A Multi-Center Observation Study on Medication-Related Osteonecrosis of the Jaw (MRONJ) in Patients with Osteoporosis, and Other Non-Malignant Bone Diseases, in Northwestern Italy over 16 Years
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patients’ Characteristics
3.2. Drug and Duration of Therapy
3.3. Clinical Manifestation
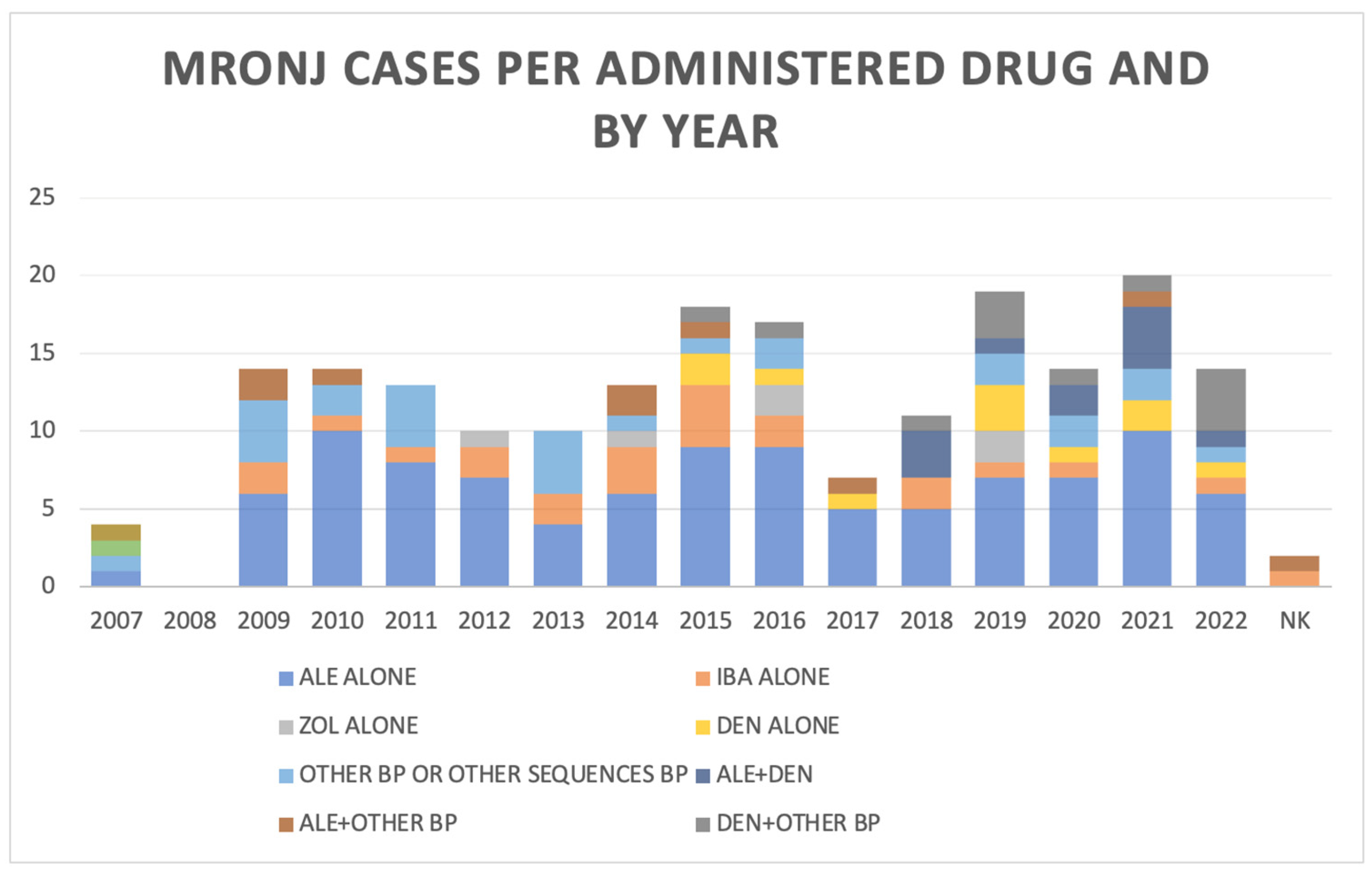
3.4. Number of ONJ Cases by Period of Data Collection
3.5. Survival Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—20 14 update. J. Oral. Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Miksad, R.A.; Lai, K.C.; Dodson, T.B.; Woo, S.B.; Treister, N.S.; Akinyemi, O.; Bihrle, M.; Maytal, G.; August, M.; Gazelle, G.S.; et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist 2011, 16, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral. Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Mauceri, R.; Bedogni, A.; Fusco, V. Re: AAOMS Position Paper on Medication-Related Osteonecrosis of the Jaw-2022 Update. J. Oral. Maxillofac. Surg. 2022, 80, 1723–1724. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Mercer, E.; Woo, S.B.; Avorn, J.; Schneeweiss, S.; Treister, N. Defining the epidemiology of bisphosphonate-associated osteonecrosis of the jaw: Prior work and current challenges. Osteoporos. Int. 2013, 24, 237–244. [Google Scholar] [CrossRef]
- Bedogni, A.; Mauceri, R.; Fusco, V.; Bertoldo, F.; Bettini, G.; Di Fede, O.; Lo Casto, A.; Marchetti, C.; Panzarella, V.; Saia, G.; et al. Italian position paper (SIPMO-SICMF) on medication-related osteonecrosis of the jaw (MRONJ). Oral Dis. 2024, 30, 3679–3709. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; O’Ryan, F.S.; Gordon, N.P.; Yang, J.; Hui, R.L.; Martin, D.; Hutchinson, M.; Lathon, P.V.; Sanchez, G.; Silver, P.; et al. Predicting Risk of Osteonecrosis of the Jaw with Oral Bisphosphonate Exposure (PROBE) Investigators. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J. Oral. Maxillofac. Surg. 2010, 68, 243–253. [Google Scholar] [CrossRef]
- Fellows, J.L.; Rindal, D.B.; Barasch, A.; Gullion, C.M.; Rush, W.; Pihlstrom, D.J.; Richman, J.; The DPBRN Collaborative Group. ONJ in two dental practice-based research network regions. J. Dent. Res. 2011, 90, 433–438. [Google Scholar] [CrossRef]
- Gammelager, H.; Erichsen, R.; Antonsen, S.; Nørholt, S.E.; Neumann-Jensen, B.; Ehrenstein, V.; Acquavella, J.; Sørensen, H.T. Positive predictive value of the International Classification of Diseases, 10th revision, codes to identify osteonecrosis of the jaw in patients with cancer. Cancer Epidemiol. 2012, 36, 381–383. [Google Scholar] [CrossRef]
- Gammelager, H.; Sværke, C.; Noerholt, S.E.; Neumann-Jensen, B.; Xue, F.; Critchlow, C.; Bergdahl, J.; Lagerros, Y.T.; Kieler, H.; Tell, G.S.; et al. Validity of an algorithm to identify osteonecrosis of the jaw in women with postmenopausal osteoporosis in the Danish National Registry of Patients. Clin. Epidemiol. 2013, 5, 263–267. [Google Scholar] [CrossRef]
