Associations between Disc Hemorrhage and Primary Open-Angle Glaucoma Based on Genome-Wide Association and Mendelian Randomization Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Genotyping
2.3. Genome-Wide Association Study
2.4. Selection of the Genetic Instrumental Variables for Mendelian Randomization
2.5. Statistics for Mendelian Randomization
3. Results
3.1. Characteristics of the Study Participants
3.2. Genome-Wide Association Study
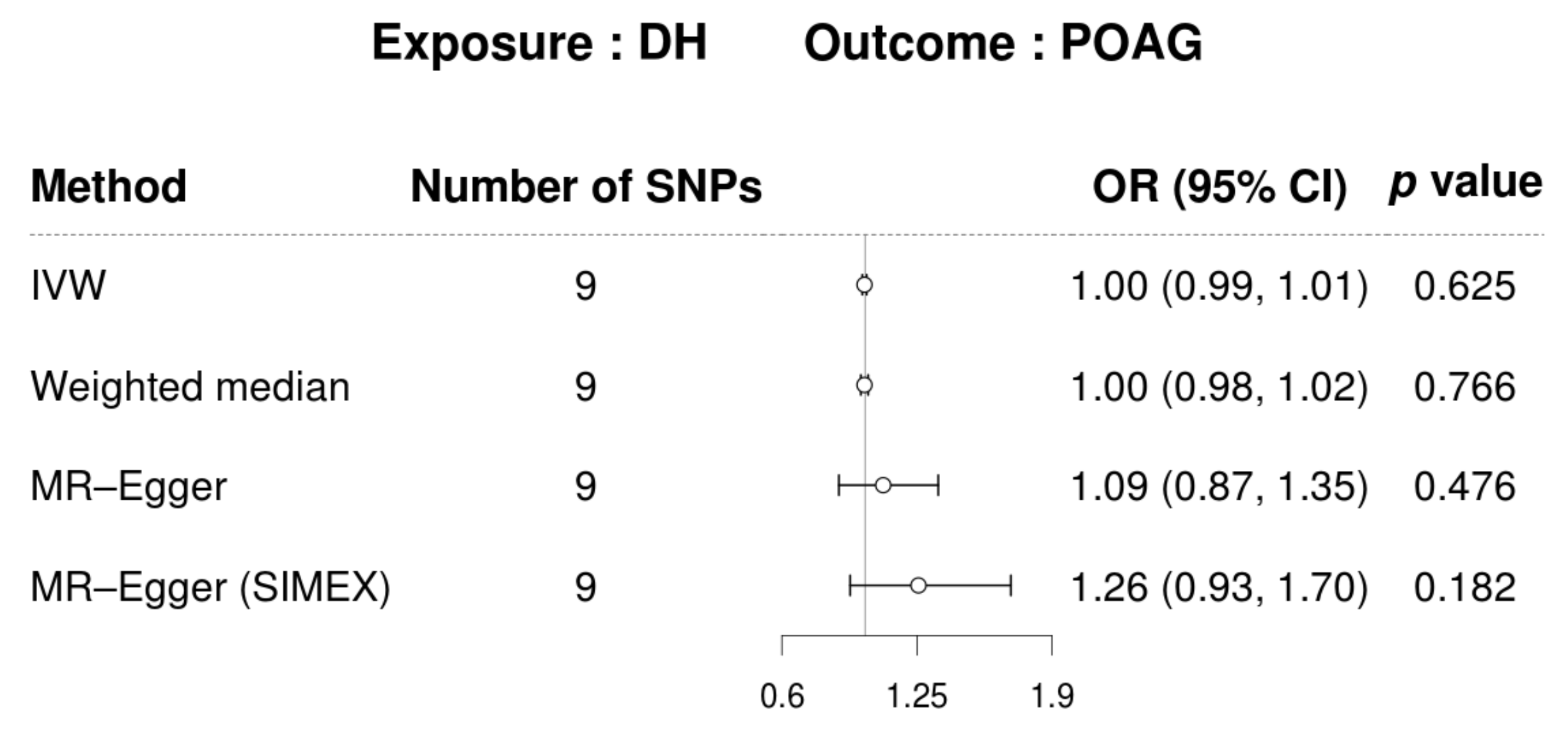
3.3. Mendelian Randomization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Addicks, E.M.; Green, W.R.; Maumenee, A.E. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Hohman, R.M.; Addicks, E.M.; Massof, R.W.; Green, W.R. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am. J. Ophthalmol. 1983, 95, 673–691. [Google Scholar] [CrossRef]
- Jonas, J.B.; Berenshtein, E.; Holbach, L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5189–5195. [Google Scholar] [CrossRef]
- Jonas, J.B.; Berenshtein, E.; Holbach, L. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2660–2665. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Kim, T.W.; Weinreb, R.N. Lamina cribrosa depth in healthy eyes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Strouthidis, N.G.; Fortune, B.; Yang, H.; Sigal, I.A.; Burgoyne, C.F. Effect of acute intraocular pressure elevation on the monkey optic nerve head as detected by spectral domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9431–9437. [Google Scholar] [CrossRef] [PubMed]
- Fatehee, N.; Yu, P.K.; Morgan, W.H.; Cringle, S.J.; Yu, D.Y. The impact of acutely elevated intraocular pressure on the porcine optic nerve head. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6192–6198. [Google Scholar] [CrossRef]
- Musch, D.C.; Gillespie, B.W.; Lichter, P.R.; Niziol, L.M.; Janz, N.K.; Investigators, C.S. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology 2009, 116, 200–207. [Google Scholar] [CrossRef]
- Leske, M.C. Ocular perfusion pressure and glaucoma: Clinical trial and epidemiologic findings. Curr. Opin. Ophthalmol. 2009, 20, 73–78. [Google Scholar] [CrossRef]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000, 130, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1998, 126, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.S.; Suelves, A.M.; Baheti, U.; Foster, C.S. Glaucoma and uveitis. Surv. Ophthalmol. 2013, 58, 1–10. [Google Scholar] [CrossRef]
