława, A.; Szumera, I.; Marciniak, R.; Niewiadomska, E.; Kaspera, W.; Krawczyk, L.; Ładziński, P.; Grabarek, B.O.; Jałowiecki, P. Variations in Values of State, Response Entropy and Haemodynamic Parameters Associated with Development of Different Epileptiform Patterns during Volatile Induction of General Anaesthesia with Two Different Anaesthetic Regimens Using Sevoflurane in Comparison with Intravenous Induct: A Comparative Study. Brain Sci. 2020, 10, 366. [Google Scholar] [CrossRef]
- Stasiowski, M.; Missir, A.; Pluta, A.; Szumera, I.; Stasiak, M.; Szopa, W.; Błaszczyk, B.; Możdżyński, B.; Majchrzak, K.; Tymowski, M.; et al. Influence of Infiltration Anaesthesia on Perioperative Outcomes Following Lumbar Discectomy under Surgical Pleth Index-Guided General Anaesthesia: A Preliminary Report from a Randomised Controlled Prospective Trial. Adv. Med. Sci. 2020, 65, 149–155. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Szumera, I.; Wardas, P.; Król, S.; Żak, J.; Missir, A.; Pluta, A.; Niewiadomska, E.; Krawczyk, L.; Jałowiecki, P.; et al. Adequacy of Anesthesia and Pupillometry for Endoscopic Sinus Surgery. J. Clin. Med. 2021, 10, 4683. [Google Scholar] [CrossRef]
- Gruenewald, M.; Ilies, C. Monitoring the Nociception-Anti-Nociception Balance. Best. Pract. Res. Clin. Anaesthesiol. 2013, 27, 235–247. [Google Scholar] [CrossRef]
- Ilies, C.; Ludwigs, J.; Gruenewald, M.; Thee, C.; Hanf, J.; Hanss, R.; Steinfath, M.; Bein, B. The Effect of Posture and Anaesthetic Technique on the Surgical Pleth Index. Anaesthesia 2012, 67, 508–513. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Meidert, A.S.; Saugel, B. Techniques for Non-Invasive Monitoring of Arterial Blood Pressure. Front. Med. 2017, 4, 231. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, W.; Shi, Q.; Bao, F.; Xu, J. Effect of Surgical Pleth Index-Guided Analgesia versus Conventional Analgesia Techniques on Fentanyl Consumption under Multimodal Analgesia in Laparoscopic Cholecystectomy: A Prospective, Randomized and Controlled Study. BMC Anesthesiol. 2021, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T.; Schneider, M.; Gruenewald, M.; Goyal, R.K.; Teo, S.R.; Hruby, J. Surgical Pleth Index: Prospective Validation of the Score to Predict Moderate-to-Severe Postoperative Pain. Br. J. Anaesth. 2019, 123, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T.; Burke, J.; Hruby, J. Surgical Pleth Index: Prediction of Postoperative Pain and Influence of Arousal. Br. J. Anaesth. 2016, 117, 371–374. [Google Scholar] [CrossRef]
- Brodner, G.; Gogarten, W.; Van Aken, H.; Hahnenkamp, K.; Wempe, C.; Freise, H.; Cosanne, I.; Huppertz-Thyssen, M.; Ellger, B. Efficacy of Intravenous Paracetamol Compared to Dipyrone and Parecoxib for Postoperative Pain Management after Minor-to-Intermediate Surgery: A Randomised, Double-Blind Trial. Eur. J. Anaesthesiol. 2011, 28, 125–132. [Google Scholar] [CrossRef]
- McNicol, E.D.; Tzortzopoulou, A.; Cepeda, M.S.; Francia, M.B.D.; Farhat, T.; Schumann, R. Single-Dose Intravenous Paracetamol or Propacetamol for Prevention or Treatment of Postoperative Pain: A Systematic Review and Meta-Analysis. Br. J. Anaesth. 2011, 106, 764–775. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.; Chandrasena, C.; Schug, S.A. Nonopioid Analgesics for Postoperative Pain Management. Curr. Opin. Anaesthesiol. 2014, 27, 513–519. [Google Scholar] [CrossRef]
