The Role of Gut Microbiota and Circadian Rhythm Oscillation of Hepatic Ischemia–Reperfusion Injury in Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Mouse Diabetes Model
2.3. Model of Warm Liver IRI
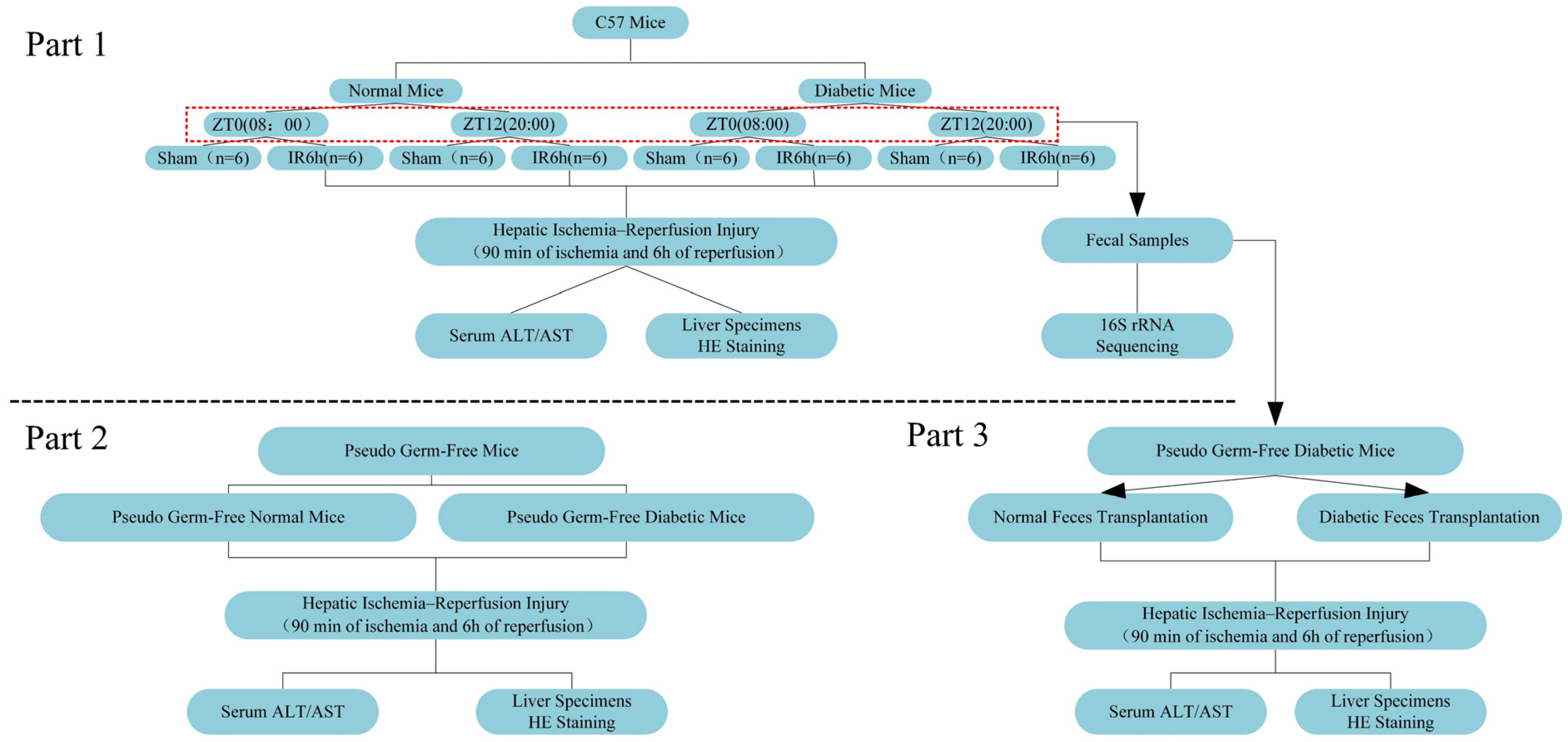
2.4. Pseudo Germ-Free Mice Experiment and Fecal Microbiota Transplantation
2.5. Bacterial Composition Analysis
2.6. Blood Sample and H&E Staining
2.7. Experimental Design
2.8. Statistical Analysis
3. Results
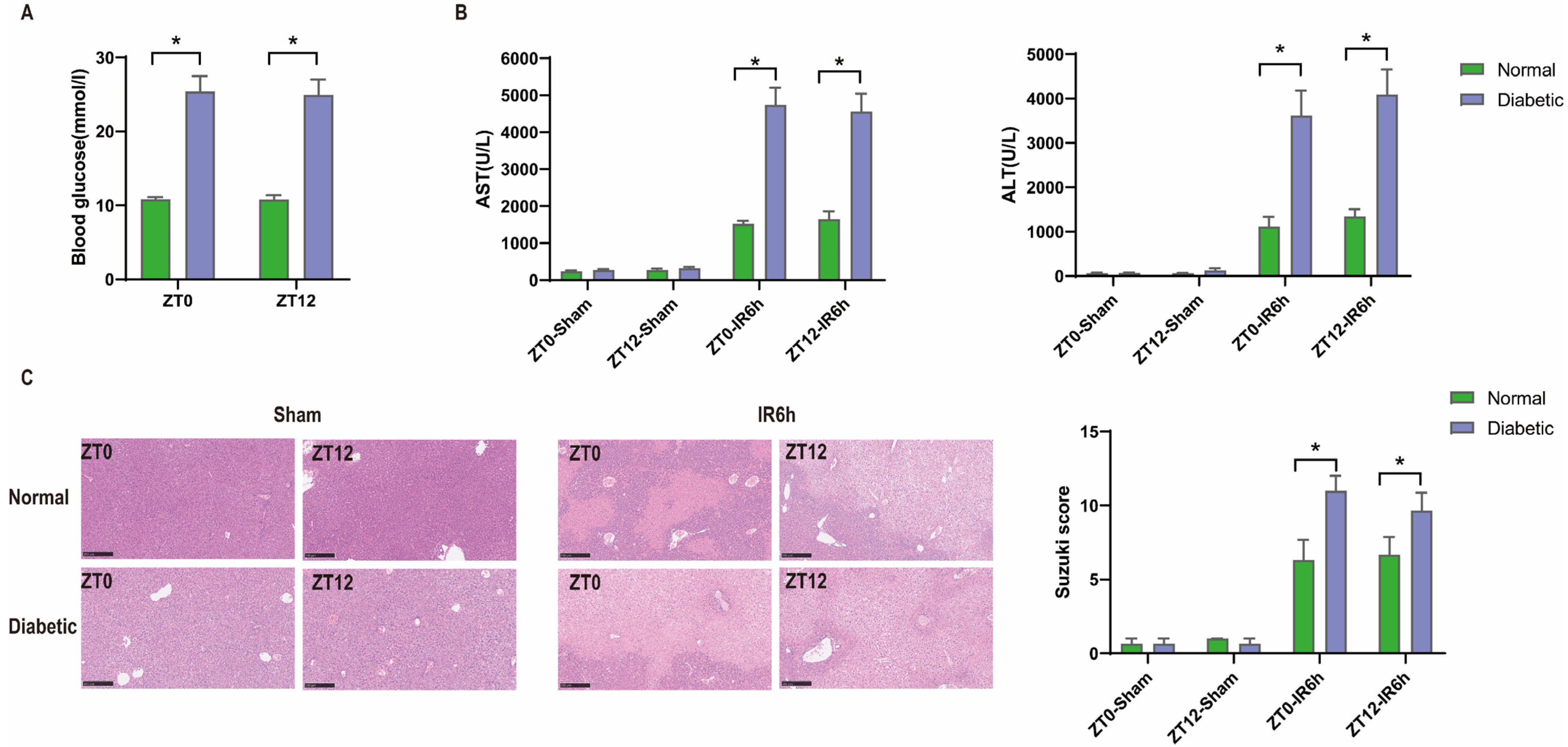
3.1. Diurnal Difference Does Not Affect HIRI in Diabetic Mice, and Hyperglycemia Aggravates HIRI
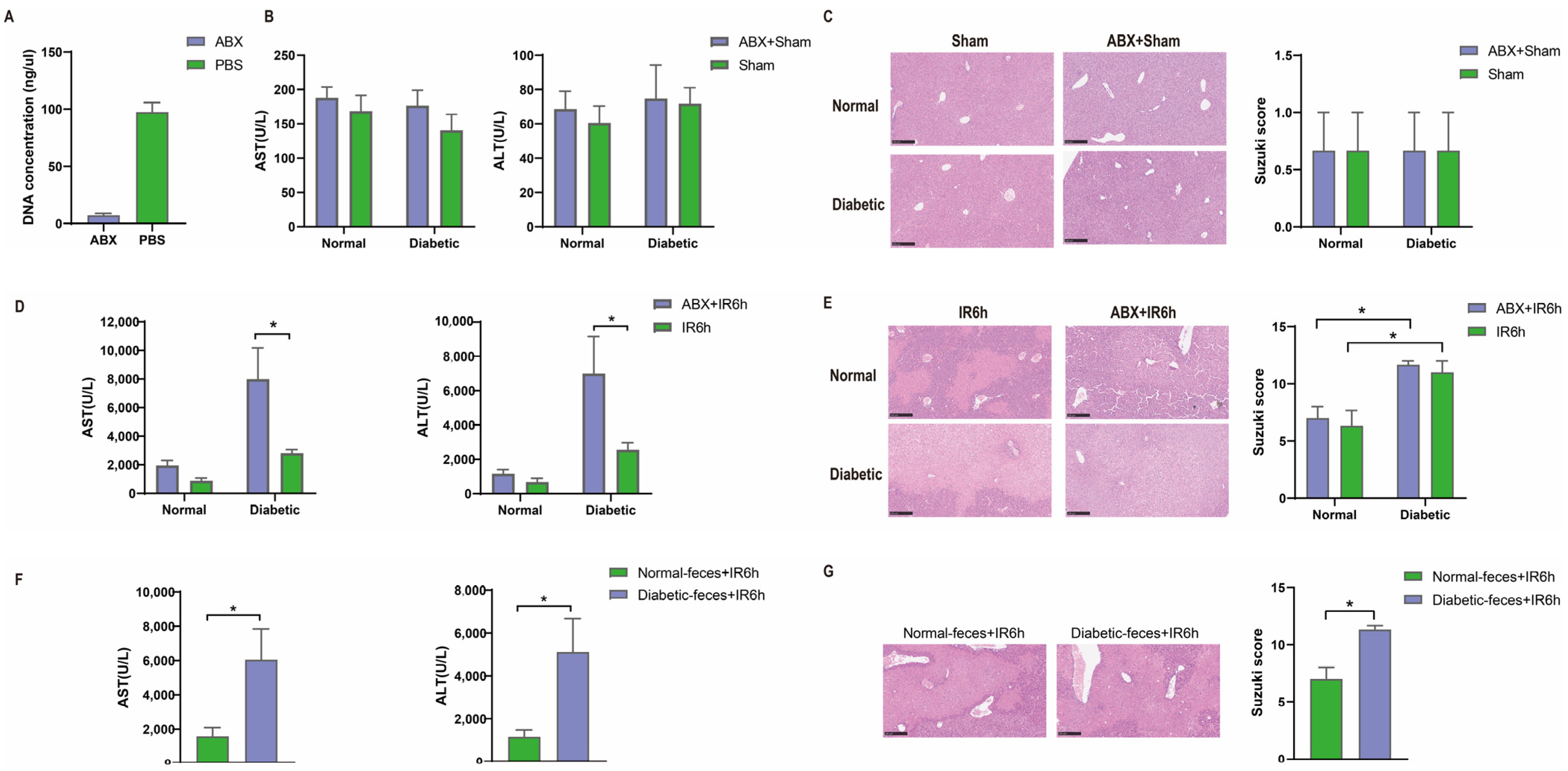
3.2. Hepatic Ischemia–Reperfusion Injury Aggravated in Pseudo Germ-Free Mice
3.3. Diabetic Fecal Microbiota Transplantation Aggravates Hepatic Ischemia–Reperfusion Injury
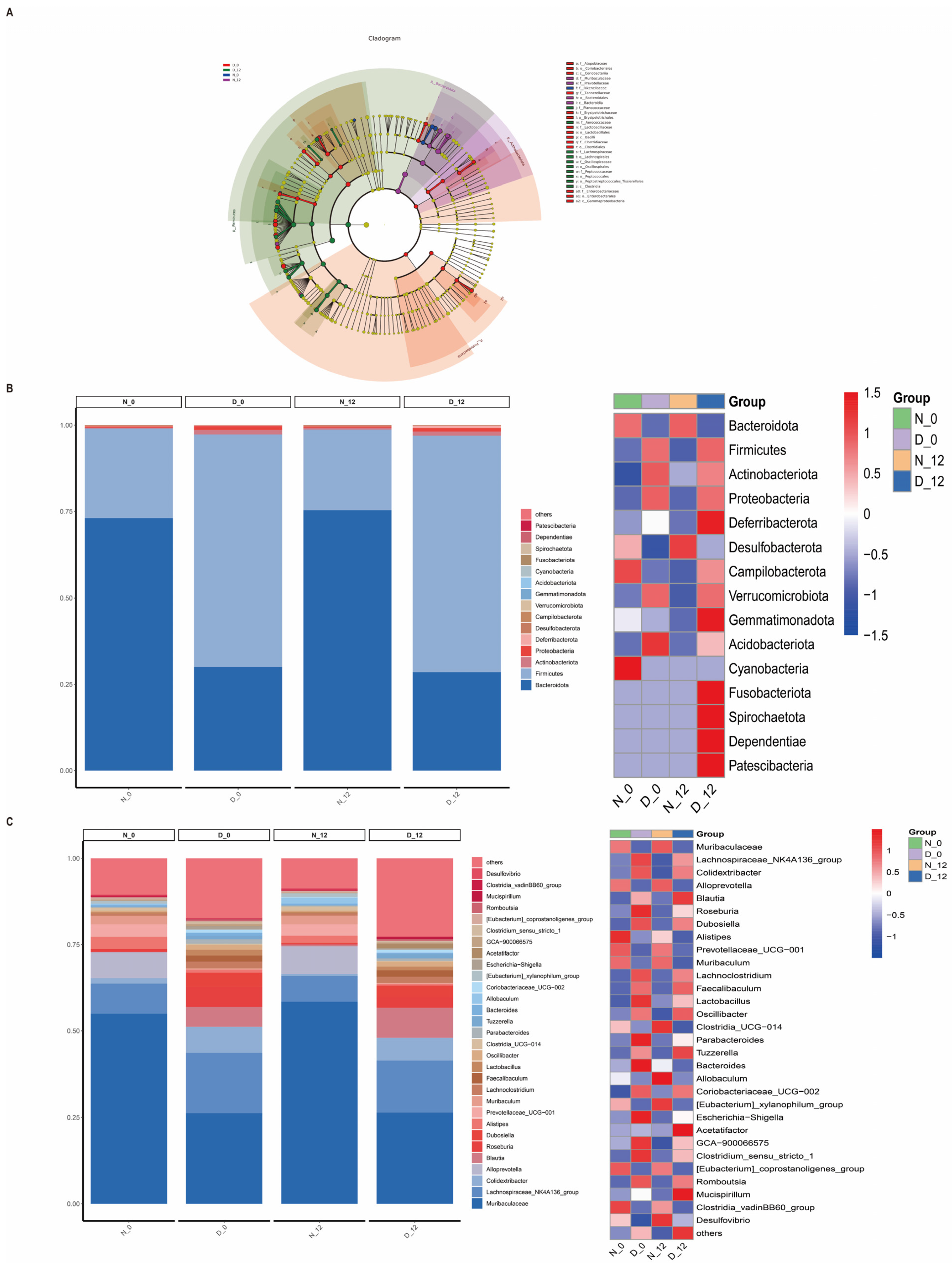
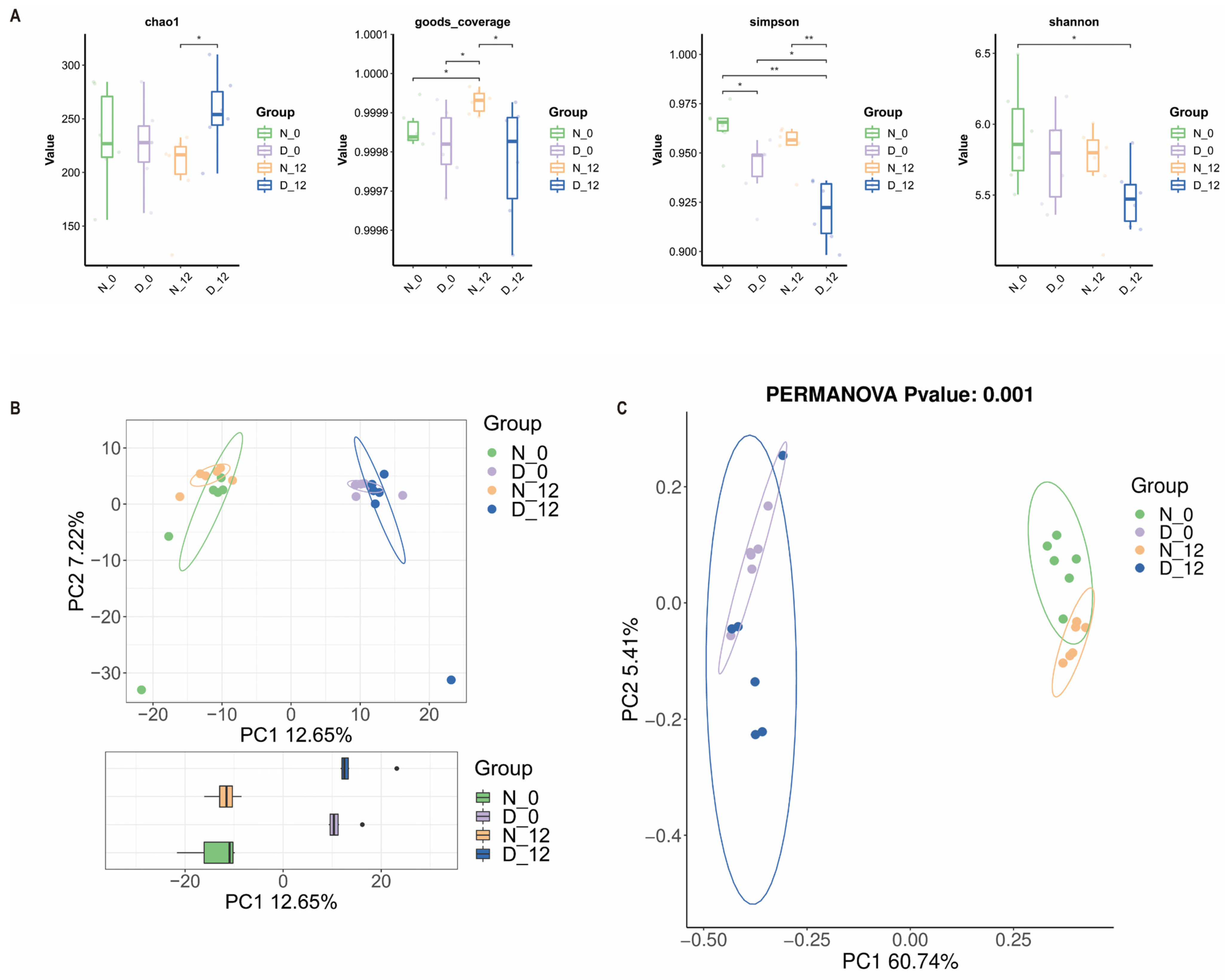
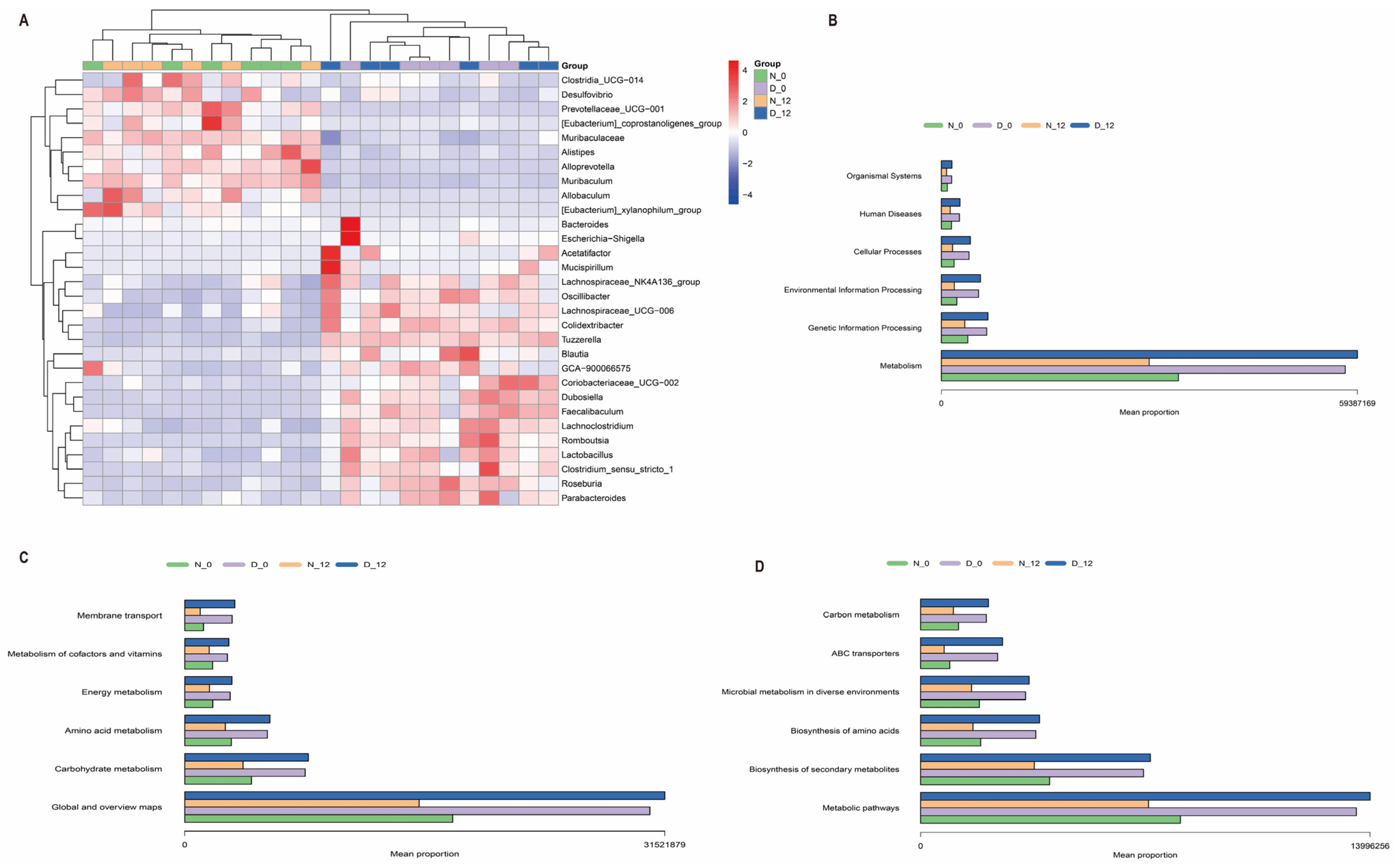
3.4. Alterations in the Gut Microbiota Composition between the Diabetic and Control Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruan, W.; Yuan, X.; Eltzschig, H.K. Circadian rhythm as a therapeutic target. Nat. Rev. Drug Discov. 2021, 20, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.; Sassone-Corsi, P.; Javeed, N.; Matveyenko, A.V.; Qian, J.; Thomas, A.P.; Schroeder, A.M.; Rakshit, K.; Colwell, C.S.; van Breukelen, F.; et al. Metabolism and the circadian clock converge. Physiol. Rev. 2013, 93, 107–135. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, Y.; Li, Z.; Sun, T.; Li, Z.; Manyande, A.; Xiang, H.; Xiong, J. Gut microbiota regulates circadian oscillation in hepatic ischemia-reperfusion injury-induced cognitive impairment by interfering with hippocampal lipid metabolism in mice. Hepatol. Int. 2023, 17, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Xu, Y.; Li, S.; Zhan, G.; Hua, D.; Tan, J.; Chi, X.; Xiang, H.; Guo, F.; Luo, A. Contribution of preoperative gut microbiota in postoperative neurocognitive dysfunction in elderly patients undergoing orthopedic surgery. Front. Aging Neurosci. 2023, 15, 1108205. [Google Scholar] [CrossRef]
- Yang, B.; Sun, T.; Chen, Y.; Xiang, H.; Xiong, J.; Bao, S. The Role of Gut Microbiota in Mice With Bile Duct Ligation-Evoked Cholestatic Liver Disease-Related Cognitive Dysfunction. Front. Microbiol. 2022, 13, 909461. [Google Scholar] [CrossRef]
- Sun, T.; Du, H.; Li, Z.; Xiong, J.; Liu, Y.; Li, Y.; Zhang, W.; Liang, F.; He, J.; Liu, X.; et al. Decoding the contributions of gut microbiota and cerebral metabolism in acute liver injury mice with and without cognitive dysfunction. CNS Neurosci. Ther. 2022, 29, 31–42. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Sun, T.; He, Z.; Xiang, H.; Xiong, J. Gut microbiota-generated short-chain fatty acids are involved in para-chlorophenylalanine-induced cognitive disorders. Front. Microbiol. 2022, 13, 1028913. [Google Scholar] [CrossRef]
