Inflammation and Fibrosis in Sleep-Disordered Breathing after Acute Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Work
2.3. Assessment of Sleep-Disordered Breathing
2.4. Statistical Analysis
3. Results
3.1. Patient Population
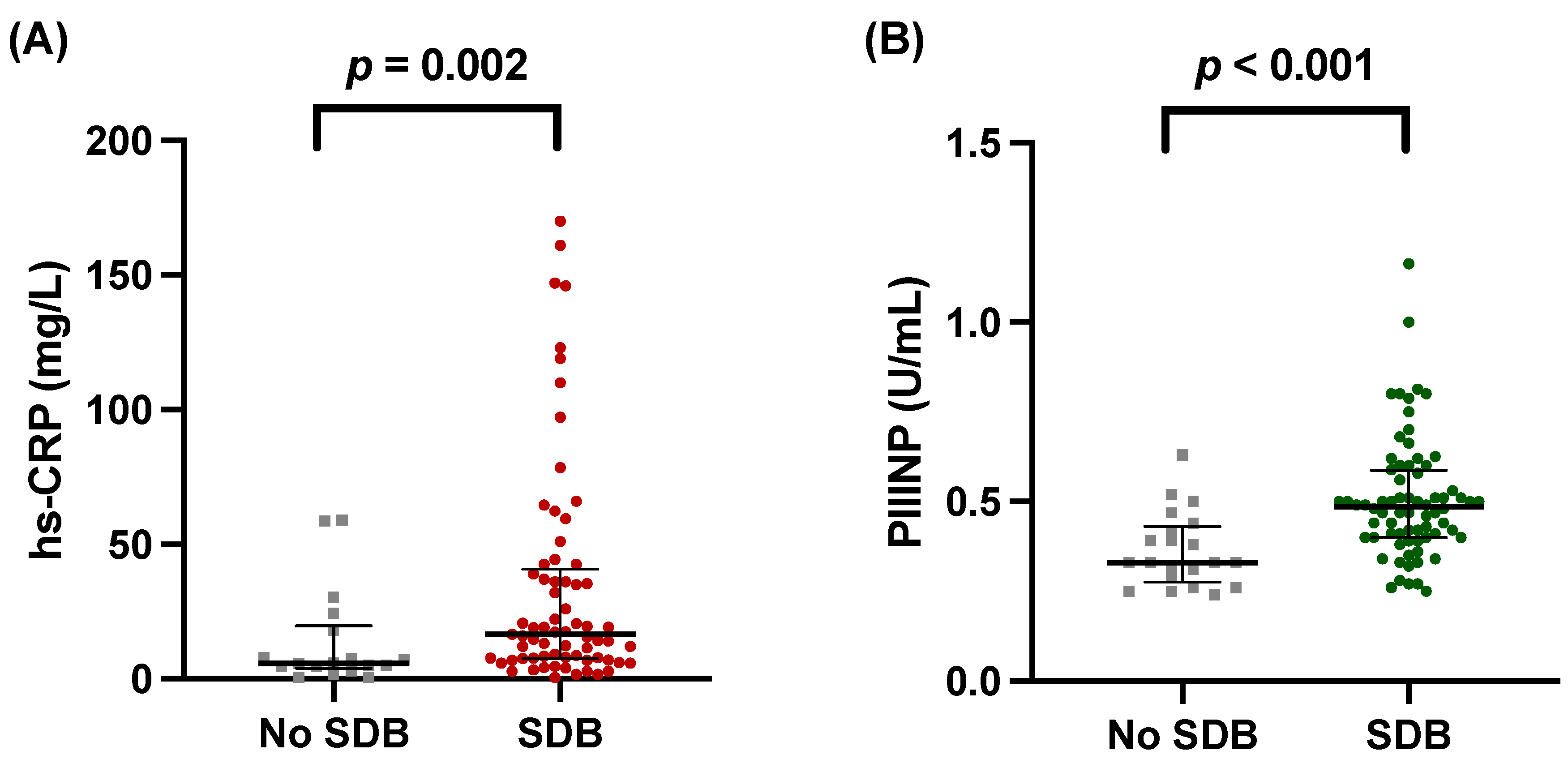
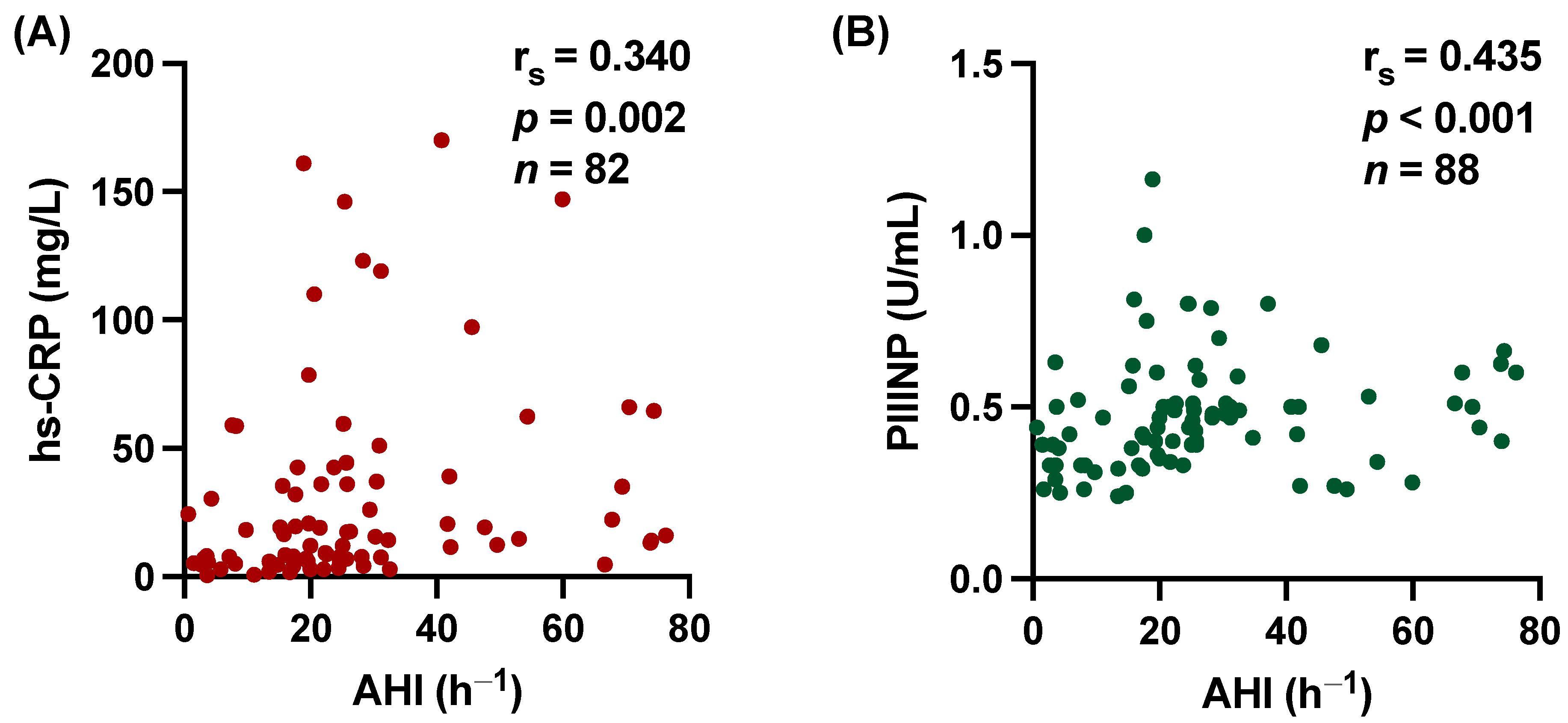
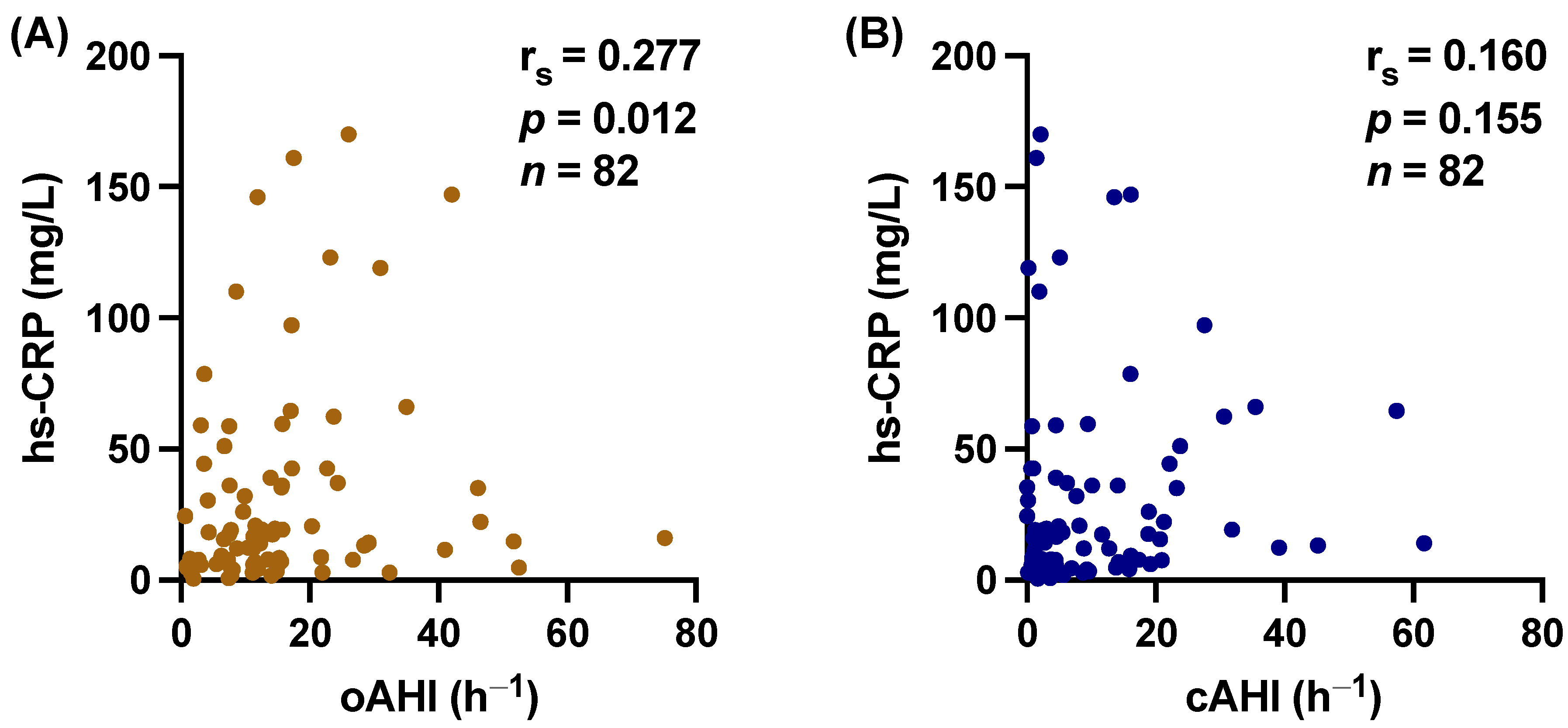
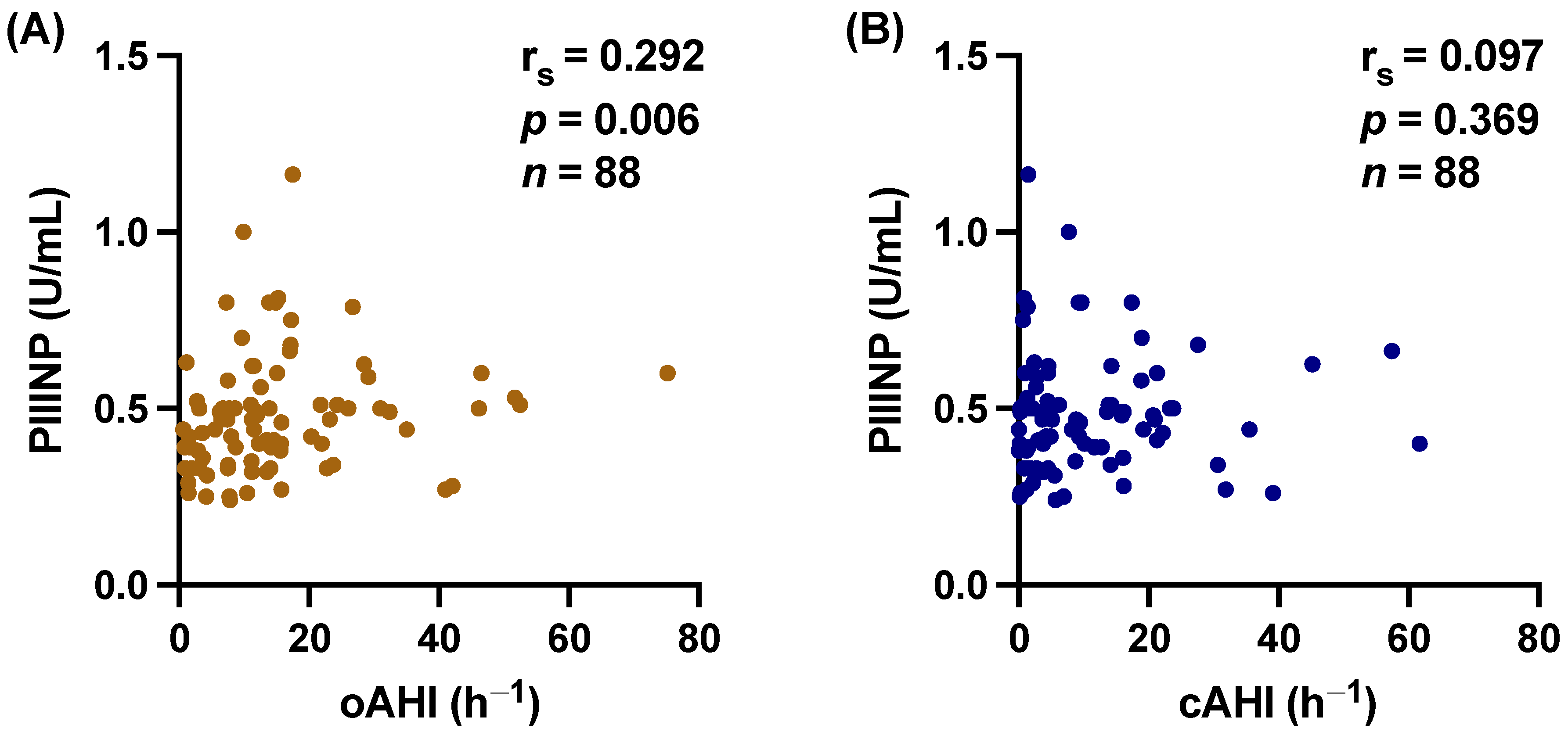
3.2. hs-CRP and PIIINP Correlate with SDB
3.3. Central vs. Obstructive Sleep Apnea
3.4. Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frantz, S.; Hundertmark, M.J.; Schulz-Menger, J.; Bengel, F.M.; Bauersachs, J. Left Ventricular Remodelling Post-Myocardial Infarction: Pathophysiology, Imaging, and Novel Therapies. Eur. Heart J. 2022, 43, 2549–2561. [Google Scholar] [CrossRef]
- Hall, C.; Gehmlich, K.; Denning, C.; Pavlovic, D. Complex Relationship between Cardiac Fibroblasts and Cardiomyocytes in Health and Disease. J. Am. Heart Assoc. 2021, 10, e019338. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.E.; Laurent, G.J. Collagen Turnover and Its Regulation in the Normal and Hypertrophying Heart. Eur. Heart J. 1995, 16, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Wittig, C.; Szulcek, R. Extracellular Matrix Protein Ratios in the Human Heart and Vessels: How to Distinguish Pathological From Physiological Changes? Front. Physiol. 2021, 12, 708656. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Kivirikko, K.I.; Tuderman, L.; Guzman, N.A. The Biosynthesis of Collagen and Its Disorders. N. Engl. J. Med. 1979, 301, 77–85. [Google Scholar] [CrossRef]