- Sener, M.; Kocum, A.; Caliskan, E.; Yilmaz, I.; Caylakli, F.; Aribogan, A. Administration of Paracetamol versus Dipyrone by Intravenous Patient-Controlled Analgesia for Postoperative Pain Relief in Children after Tonsillectomy. Braz. J. Anesthesiol. 2015, 65, 476–482. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Tong, Y.; Wang, Y.-X.; Zhao, P.-Q.; Wang, Z.-Y. A Prospective, Randomised, Double-Masked Comparison of Local Anaesthetic Agents for Vitrectomy. Br. J. Ophthalmol. 2017, 101, 1016–1021. [Google Scholar] [CrossRef]
- Knudsen, K.; Beckman Suurküla, M.; Blomberg, S.; Sjövall, J.; Edvardsson, N. Central Nervous and Cardiovascular Effects of i.v. Infusions of Ropivacaine, Bupivacaine and Placebo in Volunteers. Br. J. Anaesth. 1997, 78, 507–514. [Google Scholar] [CrossRef]
- Scott, D.B.; Lee, A.; Fagan, D.; Bowler, G.M.; Bloomfield, P.; Lundh, R. Acute Toxicity of Ropivacaine Compared with That of Bupivacaine. Anesth. Analg. 1989, 69, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Jaichandran, V.V.; Raman, R.; Gella, L.; Sharma, T. Local Anesthetic Agents for Vitreoretinal Surgery: No Advantage to Mixing Solutions. Ophthalmology 2015, 122, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Bapat, M.; Braganza, S.; Thirumalesh, M.B. Effect of Adding Dexmedetomidine to 0.75% Ropivacaine in Peribulbar Block for Vitreoretinal Surgery. J. Anaesthesiol. Clin. Pharmacol. 2022, 38, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Jaichandran, V.V.; Srinivasan, S.; Raman, S.; Jagadeesh, V.; Raman, R. A Prospective Comparison of the Efficacy of 0.5% Bupivacaine vs 0.75% Ropivacaine in Peribulbar Anesthesia for Vitreoretinal Surgery. Indian. J. Ophthalmol. 2020, 68, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, M.; Magni, G.; Marraro, G. A Prospective Randomized Double-Blinded Controlled Study of Ropivacaine 0.75% versus Bupivacaine 0.5%-Mepivacaine 2% for Peribulbar Anesthesia. Reg. Anesth. Pain Med. 2000, 25, 195–200. [Google Scholar] [CrossRef]
- Mattia, C.; Coluzzi, F. What Anesthesiologists Should Know about Paracetamol (Acetaminophen). Minerva Anestesiol. 2009, 75, 644–653. [Google Scholar]
- Sadrolsadat, S.H.; Yousefshahi, F.; Ostadalipour, A.; Mohammadi, F.Z.; Makarem, J. Effect of Intravenous Acetaminophen on Postoperative Pain in Vitrectomy: A Randomized, Double-Blind, Clinical Trial. Anesth. Pain Med. 2017, 7, e13639. [Google Scholar] [CrossRef]
- Smith, H.S. Potential Analgesic Mechanisms of Acetaminophen. Pain Physician 2009, 12, 269–280. [Google Scholar] [CrossRef]
- Graham, G.G.; Scott, K.F. Mechanism of Action of Paracetamol. Am. J. Ther. 2005, 12, 46–55. [Google Scholar] [CrossRef]
- Raffa, R.B.; Walker, E.A.; Sterious, S.N. Opioid Receptors and Acetaminophen (Paracetamol). Eur. J. Pharmacol. 2004, 503, 209–210. [Google Scholar] [CrossRef]
- Ottani, A.; Leone, S.; Sandrini, M.; Ferrari, A.; Bertolini, A. The Analgesic Activity of Paracetamol Is Prevented by the Blockade of Cannabinoid CB1 Receptors. Eur. J. Pharmacol. 2006, 531, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Roca-Vinardell, A.; Ortega-Alvaro, A.; Gibert-Rahola, J.; Micó, J.A. The Role of 5-HT1A/B Autoreceptors in the Antinociceptive Effect of Systemic Administration of Acetaminophen. Anesthesiology 2003, 98, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Bujalska, M. Effect of Nitric Oxide Synthase Inhibition on Antinociceptive Action of Different Doses of Acetaminophen. Pol. J. Pharmacol. 2004, 56, 605–610. [Google Scholar] [PubMed]