- Heath-Heckman, E.A.; Peyer, S.M.; Whistler, C.A.; Apicella, M.A.; Goldman, W.E.; McFall-Ngai, M.J. Bacterial bioluminescence regulates expression of a host cryptochrome gene in the squid-Vibrio symbiosis. mBio 2013, 4, e00167-13. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef]
- Lentsch, A.B.; Kato, A.; Yoshidome, H.; McMasters, K.M.; Edwards, M.J. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 2000, 32, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Zhou, H.M.; Zhu, J.J.; Rao, J.H.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Lu, L.; Zhai, Y. Hyperglycemia and liver ischemia reperfusion injury: A role for the advanced glycation endproduct and its receptor pathway. Am. J. Transplant. 2015, 15, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Behrends, M.; Martinez-Palli, G.; Niemann, C.U.; Cohen, S.; Ramachandran, R.; Hirose, R. Acute hyperglycemia worsens hepatic ischemia/reperfusion injury in rats. J. Gastrointest. Surg. 2010, 14, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xie, L.; Li, L.; Chen, X.; Yao, T.; Tian, Y.; Li, Q.; Wang, K.; Huang, C.; Li, C.; et al. The gut microbial metabolite, 3,4-dihydroxyphenylpropionic acid, alleviates hepatic ischemia/reperfusion injury via mitigation of macrophage pro-inflammatory activity in mice. Acta Pharm. Sin. B 2022, 12, 182–196. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Grasset, E.; Puel, A.; Charpentier, J.; Collet, X.; Christensen, J.E.; Terce, F.; Burcelin, R. A Specific Gut Microbiota Dysbiosis of Type 2 Diabetic Mice Induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell Metab. 2017, 25, 1075–1090.e1075. [Google Scholar] [CrossRef]
- Xing, H.C.; Li, L.J.; Xu, K.J.; Shen, T.; Chen, Y.B.; Sheng, J.F.; Chen, Y.; Fu, S.Z.; Chen, C.L.; Wang, J.G.; et al. Protective role of supplement with foreign Bifidobacterium and Lactobacillus in experimental hepatic ischemia-reperfusion injury. J. Gastroenterol. Hepatol. 2006, 21, 647–656. [Google Scholar] [CrossRef]
- Chassaing, B.; Etienne-Mesmin, L.; Gewirtz, A.T. Microbiota-liver axis in hepatic disease. Hepatology 2014, 59, 328–339. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, J.; Wang, W.; Ding, Y.; Sun, Z.; Zhang, Q.; Wang, Y.; Xie, H.; Yan, S.; Zheng, S. Mesenchymal stem cells improve mouse non-heart-beating liver graft survival by inhibiting Kupffer cell apoptosis via TLR4-ERK1/2-Fas/FasL-caspase3 pathway regulation. Stem Cell Res. Ther. 2016, 7, 157. [Google Scholar] [CrossRef]
- Ding, G.; Li, X.; Hou, X.; Zhou, W.; Gong, Y.; Liu, F.; He, Y.; Song, J.; Wang, J.; Basil, P.; et al. REV-ERB in GABAergic neurons controls diurnal hepatic insulin sensitivity. Nature 2021, 592, 763–767. [Google Scholar] [CrossRef]