- Drance, S.M. Disc hemorrhages in the glaucomas. Surv. Ophthalmol. 1989, 33, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C.; Heijl, A.; Hussein, M.; Bengtsson, B.; Hyman, L.; Komaroff, E.; Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch. Ophthalmol. 2003, 121, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Budenz, D.L.; Anderson, D.R.; Feuer, W.J.; Beiser, J.A.; Schiffman, J.; Parrish, R.K., II; Piltz-Seymour, J.R.; Gordon, M.O.; Kass, M.A.; Ocular Hypertension Treatment Study Group. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology 2006, 113, 2137–2143. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Alencar, L.M.; Sample, P.A.; Zangwill, L.M.; Susanna, R., Jr.; Weinreb, R.N. The relationship between intraocular pressure reduction and rates of progressive visual field loss in eyes with optic disc hemorrhage. Ophthalmology 2010, 117, 2061–2066. [Google Scholar] [CrossRef]
- Drance, S.M.; Begg, I.S. Sector haemorrhage—A probable acute ischaemic disc change in chronic simple glaucoma. Can. J. Ophthalmol. 1970, 5, 137–141. [Google Scholar]
- Sonnsjo, B.; Dokmo, Y.; Krakau, T. Disc haemorrhages, precursors of open angle glaucoma. Prog. Retin. Eye Res. 2002, 21, 35–56. [Google Scholar] [CrossRef]
- Ozturker, Z.K.; Munro, K.; Gupta, N. Optic disc hemorrhages in glaucoma and common clinical features. Can. J. Ophthalmol. 2017, 52, 583–591. [Google Scholar] [CrossRef]
- Suh, M.H.; Park, K.H. Pathogenesis and clinical implications of optic disk hemorrhage in glaucoma. Surv. Ophthalmol. 2014, 59, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Shihab, Z.M.; Lee, P.F.; Hay, P. The significance of disc hemorrhage in open-angle glaucoma. Ophthalmology 1982, 89, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Drance, S.; Anderson, D.R.; Schulzer, M.; Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am. J. Ophthalmol. 2001, 131, 699–708. [Google Scholar] [CrossRef] [PubMed]
- An, D.; House, P.; Barry, C.; Turpin, A.; McKendrick, A.M.; Chauhan, B.C.; Manners, S.; Graham, S.; Yu, D.Y.; Morgan, W.H. Recurrent Optic Disc Hemorrhage and Its Association with Visual Field Deterioration in Glaucoma. Ophthalmol. Glaucoma 2020, 3, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Diehl, D.L.; Quigley, H.A.; Miller, N.R.; Sommer, A.; Burney, E.N. Prevalence and significance of optic disc hemorrhage in a longitudinal study of glaucoma. Arch. Ophthalmol. 1990, 108, 545–550. [Google Scholar] [CrossRef]
- Leske, M.C.; Heijl, A.; Hyman, L.; Bengtsson, B.; Dong, L.; Yang, Z.; EMGT Group. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007, 114, 1965–1972. [Google Scholar] [CrossRef]
- Bengtsson, B.; Leske, M.C.; Yang, Z.; Heijl, A.; EMGT Group. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology 2008, 115, 2044–2048. [Google Scholar] [CrossRef]
- de Beaufort, H.C.; De Moraes, C.G.; Teng, C.C.; Prata, T.S.; Tello, C.; Ritch, R.; Liebmann, J.M. Recurrent disc hemorrhage does not increase the rate of visual field progression. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 839–844. [Google Scholar] [CrossRef]