- Cleutjens, J.P.M.; Kandala, J.C.; Guarda, E.; Guntaka, R.V.; Weber, K.T. Regulation of Collagen Degradation in the Rat Myocardium after Infarction. J. Mol. Cell. Cardiol. 1995, 27, 1281–1292. [Google Scholar] [CrossRef]
- Poulsen, S.H.; Høst, N.B.; Jensen, S.E.; Egstrup, K. Relationship between Serum Amino-Terminal Propeptide of Type III Procollagen and Changes of Left Ventricular Function after Acute Myocardial Infarction. Circulation 2000, 101, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Høst, N.B.; Jensen, L.T.; Bendixen, P.M.; Jensen, S.E.; Koldkjaer, O.G.; Simonsen, E.E. The Aminoterminal Propeptide of Type III Procollagen Provides New Information on Prognosis after Acute Myocardial Infarction. Am. J. Cardiol. 1995, 76, 869–873. [Google Scholar] [CrossRef]
- Agarwal, R.; Duffin, K.L.; Laska, D.A.; Voelker, J.R.; Breyer, M.D.; Mitchell, P.G. A Prospective Study of Multiple Protein Biomarkers to Predict Progression in Diabetic Chronic Kidney Disease. Nephrol. Dial. Transplant. 2014, 29, 2293–2302. [Google Scholar] [CrossRef]
- Duprez, D.A.; Gross, M.D.; Kizer, J.R.; Ix, J.H.; Hundley, W.G.; Jacobs, D.R. Predictive Value of Collagen Biomarkers for Heart Failure with and without Preserved Ejection Fraction: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Heart Assoc. 2018, 7, 13–16. [Google Scholar] [CrossRef]