- Bergdahl, J.; Jarnbring, F.; Ehrenstein, V.; Gammelager, H.; Granath, F.; Kieler, H.; Svensson, M.; Tell, G.S.; Lagerros, Y.T. Evaluation of an algorithm ascertaining cases of osteonecrosis of the jaw in the Swedish National Patient Register. Clin. Epidemiol. 2013, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.S.; Zhou, J.; Kuo, Y.F.; Baillargeon, J. Risk of Jaw Osteonecrosis After Intravenous Bisphosphonates in Cancer Patients and Patients Without Cancer. Mayo Clin. Proc. 2017, 92, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Veszelyné Kotán, E.; Bartha-Lieb, T.; Parisek, Z.; Meskó, A.; Vaszilkó, M.; Hankó, B. Database analysis of the risk factors of bisphosphonate-related osteonecrosis of the jaw in Hungarian patients. BMJ Open 2019, 9, e025600. [Google Scholar] [CrossRef] [PubMed]
- Schiodt, M.; Larsson Wexell, C.; Herlofson, B.B.; Giltvedt, K.M.; Norholt, S.E.; Ehrenstein, V. Existing data sources for clinical epidemiology: Scandinavian Cohort for osteonecrosis of the jaw—work in progress and challenges. Clin. Epidemiol. 2015, 7, 107–116. [Google Scholar] [CrossRef]
- Krüger, T.B.; Sharikabad, M.N.; Herlofson, B.B. Bisphosphonate-related osteonecrosis of the jaw in four Nordic countries and an indication of under-reporting. Acta Odontol. Scand. 2013, 71, 1386–1390. [Google Scholar] [CrossRef]
- Hallmer, F.; Andersson, G.; Götrick, B.; Warfvinge, G.; Anderud, J.; Bjørnland, T. Prevalence, initiating factor, and treatment outcome of medication-related osteonecrosis of the jaw-a 4-year prospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 477–485. [Google Scholar] [CrossRef]
- Mavrokokki, T.; Cheng, A.; Stein, B.; Goss, A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J. Oral Maxillofac. Surg. 2007, 65, 415–423. [Google Scholar] [CrossRef]
- Corraini, P.; Heide-Jørgensen, U.; Schiødt, M.; Nørholt, S.E.; Acquavella, J.; Sørensen, H.T.; Ehrenstein, V. Osteonecrosis of the jaw and survival of patients with cancer: A nationwide cohort study in Denmark. Cancer Med. 2017, 6, 2271–2277. [Google Scholar] [CrossRef]
- Fusco, V.; Galassi, C.; Berruti, A.; Ortega, C.; Ciuffreda, L.; Scoletta, M.; Goia, F.; Migliario, M.; Baraldi, A.; Boccadoro, M.; et al. Decreasing frequency of osteonecrosis of the jaw in cancer and myeloma patients treated with bisphosphonates: The experience of the oncology network of piedmont and aosta valley (north-Western Italy). ISRN Oncol. 2013, 2013, 672027. [Google Scholar] [CrossRef]
- Fusco, V.; Cabras, M.; Erovigni, F.; Dell’Acqua, A.; Arduino, P.G.; Pentenero, M.; Appendino, P.; Basano, L.; Ferrera, F.D.; Fasciolo, A.; et al. A multicenter observational study on Medication-Related Osteonecrosis of the Jaw (MRONJ) in advanced cancer and myeloma patients of a cancer network in North-Western Italy. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e466–e473. [Google Scholar] [CrossRef]
- Acquavella, J.; Ehrenstein, V.; Schiødt, M.; Heide-Jørgensen, U.; Kjellman, A.; Hansen, S.; Larsson Wexell, C.; Herlofson, B.B.; Noerholt, S.E.; Ma, H.; et al. Design and methods for a Scandinavian pharmacovigilance study of osteonecrosis of the jaw and serious infections among cancer patients treated with antiresorptive agents for the prevention of skeletal-related events. Clin. Epidemiol. 