- Doleman, B.; Read, D.; Lund, J.N.; Williams, J.P. Preventive Acetaminophen Reduces Postoperative Opioid Consumption, Vomiting, and Pain Scores After Surgery: Systematic Review and Meta-Analysis. Reg. Anesth. Pain Med. 2015, 40, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Toms, L.; McQuay, H.J.; Derry, S.; Moore, R.A. Single Dose Oral Paracetamol (Acetaminophen) for Postoperative Pain in Adults. Cochrane Database Syst. Rev. 2008, 2008, CD004602. [Google Scholar] [CrossRef]
- Apfel, C.C.; Turan, A.; Souza, K.; Pergolizzi, J.; Hornuss, C. Intravenous Acetaminophen Reduces Postoperative Nausea and Vomiting: A Systematic Review and Meta-Analysis. Pain 2013, 154, 677–689. [Google Scholar] [CrossRef]
- Oscier, C.D.; Milner, Q.J.W. Peri-Operative Use of Paracetamol. Anaesthesia 2009, 64, 65–72. [Google Scholar] [CrossRef]
- Steffen, P.; Schuhmacher, I.; Weichel, T.; Georgieff, M.; Seeling, W. Differential administration of non-opioids in postoperative analgesia, I. Quantification of the analgesic effect of metamizole using patient-controlled analgesia. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 1996, 31, 143–147. [Google Scholar] [CrossRef]
- Zukowski, M.; Kotfis, K. Safety of metamizole and paracetamol for acute pain treatment. Anestezjol. Intens. Ter. 2009, 41, 170–175. [Google Scholar]
- Konijnenbelt-Peters, J.; van der Heijden, C.; Ekhart, C.; Bos, J.; Bruhn, J.; Kramers, C. Metamizole (Dipyrone) as an Alternative Agent in Postoperative Analgesia in Patients with Contraindications for Nonsteroidal Anti-Inflammatory Drugs. Pain Pract. 2017, 17, 402–408. [Google Scholar] [CrossRef]
- Andrade, S.; Bartels, D.B.; Lange, R.; Sandford, L.; Gurwitz, J. Safety of Metamizole: A Systematic Review of the Literature. J. Clin. Pharm. Ther. 2016, 41, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Blanca-López, N.; Pérez-Sánchez, N.; Agúndez, J.A.; García-Martin, E.; Torres, M.J.; Cornejo-García, J.A.; Perkins, J.R.; Miranda, M.A.; Andreu, I.; Mayorga, C.; et al. Allergic Reactions to Metamizole: Immediate and Delayed Responses. Int. Arch. Allergy Immunol. 2016, 169, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Juste, J.F.M.; Garces, T.R.; Enguita, R.G.; Blasco, P.C.; Trallero, J.A. Cardiac Complications in a Metamizole-Induced Type I Kounis Syndrome. Braz. J. Anesthesiol. 2016, 66, 194–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shanthanna, H.; Uppal, V.; Joshi, G.P. Intraoperative Nociception Monitoring. Anesthesiol. Clin. 2021, 39, 493–506. [Google Scholar] [CrossRef]
- Ledowski, T. Objective Monitoring of Nociception: A Review of Current Commercial Solutions. Br. J. Anaesth. 2019, 123, e312–e321. [Google Scholar] [CrossRef]
- Struys, M.M.R.F.; Vanpeteghem, C.; Huiku, M.; Uutela, K.; Blyaert, N.B.K.; Mortier, E.P. Changes in a Surgical Stress Index in Response to Standardized Pain Stimuli during Propofol-Remifentanil Infusion. Br. J. Anaesth. 2007, 99, 359–367. [Google Scholar] [CrossRef]
- Wennervirta, J.; Hynynen, M.; Koivusalo, A.-M.; Uutela, K.; Huiku, M.; Vakkuri, A. Surgical Stress Index as a Measure of Nociception/Antinociception Balance during General Anesthesia. Acta Anaesthesiol. Scand. 2008, 52, 1038–1045. [Google Scholar] [CrossRef]
- Ahonen, J.; Jokela, R.; Uutela, K.; Huiku, M. Surgical Stress Index Reflects Surgical Stress in Gynaecological Laparoscopic Day-Case Surgery. Br. J. Anaesth. 2007, 98, 456–461. [Google Scholar] [CrossRef]
- Ledowski, T.; Pascoe, E.; Ang, B.; Schmarbeck, T.; Clarke, M.W.; Fuller, C.; Kapoor, V. Monitoring of Intra-Operative Nociception: Skin Conductance and Surgical Stress Index versus Stress Hormone Plasma Levels. Anaesthesia 2010, 65, 1001–1006. [Google Scholar] [CrossRef]