- Zannad, F.; Rossignol, P.; Iraqi, W. Extracellular Matrix Fibrotic Markers in Heart Failure. Heart Fail. Rev. 2010, 15, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Buchner, S.; Satzl, A.; Debl, K.; Hetzenecker, A.; Luchner, A.; Husser, O.; Hamer, O.W.; Poschenrieder, F.; Fellner, C.; Zeman, F.; et al. Impact of Sleep-Disordered Breathing on Myocardial Salvage and Infarct Size in Patients with Acute Myocardial Infarction. Eur. Heart J. 2014, 35, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Arzt, M.; Hetzenecker, A.; Steiner, S.; Buchner, S. Sleep-Disordered Breathing and Coronary Artery Disease. Can. J. Cardiol. 2015, 31, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-de-la-Torre, M.; Campos-Rodriguez, F.; Barbé, F. Obstructive Sleep Apnoea and Cardiovascular Disease. Lancet Respir. Med. 2013, 1, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Hetzenecker, A.; Stadler, S.; Oldenburg, O.; Hamer, O.W.; Zeman, F.; Bruch, L.; Seidel, M.; Buchner, S.; Arzt, M. Rationale and Design of the Randomised Treatment of Sleep Apnoea Early after Myocardial Infarction with Adaptive Servo-Ventilation Trial (TEAM-ASV I). Trials 2020, 21, 129. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Lang, T. Documenting Research in Scientific Articles: Guidelines for Authors: 3. Reporting Multivariate Analyses. Chest 2007, 131, 628–632. [Google Scholar] [CrossRef]
- Spearman, C. The Proof and Measurement of Association between Two Things. Am. J. Psychol. 1904, 15, 72–101. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; p. xxi. ISBN 9780805802832. [Google Scholar]