2016, 20, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Tennis, P.; Rothman, K.J.; Bohn, R.L.; Tan, H.; Zavras, A.; Laskarides, C.; Calingaert, B.; Anthony, M.S. Incidence of osteonecrosis of the jaw among users of bisphosphonates with selected cancers or osteoporosis. Pharmacoepidemiol. Drug. Saf. 2012, 21, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, Y.K.; Kim, T.Y.; Ha, Y.C.; Jang, S.; Kim, H.Y. Incidence of and risk for osteonecrosis of the jaw in Korean osteoporosis patients treated with bisphosphonates: A nationwide cohort-study. Bone 2021, 143, 115650. [Google Scholar] [CrossRef] [PubMed]
- Nashi, M.; Kishimoto, H.; Kobayashi, M.; Tachibana, A.; Suematsu, M.; Fujiwara, S.; Ota, Y.; Hashitani, S.; Shibatsuji, T.; Nishida, T.; et al. Incidence of antiresorptive agent-related osteonecrosis of the jaw: A multicenter retrospective epidemiological study in Hyogo Prefecture, Japan. J. Dent. Sci. 2023, 18, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Inada, A.; Hosohata, K.; Oyama, S.; Niinomi, I.; Mori, Y.; Yamaguchi, Y.; Uchida, M.; Iwanaga, K. Evaluation of medication-related osteonecrosis of the jaw using the Japanese Adverse Drug Event Report database. Ther. Clin. Risk. Manag. 2018, 15, 59–64. [Google Scholar] [CrossRef]
- Galis, B.; Zajko, J.; Hirjak, D.; Vanko, L.; Kupcova, I.; Jurkemik, J.; Gengelova, P.; Mikuskova, K.; Halmova, K.; Riznic, M.; et al. Is the prevalence of the medication-related osteonecrosis of the jaws underestimated evaluation in oncological and non-oncological disease. Bratisl. Lek. Listy. 2017, 118, 724–731. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Qi, S.; Liao, X.; Liu, D.; Pan, J. Clinical analysis of medication related osteonecrosis of the jaws: A growing severe complication in China. J. Dent. Sci. 2018, 13, 190–197. [Google Scholar] [CrossRef]
| N | % | |
|---|---|---|
| Total | 198 | |
| Age | Mean: 75 years (SD 9.91 years) | |
| Gender | ||
| Female | 192 | 97 |
| Male | 6 | 3 |
| Bone disease | ||
| Osteoporosis | 173 | 87 |
| Rheumatoid arthritis | 9 | 5 |
| Osteoporosis and rheumatoid arthritis | 8 | 4 |
| Other osteo-metabolic disease * | 8 | 4 |
| Antiresorptive treatment | ||
| Alendronate only | 100 | 50.5 |
| Ibandronate only | 23 | 11.6 |
| Zoledronic acid only Denosumab only | 6 11 | 3 5.6 |
| Sequence ALE/DEN or vice versa | 11 | 5.6 |
| Sequence ALE/oBP or vice versa Sequence DEN/oBP or vice versa | 9 12 | 4.5 6.1 |
| Other BPs or other sequences | 26 | 13.1 |
| DRUG/YEAR | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | NK | TOT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALENDRONATE | 1 | 0 | 6 | 10 | 8 | 7 | 4 | 6 | 9 | 9 | 5 | 5 | 7 | 7 | 10 | 6 | 0 | 100 |
| ZOLEDRONATE | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 6 |
| DENOSUMAB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 3 | 1 | 2 | 1 | 0 | 11 |
| CLODRONATE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 4 |
| IBANDRONATE | 0 | 0 | 2 | 1 | 1 | 2 | 2 | 3 | 4 | 2 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 22 |
| NERIDRONATE | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| PAMIDRONATE | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| RISEDRONATE | 1 | 0 | 0 | 2 | 1 | 0 | 3 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 10 |