- Gruenewald, M.; Meybohm, P.; Ilies, C.; Höcker, J.; Hanss, R.; Scholz, J.; Bein, B. Influence of Different Remifentanil Concentrations on the Performance of the Surgical Stress Index to Detect a Standardized Painful Stimulus during Sevoflurane Anaesthesia. Br. J. Anaesth. 2009, 103, 586–593. [Google Scholar] [CrossRef]
- Lurati Buse, G.A.; Mauermann, E.; Ionescu, D.; Szczeklik, W.; De Hert, S.; Filipovic, M.; Beck-Schimmer, B.; Spadaro, S.; Matute, P.; Bolliger, D.; et al. Risk Assessment for Major Adverse Cardiovascular Events after Noncardiac Surgery Using Self-Reported Functional Capacity: International Prospective Cohort Study. Br. J. Anaesth. 2023, 130, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Gola, W.; Bialka, S.; Zajac, M.; Misiolek, H. Cardiac Arrest after Small Doses Ropivacaine: Local Anesthetic Systemic Toxicity in the Course of Continuous Femoral Nerve Blockade. Int. J. Environ. Res. Public Health 2022, 19, 12223. [Google Scholar] [CrossRef] [PubMed]
- Gioia, L.; Fanelli, G.; Casati, A.; Nuti, U.; Mennella, R.; Scarioni, M.; Cerchierini, E.; Sciascia, A.; Garassino, A.; Torri, G.; et al. A Prospective, Randomized, Double-Blinded Comparison of Ropivacaine 0.5%, 0.75%, and 1% Ropivacaine for Peribulbar Block. J. Clin. Anesth. 2004, 16, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Czajka, S.; Putowski, Z.; Krzych, Ł.J. Post-Induction Hypotension and Intraoperative Hypotension as Potential Separate Risk Factors for the Adverse Outcome: A Cohort Study. J. Anesth. 2023, 37, 442–450. [Google Scholar] [CrossRef]
- Travieso-Gonzalez, A.; Núñez-Gil, I.J.; Riha, H.; Donaire, J.A.G.; Ramakrishna, H. Management of Arterial Hypertension: 2018 ACC/AHA Versus ESC Guidelines and Perioperative Implications. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3496–3503. [Google Scholar] [CrossRef]
- Jain, N.; Gera, A.; Sharma, B.; Sood, J.; Chugh, P. Comparison of Surgical Pleth Index-Guided Analgesia Using Fentanyl versus Conventional Analgesia Technique in Laparoscopic Cholecystectomy. Minerva Anestesiol. 2019, 85, 358–365. [Google Scholar] [CrossRef]
- Krebs, E.E.; Carey, T.S.; Weinberger, M. Accuracy of the Pain Numeric Rating Scale as a Screening Test in Primary Care. J. Gen. Intern. Med. 2007, 22, 1453–1458. [Google Scholar] [CrossRef]
- Oh, S.K.; Won, Y.J.; Lim, B.G. Surgical Pleth Index Monitoring in Perioperative Pain Management: Usefulness and Limitations. Korean J. Anesthesiol. 2023, 77, 31–45. [Google Scholar] [CrossRef]
- Laferrière-Langlois, P.; Morisson, L.; Jeffries, S.; Duclos, C.; Espitalier, F.; Richebé, P. Depth of Anesthesia and Nociception Monitoring: Current State and Vision for 2050. Anesth. Analg. 2024, 138, 295–307. [Google Scholar] [CrossRef]
- Won, Y.J.; Lim, B.G.; Kim, Y.S.; Lee, M.; Kim, H. Usefulness of Surgical Pleth Index-Guided Analgesia during General Anesthesia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Int. Med. Res. 2018, 46, 4386–4398. [Google Scholar] [CrossRef]
- Ribotsky, B.M.; Berkowitz, K.D.; Montague, J.R. Local Anesthetics. Is There an Advantage to Mixing Solutions? J. Am. Podiatr. Med. Assoc. 1996, 86, 487–491. [Google Scholar] [CrossRef]
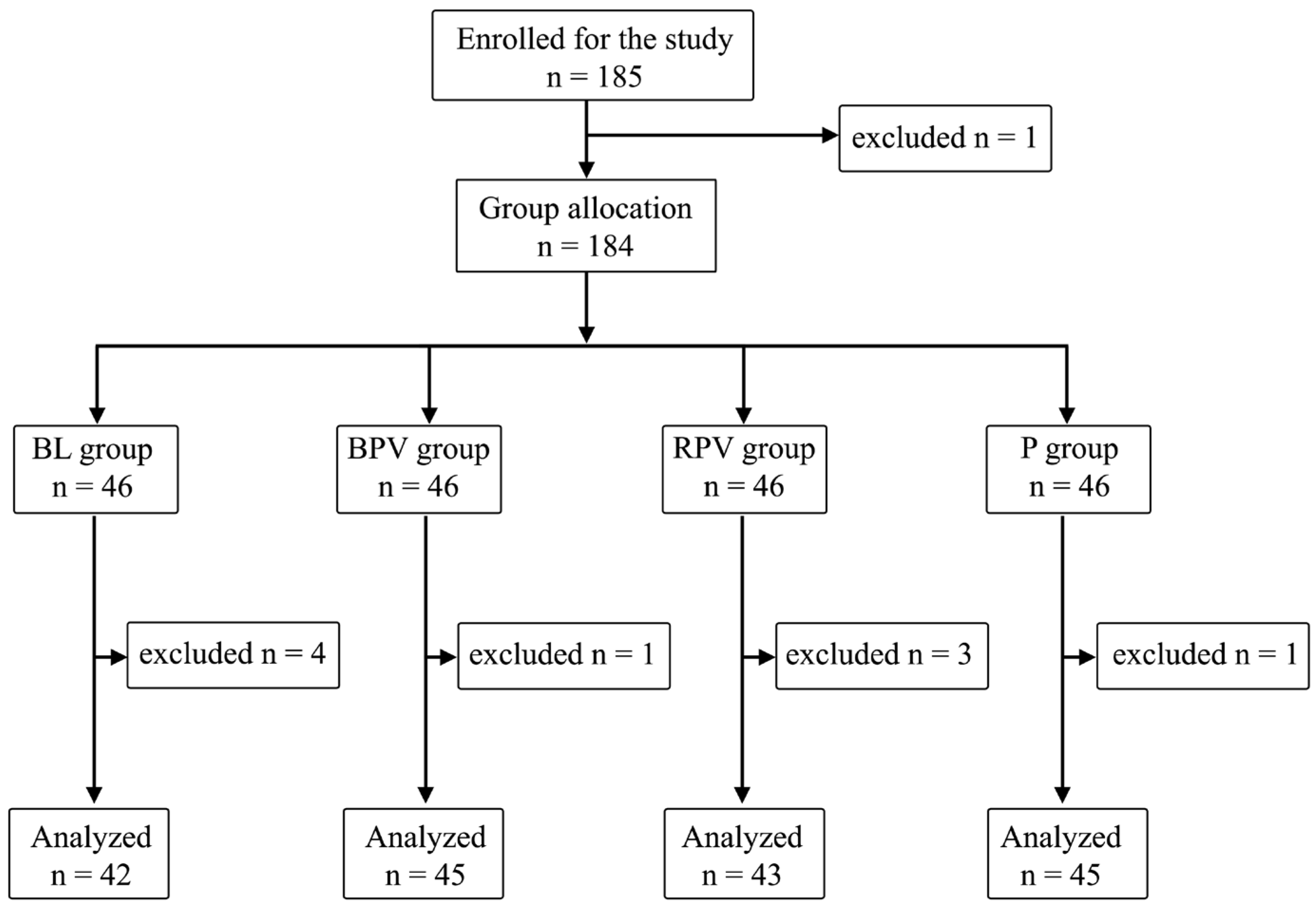

| Metrics | Total n = 175 (100%) | BL n = 42 (24%) | BPV n = 45 (25.71%) | RPV n = 43 (24.57%) | P n = 45 (25.71%) | p-Value | |
|---|---|---|---|---|---|---|---|