- Arnaud, C.; Bochaton, T.; Pépin, J.L.; Belaidi, E. Obstructive Sleep Apnoea and Cardiovascular Consequences: Pathophysiological Mechanisms. Arch. Cardiovasc. Dis. 2020, 113, 350–358. [Google Scholar] [CrossRef]
- Fisser, C.; Götz, K.; Hetzenecker, A.; Debl, K.; Zeman, F.; Hamer, O.W.; Poschenrieder, F.; Fellner, C.; Stadler, S.; Maier, L.S.; et al. Obstructive Sleep Apnoea but Not Central Sleep Apnoea Is Associated with Left Ventricular Remodelling after Acute Myocardial Infarction. Clin. Res. Cardiol. 2020, 110, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Jullian-Desayes, I.; Joyeux-Faure, M.; Tamisier, R.; Launois, S.; Borel, A.L.; Levy, P.; Pepin, J.L. Impact of Obstructive Sleep Apnea Treatment by Continuous Positive Airway Pressure on Cardiometabolic Biomarkers: A Systematic Review from Sham CPAP Randomized Controlled Trials. Sleep Med. Rev. 2015, 21, 23–38. [Google Scholar] [CrossRef]
- Thunström, E.; Glantz, H.; Yucel-Lindberg, T.; Lindberg, K.; Saygin, M.; Peker, Y. Corrigendum: CPAP Does Not Reduce Inflammatory Biomarkers in Patients with Coronary Artery Disease and Nonsleepy Obstructive Sleep Apnea: A Randomized Controlled Trial. Sleep 2017, 40, zsx157. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Glantz, H.; Eulenburg, C.; Wegscheider, K.; Herlitz, J.; Thunström, E. Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea: The RICCADSA Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Arzt, M.; Fox, H.; Stadler, S.; Hetzenecker, A.; Hamer, O.; Poschenrieder, F.; Wiest, C.; Tanacli, R.; Kelle, S.; Bruch, L.; et al. Treatment of Sleep Apnoea Early After Myocardial Infarction With Adaptive Servo-Ventilation (TEAM-ASV I): A Multicentre Randomised Controlled Trial. In A18. BREAKING NEWS IN OSA: NEW APPROACHES AND NEW TRIALS; American Thoracic Society: New York, NY, USA, 2023; Volume 207, p. A1047. [Google Scholar] [CrossRef]
- Pupkaite, J.; Sedlakova, V.; Eren Cimenci, C.; Bak, M.; McLaughlin, S.; Ruel, M.; Alarcon, E.I.; Suuronen, E.J. Delivering More of an Injectable Human Recombinant Collagen III Hydrogel Does Not Improve Its Therapeutic Efficacy for Treating Myocardial Infarction. ACS Biomater. Sci. Eng. 2020, 6, 4256–4265. [Google Scholar] [CrossRef]
- Sainio, A.; Järveläinen, H. Extracellular Matrix-Cell Interactions: Focus on Therapeutic Applications. Cell. Signal. 2020, 66, 109487. [Google Scholar] [CrossRef]
- Mohindra, P.; Zhong, J.X.; Fang, Q.; Cuylear, D.L.; Huynh, C.; Qiu, H.; Gao, D.; Kharbikar, B.N.; Huang, X.; Springer, M.L.; et al. Local Decorin Delivery via Hyaluronic Acid Microrods Improves Cardiac Performance, Ventricular Remodeling after Myocardial Infarction. NPJ Regen. Med. 2023, 8, 60. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Temirkhanova, K.; Saparov, A. Novel Therapies for the Treatment of Cardiac Fibrosis Following Myocardial Infarction. Biomedicines 2022, 10, 2178. [Google Scholar] [CrossRef]
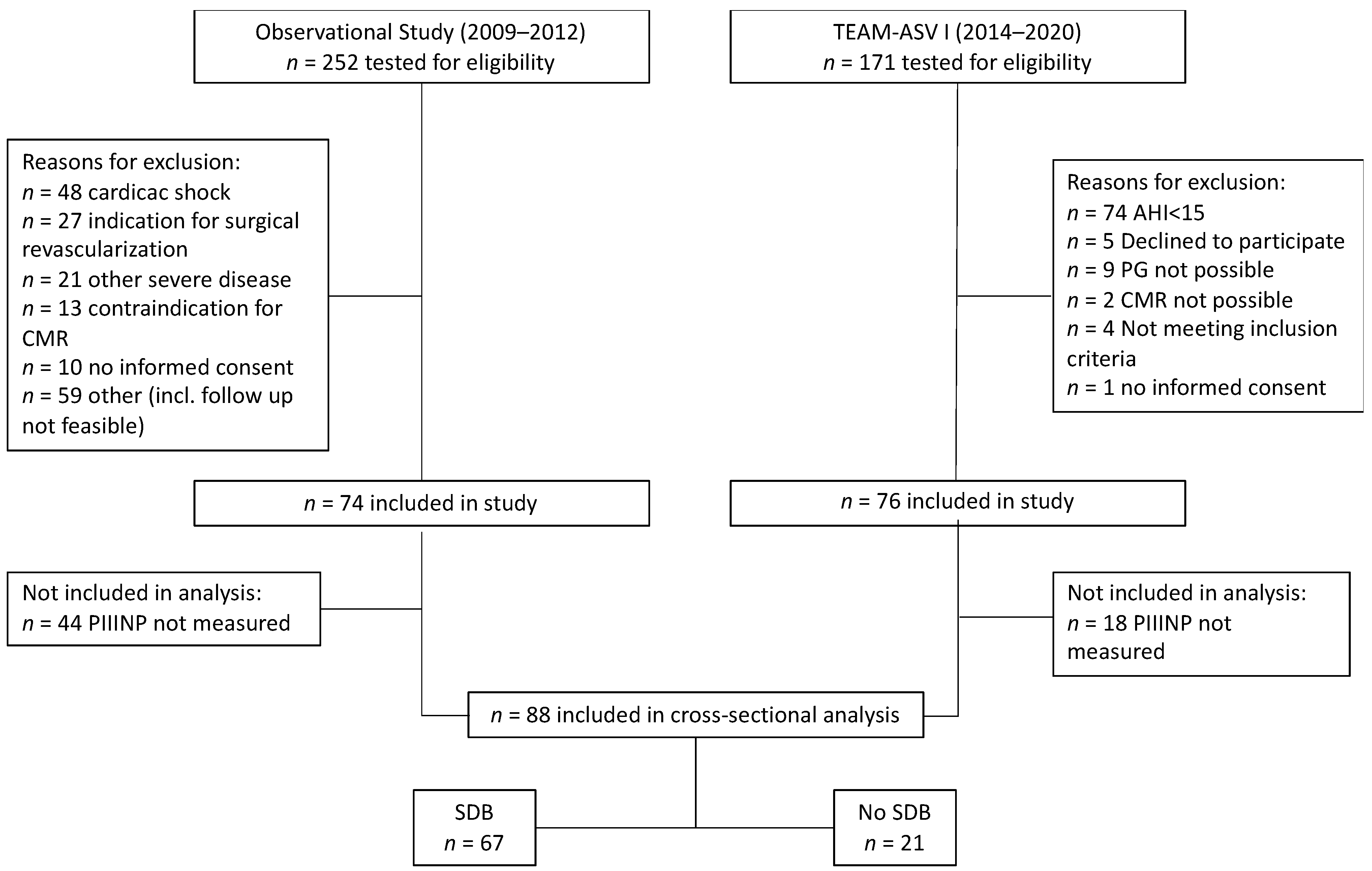