- Furlanetto, R.L.; De Moraes, C.G.; Teng, C.C.; Liebmann, J.M.; Greenfield, D.S.; Gardiner, S.K.; Ritch, R.; Krupin, T.; Low-Pressure Glaucoma Treatment Study Group. Risk factors for optic disc hemorrhage in the low-pressure glaucoma treatment study. Am. J. Ophthalmol. 2014, 157, 945–952. [Google Scholar] [CrossRef]
- Shukla, A.G.; Sirinek, P.E.; De Moraes, C.G.; Blumberg, D.M.; Cioffi, G.A.; Skaat, A.; Girkin, C.A.; Weinreb, R.N.; Zangwill, L.M.; Hood, D.C.; et al. Disc Hemorrhages Are Associated with the Presence and Progression of Glaucomatous Central Visual Field Defects. J. Glaucoma 2020, 29, 429–434. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, K.H. Lamina cribrosa defects in eyes with glaucomatous disc haemorrhage. Acta Ophthalmol. 2016, 94, e468–e473. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Lee, J.; Jung, Y.; Park, C.K. Optic Disc Hemorrhage and Lamina Cribrosa Defects in Glaucoma Progression. Sci. Rep. 2017, 7, 3489. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Kim, T.W.; Weinreb, R.N.; Kim, Y.A.; Kim, M. Relationship of intraocular pressure and frequency of spontaneous retinal venous pulsation in primary open-angle glaucoma. Ophthalmology 2012, 119, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, T.W.; Weinreb, R.N.; Lee, E.J.; Seo, J.H. Spontaneous retinal venous pulsation and disc hemorrhage in open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2822–2826. [Google Scholar] [CrossRef][Green Version]
- Lee, E.J.; Kee, H.J.; Han, J.C.; Kee, C. Evidence-based understanding of disc hemorrhage in glaucoma. Surv. Ophthalmol. 2021, 66, 412–422. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 2015, 181, 251–260. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, Y. Causal Association between Iritis or Uveitis and Glaucoma: A Two-Sample Mendelian Randomisation Study. Genes 2023, 14, 642. [Google Scholar] [CrossRef]
- Hanyuda, A.; Goto, A.; Nakatochi, M.; Sutoh, Y.; Narita, A.; Nakano, S.; Katagiri, R.; Wakai, K.; Takashima, N.; Koyama, T.; et al. Association Between Glycemic Traits and Primary Open-Angle Glaucoma: A Mendelian Randomization Study in the Japanese Population. Am. J. Ophthalmol. 2022, 245, 193–201. [Google Scholar] [CrossRef]
- Kim, J.; Aschard, H.; Kang, J.H.; Lentjes, M.A.H.; Do, R.; Wiggs, J.L.; Khawaja, A.P.; Pasquale, L.R.; Modifiable Risk Factors for Glaucoma Collaboration. Intraocular Pressure, Glaucoma, and Dietary Caffeine Consumption: A Gene-Diet Interaction Study from the UK Biobank. Ophthalmology 2021, 128, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, F.; Liu, X.; Lin, X.; Yin, H.; Tang, Q.; Jiang, L.; Yao, K. Appraising the Effects of Metabolic Traits on the Risk of Glaucoma: A Mendelian Randomization Study. Metabolites 2023, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhong, Z.; Wang, Q.; Su, G.; Cao, Q.; Kijlstra, A.; Yang, P. Genetically predicted fasting blood glucose level plays a causal role in intraocular pressure: A Mendelian randomisation study. Clin. Exp. Ophthalmol. 