| Age X ± Sd Me (IQR) | [years] | 66.1 ± 9.5 67 (11) | 66.3 ± 11.4 66.5 (13) | 66.29 ± 7.6 68 (8) | 66.23 ± 9.7 68 (12) | 65.24 ± 9.6 66 (9) | 0.83 NS |
| Gender n (%) | Female | 99 (56.57) | 26 (61.9) | 23 (51.1) | 19 (44.2) | 31 (68.9) | 0.09 NS |
| Male | 76 (43.43) | 16 (38.1) | 22 (48.9) | 24 (55.8) | 14 (31.1) | ||
| Anthropometric Data | Total n = 175 (100%) | BL n = 42 (24%) | BPV n = 45 (25.71%) | RPV n = 43 (24.57%) | P n = 45 (25.71%) | p-Value | |
| Height X ± Sd Me (IQR) | [cm] | 166.2 ± 9 165 (13) | 165.7 ± 8.3 164.5 (10) | 168.9 ± 9 168 (11) | 166.7 ± 9.94 168 (18) | 163.7 ± 8.1 163 (11) | 0.07 NS |
| Weight X ± Sd Me (IQR) | [kg] | 77.4 ± 14.4 76 (20) | 77.3 ± 16.1 73 (14) | 80.3 ± 13.3 79 (21) | 78.1 ± 15 76 (19) | 74.2 ± 13.1 74 (19) | 0.22 NS |
| BMI X ± Sd Me (IQR) | [kg/m2] | 28 ± 4.7 27.7 (5.9) | 28.2 ± 5.5 26.8 (4.8) | 28 ± 3.3 27.5 (4.9) | 28.1 ± 4.7 28.1 (7) | 27.8 ± 5 27.9 (7) | 0.86 NS |
| BMI n (%) | norm | 51 (30) | 12 (29.3) | 9 (22) | 14 (32.6) | 16 (35.6) | 0.6 NS |
| overweight | 69 (40.6) | 20 (48.8) | 19 (46.3) | 15 (34.9) | 15 (33.3) | 0.4 NS | |
| obesity | 50 (29.4) | 9 (22) | 13 (31.7) | 14 (32.6) | 14 (31.1) | 0.7 NS | |
| Type of VRS | Total n = 175 (100%) | BL n = 42 (24%) | BPV n = 45 (25.71%) | RPV n = 43 (24.57%) | P n = 45 (25.71%) | p-Value |
|---|---|---|---|---|---|---|
| Pars Plana Vitrectomy Yes (%) | 62 (35.4) | 18 (42.9) | 14 (31.1) | 12 (27.9) | 18 (40) | 0.41 NS |
| Phacovitrectomy Yes (%) | 113 (64.6) | 24 (57.1) | 31 (68.9) | 31 (72.1) | 27 (60) | 0.41 NS |
| The frequency of surgical maneuvers during VRS | ||||||
| Speculum Adjustment Yes (%) | 175 (100) | 42 (100) | 45 (100) | 43 (100) | 45 (100) | - |
| Trocars’ Insertion Yes (%) | 175 (100) | 42 (100) | 45 (100) | 43 (100) | 45 (100) | - |
| Vitrectom Insertion Yes (%) | 28 ± 4.7 27.7 (5.9) | 28.2 ± 5.5 26.8 (4.8) | 28 ± 3.3 27.5 (4.9) | 28.1 ± 4.7 28.1 (7) | 27.8 ± 5 27.9 (7) | 0.86 NS |
| Staining Agent Injection Yes (%) | 155 (88.6) | 34 (81) | 44 (97.8) | 37 (86) | 40 (88.9) | BL vs. BPV, p = 0.03 |
| Peeling Yes (%) | 155 (88.6) | 34 (81) | 44 (97.8) | 37 (86) | 40 (88.9) | BL vs. BPV, p = 0.03 |
| Gas-Liquid Exchange Yes (%) | 52 (29.7) | 13 (31) | 11 (24.4) | 10 (23.3) | 18 (40) | 0.29 NS |
| Endolaser Treatment Yes (%) | 116 (66.3) | 28 (66.7) | 29 (64.4) | 28 (65.1) | 31 (68.9) | 0.97 NS |
| Silicon Oil Injection Yes (%) | 42 (24) | 8 (19) | 10 (22.2) | 11 (25.6) | 13 (28.9) | 0.73 NS |
| Indentation Yes (%) | 47 (26.9) | 14 (33.3) | 3 (6.7) | 6 (14) | 24 (53.3) | BL vs. BPV, p = 0.004; BPV vs. P, p < 0.001; RPV vs. P, p < 0.001 |
| Subconjunctival Injection Yes (%) | 175 (100) | 42 (100) | 45 (100) | 43 (100) | 45 (100) | - |
| Trocars’ Removal Yes (%) | 175 (100) | 42 (100) | 45 (100) | 43 (100) | 45 (100) | - |
| Speculum Removal Yes (%) | 175 (100) | 42 (100) | 45 (100) | 43 (100) | 45 (100) | - |
| VRS | Total n = 175 (100%) | BL n = 42 (24%) | BPV n = 45 (25.71%) | RPV n = 43 (24.57%) | P n = 45 (25.71%) | p-Value | |
|---|---|---|---|---|---|---|---|
| Time of VRS X ± Sd Me (IQR) | [min] | 51.32 ± 18.32 48 (25) | 59.4 ± 22.74 59.5 (38) | 45.73 ± 15.38 43 (18) | 47.74 ± 16.05 43 (24) | 52.78 ± 15.93 51 (22) | BL vs. BPV, p = 0.01 |
| Dose of IROA using FNT X ± Sd Me (IQR) | [mcg] | 121.08 ± 76.78 100 (100) | 131 ± 92.12 100 (150) | 116.67 ± 64.77 100 (100) | 111.36 ± 65.34 100 (100) | 122.37 ± 80.28 100 (100) | 0.97 NS |
| Number of patients requiring IROA | Yes (%) | 120 (68.57) | 30 (71.43) | 30 (66.67) | 22 (51.16) | 38 (84.44) | RPV vs. P, p = 0.002 |