| All Patients n = 88 | No SDB n = 21 | SDB n = 67 | p Value | |
|---|---|---|---|---|
| Age, years | 57 (±10) | 52 (±9) | 58 (±9) | 0.011 |
| Female gender, n (%) | 16 (18) | 4 (19) | 12 (18) | 0.100 |
| BMI, kg/m2 | 30 (±5) | 27 (±2) | 30 (±5) | 0.011 |
| Arterial hypertension, n (%) | 48 (55) | 10 (48) | 38 (57) | 0.456 |
| Diabetes mellitus, n (%) | 15 (17) | 2 (10) | 13 (19) | 0.506 |
| Peripheral artery disease, n (%) | 4 (5) | 1 (5) | 3 (5) | 1.000 |
| Current smoker, n (%) | 43 (49) | 12 (60) | 31 (46) | 0.281 |
| AHI, h−1 | 23.2 (15.3–32.0) | 4.3 (3.3–9.0) | 25.8 (20.0–41.7) | <0.001 |
| oAHI, h−1 | 11.9 (6.8–17.5) | 2.8 (1.4–6.6) | 14.6 (10.5–23.2) | <0.001 |
| cAHI, h−1 | 5.1 (1.5–16.1) | 2.0 (0.7–4.4) | 9.3 (2.6–18.9) | <0.001 |
| ODI, h−1 | 15.5 (6.7–24.7) | 3.7 (2.3–6.7) | 18.9 (12.4–28.9) | <0.001 |
| T90%, % TRT | 2.4 (0.43–15.5) | 0.5 (0.2–15.8) | 4.7 (0.6–15.5) | 0.139 |
| LVEF, % | 52 (±9) | 50 (±8) | 52 (±9) | 0.492 |
| LVEDV index, mL/m2 | 76 (67–87) | 74 (64–84) | 76 (65–83) | 0.192 |
| LVESV index, mL/m2 | 37 (28–50) | 37 (28–50) | 37 (28–50) | 0.903 |
| STEMI, n (%) | 74 (86) | 21 (100) | 53 (82) | 0.034 |
| Multivessel disease, n (%) | 44 (50) | 10 (48) | 34 (51) | 0.803 |
| Pain-to-balloon time, h | 4.2 (2.8–10.1) | 6.8 (2.8–17.8) | 4.0 (2.8–8.5) | 0.132 |
| NT-proBNP max, pg/mL | 926 (314–1598) | 608 (291–1766) | 936 (337–1536) | 0.841 |
| CK, U/L | 218 (130–358) | 240 (186–396) | 200 (118–358) | 0.129 |