2022, 50, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, D.; Huang, Y.; Khawaja, A.P.; Foster, P.J.; Zhu, Z.; Guggenheim, J.A.; He, M. High Blood Pressure and Intraocular Pressure: A Mendelian Randomization Study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 29. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, Y.A.; Seo, J.H. Causal Association of Obesity and Dyslipidemia with Type 2 Diabetes: A Two-Sample Mendelian Randomization Study. Genes 2022, 13, 2407. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, Y. Possible Causal Association between Type 2 Diabetes and Glycaemic Traits in Primary Open-Angle Glaucoma: A Two-Sample Mendelian Randomisation Study. Biomedicines 2024, 12, 866. [Google Scholar] [CrossRef]
- Meer, E.; Qin, V.L.; Gudiseva, H.V.; McGeehan, B.; Salowe, R.; Pistilli, M.; He, J.; Daniel, E.; Ying, G.S.; Chavali, V.R.M.; et al. LMX1B Locus Associated with Low-Risk Baseline Glaucomatous Features in the POAAGG Study. Genes 2021, 12, 1252. [Google Scholar] [CrossRef]
- Sakaue, S.; Kanai, M.; Tanigawa, Y.; Karjalainen, J.; Kurki, M.; Koshiba, S.; Narita, A.; Konuma, T.; Yamamoto, K.; Akiyama, M.; et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021, 53, 1415–1424. [Google Scholar] [CrossRef]
- Lee, C.; Choe, E.K.; Choi, J.M.; Hwang, Y.; Lee, Y.; Park, B.; Chung, S.J.; Kwak, M.S.; Lee, J.E.; Kim, J.S.; et al. Health and Prevention Enhancement (H-PEACE): A retrospective, population-based cohort study conducted at the Seoul National University Hospital Gangnam Center, Korea. BMJ Open 2018, 8, e019327. [Google Scholar] [CrossRef]
- Kim, Y.A.; Yoon, J.W.; Lee, Y.; Choi, H.J.; Yun, J.W.; Bae, E.; Kwon, S.H.; Ahn, S.E.; Do, A.R.; Jin, H.; et al. Unveiling Genetic Variants Underlying Vitamin D Deficiency in Multiple Korean Cohorts by a Genome-Wide Association Study. Endocrinol. Metab. 2021, 36, 1189–1200. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, Y.H.; Kim, J.S.; Lee, J.; Kim, Y.J.; Cheong, H.S.; Kim, S.H.; Park, K.H.; Kim, D.M.; Choi, H.J.; et al. Genetic analysis of primary open-angle glaucoma-related risk alleles in a Korean population: The GLAU-GENDISK study. Br. J. Ophthalmol. 2021, 105, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Delaneau, O.; Marchini, J.; Zagury, J.F. A linear complexity phasing method for thousands of genomes. Nat. Methods 2011, 9, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Marchini, J.; Howie, B.; Myers, S.; McVean, G.; Donnelly, P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 2007, 39, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Pruim, R.J.; Welch, R.P.; Sanna, S.; Teslovich, T.M.; Chines, P.S.; Gliedt, T.P.; Boehnke, M.; Abecasis, G.R.; Willer, C.J. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics 2010, 26, 2336–2337. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G.; Crp Chd Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Holmes, M.V.; Minelli, C.; Relton, C.L.; et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.A.; Thompson, J.R. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 2016, 45, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Publisher Correction: Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 1196. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.F.; Minelli, C.; Sheehan, N.A.; Thompson, J.R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 2015, 34, 2926–2940. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lee, S.; Won, S. Causal Evaluation of Laboratory Markers in Type 2 Diabetes on Cancer and Vascular Diseases Using Various Mendelian Randomization Tools. Front. Genet. 2020, 11, 597420. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.E.; Bonnet, F.; Oldfield, M.; Jandeleit-Dahm, K. Mechanisms of diabetic vasculopathy: An overview. Am. J. Hypertens. 2001, 14, 475–486. [Google Scholar] [CrossRef]
- Lee, E.J.; Han, J.C.; Kee, C. A novel hypothesis for the pathogenesis of glaucomatous disc hemorrhage. Prog. Retin. Eye Res. 2017, 60, 20–43. [Google Scholar] [CrossRef]
- Rasker, M.T.; van den Enden, A.; Bakker, D.; Hoyng, P.F. Deterioration of visual fields in patients with glaucoma with and without optic disc hemorrhages. Arch. Ophthalmol. 1997, 115, 1257–1262. [Google Scholar] [CrossRef]
- Lichou, F.; Trynka, G. Functional studies of GWAS variants are gaining momentum. Nat. Commun. 2020, 11, 6283. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segre, A.V.; Rouhana, J.M.; et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat. Commun. 2021, 12, 1258. [Google Scholar] [CrossRef]
- Wang, Z.; Wiggs, J.L.; Aung, T.; Khawaja, A.P.; Khor, C.C. The genetic basis for adult onset glaucoma: Recent advances and future directions. Prog. Retin. Eye Res. 2022, 90, 101066. [Google Scholar] [CrossRef]
- Procknow, S.S.; Kozel, B.A. Emerging mechanisms of elastin transcriptional regulation. Am. J. Physiol. Cell Physiol. 2022, 323, C666–C677. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Cocciolone, A.J.; Wagenseil, J.E. Elastin, arterial mechanics, and stenosis. Am. J. Physiol. Cell Physiol. 2022, 322, C875–C886. [Google Scholar] [CrossRef] [PubMed]
- Paterakis, K.; Koutsias, S.; Doxani, C.; Xanthopoulou, P.; Kokkali, C.; Mpoulimari, I.; Tziastoudi, M.; Karampelas, I.; Dardiotis, E.; Hadjigeorgiou, G.; et al. Variants of the elastin (ELN) gene and susceptibility to intracranial aneurysm: A synthesis of genetic association studies using a genetic model-free approach. Int. J. Neurosci. 