| Intraoperative fluid therapy X ± Sd Me (IQR) | [mL] | 1082.85 ± 309.42 1000 (350) | 1097.56 ± 270.64 1000 (250) | 1057.21 ± 284.14 1000 (450) | 1234.39 ± 355.48 1250 (500) | 940 ± 254.75 1000 (250) | BL vs. P, p = 0.04; RPV vs. P, p < 0.001 |
| Postoperative pain perception in the PACU | |||||||
| NPRS max X ± Sd Me (IQR) | [1 ÷ 10] | 0.87 ± 1.76 0 (0) | 0.95 ± 1.78 0 (1) | 0.09 ± 0.6 0 (0) | 1.19 ± 2.07 0 (2) | 1.27 ± 1.97 0 (2) | BPV vs. P, p = 0.03 |
| Type of first postoperative pain perception n (%) | Mild | 157 (89.2) | 37 (88.1) | 44 (97.78) | 37 (86.05) | 39 (84.78) | 0.22 NS |
| Moderate | 16 (9.09) | 5 (11.9) | 1 (2.22) | 5 (11.63) | 5 (10.87) | 0.17 NS | |
| Acute | 2 (1.14) | 0 (0) | 0 (0) | 1 (2.33) | 1 (2.17) | 1.0 NS | |
| IPPP | 18 (10.23) | 5 (11.9) | 1 (2.22) | 6 (13.96) | 6 (13.04) | 0.22 NS | |
| Parameter | BL n = 42 (24%) | BPV n = 45 (25.71%) | RPV n = 43 (24.57%) | P n = 45 (25.71%) | p-Value |
|---|---|---|---|---|---|
| Stage 1—onset | |||||
| SAP (mmHg) | 146 ± 26.63 143 (35) | 151.64 ± 17.79 152 (27) | 151.91 ± 18.56 151 (25) | 153 ± 19.2 157 (28) | 0.5 NS |
| MAP (mmHg) | 108.57 ± 15.3 108.5 (24) | 112.47 ± 13.13 111 (18) | 109.63 ± 10.66 109 (15) | 110.53 ± 12.21 111 (17) | 0.68 NS |
| DAP (mmHg) | 79.26 ± 9.59 79 (14) | 83.6 ± 10.85 82 (14) | 80.16 ± 7.86 80 (11) | 78.42 ± 10.66 76 (15) | 0.14 NS |
| HR (beats/min) | 70.64 ± 11.92 72.5 (19) | 70.24 ± 9.48 69 (17) | 70.56 ± 10.18 68 (17) | 73.89 ± 11.23 74 (14) | 0.38 NS |
| SPI | 55.74 ± 20.09 62.5 (32) | 59.42 ± 16.78 65 (25) | 58.7 ± 14.96 61 (24) | 55.58 ± 16.27 51 (24) | 0.6 NS |
| Stage 2—between GA induction and start of VRS | |||||
| mean SAP (mmHg) | 124.74 ± 24.76 119.5 (32) | 129.39 ± 26.83 129 (38.3) | 120.1 ± 23.49 115.7 (34) | 134.87 ± 25.92 134 (40) | RPV vs. P, p = 0.03 |
| mean MAP (mmHg) | 92.34 ± 16.91 88.8 (23) | 96.07 ± 17.75 94.5 (23) | 89.49 ± 15.83 88 (20) | 98.43 ± 16.87 100 (26.5) | RPV vs. P, p = 0.048 |
| mean DAP (mmHg) | 70.4 ± 11.91 69 (18.3) | 74.03 ± 12.61 73 (20) | 68.34 ± 12.47 67 (15) | 72.86 ± 11.78 73 (17.3) | 0.12 NS |
| mean HR (beats/min) | 69.73 ± 11 68.2 (18.5) | 69.55 ± 9 68.8 (12) | 66.67 ± 11.51 66.4 (16.3) | 68.78 ± 9.82 68 (12.3) | 0.48 NS |
| mean SPI | 31.64 ± 9.55 29.5 (11) | 33.69 ± 12.79 28.7 (14) | 28.13 ± 9.83 25.7 (12.6) | 39.81 ± 36.83 33 (17.1) | RPV vs. P, p = 0.01 |
| mean SE | 42.45 ± 8.8 42.2 (13) | 39.89 ± 7.87 38.8 (13) | 42.47 ± 7.63 41.4 (10.6) | 40.6 ± 10.15 39.8 (16.6) | 0.34 NS |
| Stage 3—VRS | |||||
| mean SAP (mmHg) | 105.25 ± 16.51 100.2 (16.2) | 100.97 ± 13.85 99.7 (21.39) | 102.3 ± 20.47 95.2 (21.7) | 109.76 ± 22.48 106 (24.9) | RPV vs. P, p = 0.04 |
| mean MAP (mmHg) | 79.81 ± 11.09 76.9 (11.9) | 77.41 ± 9.48 75.1 (14.5) | 77.77 ± 14.45 73.6 (15.1) | 83.28 ± 12.54 81.3 (14.5) | RPV vs. P, p = 0.03 |
| mean DAP (mmHg) | 61.38 ± 8.53 60 (12.1) | 60.43 ± 8.1 59 (11.64) | 59.25 ± 11.57 57.4 (12.6) | 63.11 ± 9.95 63.4 (10.5) | RPV vs. P, p = 0.04 |
| mean HR (beats/min) | 62 ± 8.64 62.9 (12.5) | 59.26 ± 7.07 58.2 (9.65) | 59.44 ± 8.25 58.3 (13.4) | 60.78 ± 8.67 60.3 (12.4) | 0.31 NS |
| mean SPI | 31.74 ± 7.36 31.4 (9.1) | 29.53 ± 8.18 28.3 (9.31) | 27.4 ± 7.54 25.8 (8.7) | 34.85 ± 10.86 31.7 (9.8) | BL vs. RPV, p = 0.02; BPV vs. P, p = 0.04; RPV vs. P, p = 0.001 |
| mean SE | 44.77 ± 6.33 45.5 (9.3) | 42.49 ± 7.48 41.6 (6.94) | 44.66 ± 6.67 45.7 (7) | 41.2 ± 7.71 40.5 (10.6) | 0.06 NS |
| Stage 4—postoperatively | |||||