- Johnson, B.P.; Walisser, J.A.; Liu, Y.; Shen, A.L.; McDearmon, E.L.; Moran, S.M.; McIntosh, B.E.; Vollrath, A.L.; Schook, A.C.; Takahashi, J.S.; et al. Hepatocyte circadian clock controls acetaminophen bioactivation through NADPH-cytochrome P450 oxidoreductase. Proc. Natl. Acad. Sci. USA 2014, 111, 18757–18762. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011, 121, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, D.; Yao, W.; Zhu, Q.; Liu, Y.; Huang, F.; Feng, J.; Chen, X.; Huang, Y.; Chi, X.; et al. Hyperglycemia Aggravates Hepatic Ischemia Reperfusion Injury by Inducing Chronic Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 3919627. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, C.; Shen, G.; Yang, S.; Cheng, X.; Cheng, F.; Rao, J.; Wang, X. Hyperglycemia-Triggered Sphingosine-1-Phosphate and Sphingosine-1-Phosphate Receptor 3 Signaling Worsens Liver Ischemia/Reperfusion Injury by Regulating M1/M2 Polarization. Liver Transplant. 2019, 25, 1074–1090. [Google Scholar] [CrossRef] [PubMed]
- Reinke, H.; Asher, G. Circadian Clock Control of Liver Metabolic Functions. Gastroenterology 2016, 150, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Ming, H.; Lei, S.; Zhou, B.; Zhao, B.; Yu, Y.; Xue, R.; Xia, Z. Roles of HDAC3-orchestrated circadian clock gene oscillations in diabetic rats following myocardial ischaemia/reperfusion injury. Cell Death Dis. 2021, 12, 43. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118 (Suppl. 1), S23–S31. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Fang, D.; Yang, B.; Su, C.; Li, R.; Xiao, X.; et al. Gut microbiome alterations in high-fat-diet-fed mice are associated with antibiotic tolerance. Nat. Microbiol. 2021, 6, 874–884. [Google Scholar] [CrossRef]
- Monaghan, T.; Mullish, B.H.; Patterson, J.; Wong, G.K.; Marchesi, J.R.; Xu, H.; Jilani, T.; Kao, D. Effective fecal microbiota transplantation for recurrent Clostridioides difficile infection in humans is associated with increased signalling in the bile acid-farnesoid X receptor-fibroblast growth factor pathway. Gut Microbes 2019, 10, 142–148. [Google Scholar] [CrossRef]
- Andermann, T.; Antonelli, A.; Barrett, R.L.; Silvestro, D. Estimating Alpha, Beta, and Gamma Diversity Through Deep Learning. Front. Plant Sci. 2022, 13, 839407. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.; Ling, C.W.; He, Y.; Jiang, Z.; Fu, Y.; Xu, F.; Miao, Z.; Sun, T.Y.; Lin, J.S.; Zhu, H.L.; et al. Interpretable Machine Learning Framework Reveals Robust Gut Microbiome Features Associated With Type 2 Diabetes. Diabetes Care 2021, 44, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.; Hamady, M.; Yatsunenko, T.; Cantarel, B.; Duncan, A.; Ley, R.; Sogin, M.; Jones, W.; Roe, B.; Affourtit, J.