2017, 127, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Narita, A.; Nakaoka, H.; Cui, T.; Takahashi, T.; Yasuno, K.; Tajima, A.; Krischek, B.; Yamamoto, K.; Kasuya, H.; et al. Genome-wide association study to identify genetic variants present in Japanese patients harboring intracranial aneurysms. J. Hum. Genet. 2010, 55, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Pena, J.D.; Agapova, O.; Gabelt, B.T.; Levin, L.A.; Lucarelli, M.J.; Kaufman, P.L.; Hernandez, M.R. Increased elastin expression in astrocytes of the lamina cribrosa in response to elevated intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2303–2314. [Google Scholar]
- Fan, B.J.; Figuieredo Sena, D.R.; Pasquale, L.R.; Grosskreutz, C.L.; Rhee, D.J.; Chen, T.C.; Delbono, E.A.; Haines, J.L.; Wiggs, J.L. Lack of association of polymorphisms in elastin with pseudoexfoliation syndrome and glaucoma. J. Glaucoma 2010, 19, 432–436. [Google Scholar] [CrossRef]
- MacGregor, S.; Ong, J.S.; An, J.; Han, X.; Zhou, T.; Siggs, O.M.; Law, M.H.; Souzeau, E.; Sharma, S.; Lynn, D.J.; et al. Genome-wide association study of intraocular pressure uncovers new pathways to glaucoma. Nat. Genet. 2018, 50, 1067–1071. [Google Scholar] [CrossRef]
- Kim, K.E.; Park, K.H. Optic disc hemorrhage in glaucoma: Pathophysiology and prognostic significance. Curr. Opin. Ophthalmol. 2017, 28, 105–112. [Google Scholar] [CrossRef]





| Disc Hemorrhage N = 31 | Control N = 8457 | p-Value * | |
|---|---|---|---|
| Age (years) | 54.0 (46.0, 58.5) | 52.0 (46.0, 58.0) | 0.304 |
| Sex | 0.414 | ||
| Male n (%) | 21 (67.7%) | 4981 (58.9%) | |
| Female n (%) | 10 (32.3%) | 3476 (41.1%) | |
| IOP (mmHg) | |||
| Right eye | 13 (13, 15) | 12 (10, 14) | 0.023 |
| Left eye | 15 (13, 16) | 13 (11, 15) | 0.003 |
| Mean IOP | 14.5 (12.0, 15.5) | 12.5 (10.5, 14.5) | 0.007 |
| Higher IOP of both eyes | 15 (13, 16) | 13 (11, 15) | 0.004 |
| Weight (kg) | 63.7 (56.4, 72.8) | 64.8 (55.6, 73.0) | 0.698 |
| BMI (kg/m) | 23.58 (21.10, 25.06) | 23.18 (21.14, 25.13) | 0.783 |
| Systolic blood pressure (mmHg) | 120.0 (103.5, 128.5) | 115 (106, 125) | 0.535 |
| Diastolic blood pressure (mmHg) | 81.0 (70.0, 87.5) | 76 (69, 83) | 0.062 |
| Comorbidity | |||
| Diabetes, n (%) | 1 (3.23%) | 296 (3.51%) | 1 |
| Hypertension, n (%) | 2 (6.45%) | 982 (11.6%) | 0.573 |
| Laboratory examination | |||
| HbA1c (%) | 5.50 (5.35, 5.75) | 5.6 (5.4, 5.8) | 0.536 |
| Fasting glucose (mg/dL) | 95 (91, 100) | 96 (90, 103) | 0.742 |
| Total cholesterol (mg/dL) | 183.0 (169.0, 202.5) | 193 (171, 216) | 0.286 |
| LDL cholesterol (mg/dL) | 112 (100, 142) | 121 (101, 142) | 0.517 |
| HDL cholesterol (mg/dL) | 52.0 (46.5, 61.0) | 52 (45, 60) | 0.72 |
| Triglyceride (mg/dL) | 79 (61, 112) | 90 (63, 133) | 0.196 |
| Chr | SNP | Position | Allele | MAF | OR | L95 | U95 | p | Mapped Genes |
|---|---|---|---|---|---|---|---|---|---|
| 7 | rs62463744 | 73287385 | C/T | 0.06888 | 4.87 | 2.65 | 8.95 | 3.49 × 10−7 | TMEM270;ELN |