| mean SAP (mmHg) | 147.01 ± 18.82 143.8 (24.2) | 150.68 ± 150.68 152 (18) | 147.6 ± 18.41 146.8 (23.3) | 150.41 ± 18.61 153.6 (29.8) | 0.58 NS |
| meanMAP (mmHg) | 102.29 ± 14.19 102.8 (13.3) | 108.4 ± 108.4 110.5 (17.7) | 102.8 ± 13.86 103.3 (19.3) | 103.06 ± 16.12 103.3 (19.1) | 0.13 NS |
| mean DAP (mmHg) | 80.59 ± 11.81 77.3 (14.3) | 82.48 ± 82.48 83.3 (14.1) | 80.6 ± 14.54 77.2 (19.3) | 80.17 ± 14.72 78.1 (14.7) | 0.39 NS |
| mean HR (beats/min) | 72.48 ± 11.09 71.1 (13.2) | 70.08 ± 70.08 70.9 (14) | 70.09 ± 9.89 70 (12.6) | 68.55 ± 9.69 66.6 (13.9) | 0.44 NS |
| mean SPI | 55.91 ± 13.5 54.8 (23.2) | 62.05 ± 62.05 63.4 (18.3) | 58.05 ± 11.03 60.4 (18.6) | 52.8 ± 15.38 52.2 (24) | BPV vs. P, p = 0.009 |
| Parameter | BL n = 42 (24%) | BPV n = 45 (25.71%) | RPV n = 43 (24.57%) | P n = 45 (25.71%) | p-Value |
|---|---|---|---|---|---|
| Stage 2—between GA induction and start of VRS | |||||
| max SAP (mmHg) | 137.36 ± 27.16 132 (33) | 141.37 ± 28.43 140 (49) | 131.58 ± 27.05 128 (46) | 142.42 ± 25.52 147 (38) | 0.13 NS |
| max MAP (mmHg) | 101.05 ± 17.86 97 (25) | 104.81 ± 19.69 104 (31) | 96.81 ± 18.08 95 (28) | 103.51 ± 16.01 104 (21) | 0.12 NS |
| max DAP (mmHg) | 76.14 ± 12.03 75.5 (17) | 80.88 ± 14.57 78 (20) | 73.58 ± 14.62 72 (22) | 76.27 ± 11.55 78 (18) | 0.09 NS |
| max HR (beats/min) | 76.93 ± 13.4 75 (18) | 75.22 ± 10.94 73 (13) | 74.47 ± 13.03 74 (17) | 74.38 ± 11.42 73 (15) | 0.77 NS |
| max SPI | 40.02 ± 10.78 39 (12) | 45.91 ± 17.54 43 (33) | 38.86 ± 14.68 35 (18) | 43.02 ± 14.3 43 (21) | 0.19 NS |
| max SE | 48.39 ± 12.9 45 (15) | 51.76 ± 16.01 48 (18) | 53.68 ± 12.14 52 (12.5) | 46.56 ± 10.18 45 (17) | 0.08 NS |
| min SAP (mmHg) | 114.24 ± 26.09 109 (36) | 118 ± 29.72 115 (41) | 109.81 ± 23.69 106 (29) | 127.09 ± 28.23 133 (45) | RPV vs. P, p = 0.02 |
| min MAP (mmHg) | 85.74 ± 17.19 83.6 (22) | 88.77 ± 18.61 86 (24) | 82.98 ± 16.18 80 (22) | 93.44 ± 18.62 94 (29) | RPV vs. P, p = 0.04 |
| min DAP (mmHg) | 65.36 ± 12.98 64.5 (15) | 68.51 ± 13.4 67 (20) | 63.6 ± 12.27 62 (17) | 69.51 ± 12.99 67 (20) | 0.15 NS |
| min HR (beats/min) | 65.14 ± 11.18 65 (17) | 64.96 ± 9.15 63 (12) | 62.35 ± 11.23 61 (15) | 65.27 ± 9.28 65 (11) | 0.42 NS |
| min SPI | 25.14 ± 8.54 25.5 (13) | 26.33 ± 12.95 22 (12) | 21.05 ± 8.21 19 (10) | 28.09 ± 10.69 25 (12) | RPV vs. P, p = 0.002 |
| min SE | 37.01 ± 9.12 39 (11) | 29.42 ± 7.8 28 (9) | 30.45 ± 8.43 32 (9.5) | 33.97 ± 9.69 33 (13) | BL vs. BPV, p < 0.001; BL vs. RPV, p = 0.02 |
| Stage 3—VRS | |||||
| max SAP (mmHg) | 131.36 ± 26.51 125 (38) | 121.13 ± 24.22 118 (27) | 120.6 ± 31.48 111 (33) | 138.44 ± 26.79 135 (33) | BPV vs. P, p = 0.01; RPV vs. P, p = 0.001 |
| max MAP (mmHg) | 98.89 ± 17.29 94.2 (25) | 91.36 ± 16.81 90 (20) | 90.78 ± 19.98 86 (16) | 103.16 ± 17.92 102 (25) | BPV vs. P, p = 0.01; RPV vs. P, p = 0.001 |
| max DAP (mmHg) | 75.52 ± 12.81 74 (20) | 71.82 ± 12.38 69 (14) | 70.56 ± 15.11 68 (19) | 76.98 ± 13.1 75 (23) | 0.06 NS |
| max HR (beats/min) | 75.21 ± 14.37 73 (15) | 66.22 ± 8.26 65 (10) | 67.09 ± 10.84 65 (13) | 70.29 ± 10.84 68 (18) | BL vs. BPV, p = 0.005; BL vs. RPV, p = 0.02 |
| max SPI | 53.38 ± 11.5 53 (14) | 47.31 ± 13.05 47 (18) | 42.77 ± 11.87 38 (15) | 52 ± 12.84 53 (16) | BL vs. RPV, p < 0.001; RPV vs. P, p = 0.004 |
| max SE | 54.9 ± 6.91 54 (11) | 52.69 ± 10.76 50 (14) | 55.69 ± 8.49 56 (11) | 54.07 ± 7.91 56 (14) | 0.17 NS |
| min SAP (mmHg) | 87.74 ± 15.85 86.5 (17) | 88.6 ± 12.94 85 (15) | 89.16 ± 17.03 87 (21) | 90.82 ± 17.05 87 (26) | 0.91 NS |