| TOT | 2 | 0 | 9 | 13 | 11 | 10 | 9 | 11 | 16 | 15 | 6 | 7 | 15 | 10 | 14 | 9 | 1 | 158 |
| ALE + DEN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 2 | 4 | 1 | 0 | 11 | ||
| ALE + oBP | 2 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 9 | ||
| ZOL + DEN | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 4 | ||
| ZOL + oBP | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | ||
| DEN + oBP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 2 | 0 | 8 | ||
| oBP sequence | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | ||
| TOT | 5 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 1 | 4 | 4 | 4 | 6 | 5 | 1 | 40 |
| DRUG(S) | No. | Median Duration and Range (Year) | 25% Interquartile | 75% Interquartile |
|---|---|---|---|---|
| ALENDRONATE | 100 | 8.2 (1–30) | 3.7 | 11.1 |
| ZOLEDRONIC ACID | 6 | 2.5 (0–7) | 1.0 | 3.9 |
| DENOSUMAB | 11 | 2.6 (1–4) | 2.1 | 3.0 |
| IBANDRONATE | 22 | 4.8 (2–8) | 3.0 | 7.5 |
| OTHER BP ALONE | 19 | 5 (0–15) | 1.4 | 5.7 |
| ALE + DEN OR VICE VERSA | 11 | 8.1 (3–10) | 7.9 | 9.9 |
| ALE + IBAN OR VICE VERSA | 5 | 5 (3.1–6.2) | 3.8 | 5.4 |
| ALE + oBP or VICE VERSA | 4 | 7 (3–10) | 6.2 | 9.7 |
| DEN + oBP OR VICE VERSA | 12 | 7 (3–19) | 3.5 | 9 |
| OTHER BP SEQUENCES | 8 | 6 (0.5–14) | 2.8 | 8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi, D.; Arduino, P.G.; Gambino, A.; Erovigni, F.; Dell’Acqua, A.; Pera, F.; Carossa, M.; Pentenero, M.; Appendino, P.; Della Ferrera, F.; et al. A Multi-Center Observation Study on Medication-Related Osteonecrosis of the Jaw (MRONJ) in Patients with Osteoporosis, and Other Non-Malignant Bone Diseases, in Northwestern Italy over 16 Years. Biomedicines 2024, 12, 2179. https://doi.org/10.3390/biomedicines12102179
Karimi D, Arduino PG, Gambino A, Erovigni F, Dell’Acqua A, Pera F, Carossa M, Pentenero M, Appendino P, Della Ferrera F, et al. A Multi-Center Observation Study on Medication-Related Osteonecrosis of the Jaw (MRONJ) in Patients with Osteoporosis, and Other Non-Malignant Bone Diseases, in Northwestern Italy over 16 Years. Biomedicines. 2024; 12(10):2179. https://doi.org/10.3390/biomedicines12102179
Chicago/Turabian StyleKarimi, Dora, Paolo Giacomo Arduino, Alessio Gambino, Francesco Erovigni, Alessandro Dell’Acqua, Francesco Pera, Massimo Carossa, Monica Pentenero, Paolo Appendino, Francesco Della Ferrera, and et al. 2024. "A Multi-Center Observation Study on Medication-Related Osteonecrosis of the Jaw (MRONJ) in Patients with Osteoporosis, and Other Non-Malignant Bone Diseases, in Northwestern Italy over 16 Years" Biomedicines 12, no. 10: 2179. https://doi.org/10.3390/biomedicines12102179
APA StyleKarimi, D., Arduino, P. G., Gambino, A., Erovigni, F., Dell’Acqua, A., Pera, F., Carossa, M., Pentenero, M., Appendino, P., Della Ferrera, F., Fasciolo, A., Caka, M., Migliario, M., Brucoli, M., Franchi, S., Pezzimenti, A., & Fusco, V. (2024). A Multi-Center Observation Study on Medication-Related Osteonecrosis of the Jaw (MRONJ) in Patients with Osteoporosis, and Other Non-Malignant Bone Diseases, in Northwestern Italy over 16 Years. Biomedicines, 12(10), 2179. https://doi.org/10.3390/biomedicines12102179