| Creatinine, mg/dL | 0.91 (0.80–1.04) | 0.92 (0.74–1.02) | 0.91 (0.80–1.04) | 0.038 |
| eGFR, mL/min/1.73 m2 | 79 (±23) | 96 (±15) | 74 (±23) | <0.001 |
| LDL, mg/dL | 127 (±31) | 131 (±26) | 126 (±33) | 0.511 |
| Leukozyten, 109/L | 10.1 (±3.2) | 12 (±3.0) | 9.6 (±3.0) | 0.004 |
| Hemoglobin, g/dL | 14.4 (±1.4) | 15.0 (±1.1) | 14.2 (±1.5) | 0.009 |
| Thrombozyten, 109/L | 223 (203–284) | 236 (199–291) | 236 (204–276) | 0.858 |
| Univariable Linear Regression Analysis | Multivariable Linear Regression Analysis | |||||
|---|---|---|---|---|---|---|
| hs-CRP [mg/L] | B | 95% CI | p Value | B | 95% CI | p Value |
| SDB | 21.961 | 1.574 to 42.349 | 0.035 | 21.510 | 2.397 to 40.623 | 0.028 |
| Age, years | 0.318 | −0.618 to 1.255 | 0.501 | |||
| Female sex | −2.125 | −24.017 to 19.767 | 0.847 | |||
| BMI | −10.697 | −33.637 to 12.242 | 0.356 | |||
| Current smoker | 5.674 | −11.807 to 23.156 | 0.520 | |||
| Arterial hypertension | 10.350 | −6.984 to 27.684 | 0.238 | |||
| Diabetes mellitus | −0.052 | −0.147 to 0.043 | 0.280 | |||
| PAD | −26.425 | −66.279 to 13.428 | 0.191 | |||
| LVEF, % | −1.151 | −2.046 to −0.255 | 0.013 | −1.262 | −2.138 to −0.386 | 0.005 |
| eGFR, mL/min/1.73 m2 | 0.021 | −0.357 to 0.400 | 0.911 | |||
| Univariable Linear Regression Analysis | Multivariable Linear Regression Analysis | |||||
|---|---|---|---|---|---|---|
| PIIINP [U/mL] | B | 95% CI | p Value | B | 95% CI | p Value |
| SDB | 0.149 | 0.071 to 0.227 | <0.001 | 0.146 | 0.065 to 0.227 | <0.001 |