| min MAP (mmHg) | 66.38 ± 10.76 64.5 (13) | 67.91 ± 9.55 66 (10) | 68.19 ± 12.37 66 (14) | 68.64 ± 12.15 65 (17) | 0.8 NS |
| min DAP (mmHg) | 50.67 ± 8.18 49.5 (13) | 52.78 ± 7.52 52 (9) | 52.33 ± 9.99 51 (11) | 52 ± 8.66 48 (14) | 0.58 NS |
| min HR (beats/min) | 55.07 ± 8.19 57 (13) | 54 ± 7.04 53 (9) | 54.47 ± 7.83 53 (11) | 54.91 ± 8.16 54 (11) | 0.92 NS |
| min SPI | 19.36 ± 6.48 19 (8) | 19.8 ± 6.69 18 (8) | 18.51 ± 6.37 17 (8) | 23.07 ± 8.92 22 (9) | 0.06 NS |
| min SE | 35.71 ± 8.11 36 (9) | 34.04 ± 7.16 34 (7) | 34.64 ± 6.84 35 (8) | 31.82 ± 6.97 32 (7) | BL vs. P, p = 0.008 |
| Stage 4—PACU | |||||
| max SAP (mmHg) | 157.03 ± 22.44 151.5 (35.5) | 160.62 ± 18.42 161 (29) | 158.21 ± 21.86 157 (34) | 157.84 ± 19.88 162 (31.5) | 0.77 NS |
| max MAP (mmHg) | 110.4 ± 17.06 109 (22) | 116.16 ± 15.47 119 (23) | 109.5 ± 15.48 108.5 (20) | 108.59 ± 16.77 108 (22.5) | 0.12 NS |
| max DAP (mmHg) | 86.5 ± 11.1 84 (12.5) | 88.09 ± 12.4 8 9 (14) | 86.71 ± 14.28 85 (16) | 85.11 ± 15.06 83 (15.5) | 0.41 NS |
| max HR (beats/min) | 77.9 ± 11.22 79 (13) | 76.98 ± 11.1 77 (14) | 76.81 ± 11.98 77 (17) | 72.89 ± 10.83 72.5 (18.5) | 0.18 NS |
| max SPI | 64.8 ± 14.14 63 (22) | 72.82 ± 13.74 76 (18.5) | 69.93 ± 11.58 71.5 (14) | 60.91 ± 15.58 58 (27) | BL vs. BPV, p = 0.03; BPV vs. P, p = 0.001 |
| min SAP (mmHg) | 138.03 ± 18.55 135 (17.5) | 142.33 ± 14.43 143 (18) | 137.17 ± 17.96 138 (31) | 144.14 ± 18.9 144 (32) | 0.17 NS |
| min MAP (mmHg) | 96.19 ± 14.97 97 (19) | 101.09 ± 14 105 (18) | 96.39 ± 13.33 96.5 (17) | 98.73 ± 17.1 100.2 (21) | 0.2 NS |
| min DAP (mmHg) | 75.9 ± 13.16 73.5 (15) | 77.44 ± 11.58 78 (11) | 76.31 ± 14.42 74.3 (20) | 76.34 ± 15.31 74 (17.5) | 0.6 NS |
| min HR (beats/min) | 67.43 ± 10.95 65 (15) | 64.13 ± 9.07 64 (15) | 62.93 ± 10.27 60.5 (17) | 65.23 ± 8.82 64 (13) | 0.22 NS |
| min SPI | 47.3 ± 13.59 49 (21) | 50.45 ± 16.89 52 (22.5) | 46.55 ± 13.09 47 (18) | 45.07 ± 14.89 44.5 (23) | 0.26 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiowski, M.J.; Lyssek-Boroń, A.; Krysik, K.; Majer, D.; Zmarzły, N.; Grabarek, B.O. Evaluating the Efficacy of Pre-Emptive Peribulbar Blocks with Different Local Anesthetics or Paracetamol Using the Adequacy of Anesthesia Guidance for Vitreoretinal Surgeries: A Preliminary Report. Biomedicines 2024, 12, 2303. https://doi.org/10.3390/biomedicines12102303
Stasiowski MJ, Lyssek-Boroń A, Krysik K, Majer D, Zmarzły N, Grabarek BO. Evaluating the Efficacy of Pre-Emptive Peribulbar Blocks with Different Local Anesthetics or Paracetamol Using the Adequacy of Anesthesia Guidance for Vitreoretinal Surgeries: A Preliminary Report. Biomedicines. 2024; 12(10):2303. https://doi.org/10.3390/biomedicines12102303
Chicago/Turabian StyleStasiowski, Michał Jan, Anita Lyssek-Boroń, Katarzyna Krysik, Dominika Majer, Nikola Zmarzły, and Beniamin Oskar Grabarek. 2024. "Evaluating the Efficacy of Pre-Emptive Peribulbar Blocks with Different Local Anesthetics or Paracetamol Using the Adequacy of Anesthesia Guidance for Vitreoretinal Surgeries: A Preliminary Report" Biomedicines 12, no. 10: 2303. https://doi.org/10.3390/biomedicines12102303
APA StyleStasiowski, M. J., Lyssek-Boroń, A., Krysik, K., Majer, D., Zmarzły, N., & Grabarek, B. O. (2024). Evaluating the Efficacy of Pre-Emptive Peribulbar Blocks with Different Local Anesthetics or Paracetamol Using the Adequacy of Anesthesia Guidance for Vitreoretinal Surgeries: A Preliminary Report. Biomedicines, 12(10), 2303. https://doi.org/10.3390/biomedicines12102303