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.; Ley, R.; Mahowald, M.; Magrini, V.; Mardis, E.; Gordon, J. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Thaiss, C.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Deng, F.; Zhao, B.C.; Yang, X.; Lin, Z.B.; Sun, Q.S.; Wang, Y.F.; Yan, Z.Z.; Liu, W.F.; Li, C.; Hu, J.J.; et al. The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut Microbes 2021, 13, 1902719. [Google Scholar] [CrossRef]
- Ascher, S.; Wilms, E.; Pontarollo, G.; Formes, H.; Bayer, F.; Müller, M.; Malinarich, F.; Grill, A.; Bosmann, M.; Saffarzadeh, M.; et al. Gut Microbiota Restricts NETosis in Acute Mesenteric Ischemia-Reperfusion Injury. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2279–2292. [Google Scholar] [CrossRef]
- Hu, J.; Deng, F.; Zhao, B.; Lin, Z.; Sun, Q.; Yang, X.; Wu, M.; Qiu, S.; Chen, Y.; Yan, Z.; et al. Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling. Microbiome 2022, 10, 38. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Xie, R.; Wang, Y.; Ma, Y. Gut microbiota aggravate cardiac ischemia-reperfusion injury via regulating the formation of neutrophils extracellular traps. Life Sci. 2022, 303, 120670. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, S.; Lv, H.; Cao, L. Gut microbiota from mice with cerebral ischemia-reperfusion injury affects the brain in healthy mice. Aging 2021, 13, 10058–10074. [Google Scholar] [CrossRef]