| 17 | rs11658281 | 8637070 | T/G | 0.1353 | 4.03 | 2.32 | 7.00 | 7.78 × 10−7 | CCDC42 |
| 6 | rs77127203 | 166267889 | A/G | 0.2755 | 3.55 | 2.11 | 5.96 | 1.67 × 10−6 | PDE10A;LINC00473 |
| 2 | rs7589033 | 43649228 | T/C | 0.05157 | 4.83 | 2.53 | 9.20 | 1.77 × 10−6 | THADA |
| 2 | rs58526585 | 43857040 | T/C | 0.05278 | 4.76 | 2.48 | 9.11 | 2.59 × 10−6 | THADA;PLEKHH2 |
| 17 | rs113460962 | 4850748 | C/T | 0.09861 | 3.87 | 2.20 | 6.80 | 2.60 × 10−6 | PFN1 |
| 15 | rs76143071 | 50723310 | C/T | 0.0526 | 4.70 | 2.46 | 8.98 | 2.74 × 10−6 | USP8 |
| 6 | rs9462784 | 42202149 | C/T | 0.1106 | 3.78 | 2.16 | 6.61 | 3.31 × 10−6 | TRERF1 |
| 17 | rs2302320 | 4796656 | C/T | 0.1821 | 3.37 | 2.01 | 5.64 | 4.10 × 10−6 | MINK1 |
| 10 | rs140873075 | 80392732 | G/C | 0.05223 | 4.42 | 2.33 | 8.41 | 5.84 × 10−6 | LINC00595;ZMIZ1-AS1 |
| 15 | rs3803373 | 50792874 | A/G | 0.07226 | 4.09 | 2.22 | 7.55 | 6.40 × 10−6 | USP50;USP8 |
| 20 | rs78583358 | 1840763 | T/G | 0.09542 | 3.70 | 2.08 | 6.58 | 8.50 × 10−6 | LOC100289473;SIRPA |
| 10 | rs78617863 | 5523330 | G/C | 0.08089 | 3.82 | 2.11 | 6.91 | 9.10 × 10−6 | NET1;CALML5 |
| 17 | rs112718881 | 4855824 | T/C | 0.05742 | 4.43 | 2.29 | 8.56 | 9.32 × 10−6 | ENO3 |
| Trait | Heritability | Genetic Correlation with POAG | ||
|---|---|---|---|---|
| h2 ± SE | GIF | GC ± SE | p | |
| DH | 0.067 ± 0.056 | 0.993 | 0.257 ± 0.289 | 0.373 |
| Heterogeneity | Horizontal Pleiotropy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MR-Egger | MR-Egger (SIMEX) | ||||||||
| N | F | I2 (%) | p-Value * | p-Value # | p-Value † | Intercept, β(SE) | p-Value | Intercept, β(SE) | p-Value |
| 9 | 21.73 | 39.56 | 0.743 | 0.717 | 0.728 | −0.118 (0.151) | 0.462 | −0.316 (0.21) | 0.175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.H.; Lee, Y.; Choi, H.J. Associations between Disc Hemorrhage and Primary Open-Angle Glaucoma Based on Genome-Wide Association and Mendelian Randomization Analyses. Biomedicines 2024, 12, 2253. https://doi.org/10.3390/biomedicines12102253
Seo JH, Lee Y, Choi HJ. Associations between Disc Hemorrhage and Primary Open-Angle Glaucoma Based on Genome-Wide Association and Mendelian Randomization Analyses. Biomedicines. 2024; 12(10):2253. https://doi.org/10.3390/biomedicines12102253
Chicago/Turabian StyleSeo, Je Hyun, Young Lee, and Hyuk Jin Choi. 2024. "Associations between Disc Hemorrhage and Primary Open-Angle Glaucoma Based on Genome-Wide Association and Mendelian Randomization Analyses" Biomedicines 12, no. 10: 2253. https://doi.org/10.3390/biomedicines12102253
APA StyleSeo, J. H., Lee, Y., & Choi, H. J. (2024). Associations between Disc Hemorrhage and Primary Open-Angle Glaucoma Based on Genome-Wide Association and Mendelian Randomization Analyses. Biomedicines, 12(10), 2253. https://doi.org/10.3390/biomedicines12102253