- Huang, P.; Cao, J.; Chen, J.; Luo, Y.; Gong, X.; Wu, C.; Wang, Y. Crosstalk between gut microbiota and renal ischemia/reperfusion injury. Front. Cell Infect. Microbiol. 2022, 12, 1015825. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, Y.; Li, Y.; Sun, T.; Xiang, H.; He, Z. The Role of Gut Microbiota and Circadian Rhythm Oscillation of Hepatic Ischemia–Reperfusion Injury in Diabetic Mice. Biomedicines 2024, 12, 54. https://doi.org/10.3390/biomedicines12010054
Li J, Liu Y, Li Y, Sun T, Xiang H, He Z. The Role of Gut Microbiota and Circadian Rhythm Oscillation of Hepatic Ischemia–Reperfusion Injury in Diabetic Mice. Biomedicines. 2024; 12(1):54. https://doi.org/10.3390/biomedicines12010054
Chicago/Turabian StyleLi, Juan, Yanbo Liu, Yijing Li, Tianning Sun, Hongbing Xiang, and Zhigang He. 2024. "The Role of Gut Microbiota and Circadian Rhythm Oscillation of Hepatic Ischemia–Reperfusion Injury in Diabetic Mice" Biomedicines 12, no. 1: 54. https://doi.org/10.3390/biomedicines12010054
APA StyleLi, J., Liu, Y., Li, Y., Sun, T., Xiang, H., & He, Z. (2024). The Role of Gut Microbiota and Circadian Rhythm Oscillation of Hepatic Ischemia–Reperfusion Injury in Diabetic Mice. Biomedicines, 12(1), 54. https://doi.org/10.3390/biomedicines12010054