| Age, years | 0.003 | −0.001 to 0.007 | 0.089 | 0.001 | −0.002 to 0.005 | 0.437 |
| Female sex | −0.003 | −0.096 to 0.091 | 0.954 | |||
| BMI | 0.003 | −0.005 to −0.010 | 0.484 | |||
| Current smoker | −0.047 | −0.119 to 0.025 | 0.195 | |||
| Arterial hypertension | −0.026 | −0.098 to 0.046 | 0.472 | |||
| Diabetes mellitus | −0.052 | −0.147 to 0.043 | 0.280 | |||
| PAD | −0.055 | −0.227 to 0.118 | 0.531 | |||
| LVEF, % | 0.001 | −0.003 to 0.005 | 0.512 | |||
| eGFR, mL/min/1.73 m2 | 0.001 | −0.001 to 0.002 | 0.394 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pec, J.; Buchner, S.; Fox, H.; Oldenburg, O.; Stadler, S.; Maier, L.S.; Arzt, M.; Wagner, S. Inflammation and Fibrosis in Sleep-Disordered Breathing after Acute Myocardial Infarction. Biomedicines 2024, 12, 154. https://doi.org/10.3390/biomedicines12010154
Pec J, Buchner S, Fox H, Oldenburg O, Stadler S, Maier LS, Arzt M, Wagner S. Inflammation and Fibrosis in Sleep-Disordered Breathing after Acute Myocardial Infarction. Biomedicines. 2024; 12(1):154. https://doi.org/10.3390/biomedicines12010154
Chicago/Turabian StylePec, Jan, Stefan Buchner, Henrik Fox, Olaf Oldenburg, Stefan Stadler, Lars S. Maier, Michael Arzt, and Stefan Wagner. 2024. "Inflammation and Fibrosis in Sleep-Disordered Breathing after Acute Myocardial Infarction" Biomedicines 12, no. 1: 154. https://doi.org/10.3390/biomedicines12010154
APA StylePec, J., Buchner, S., Fox, H., Oldenburg, O., Stadler, S., Maier, L. S., Arzt, M., & Wagner, S. (2024). Inflammation and Fibrosis in Sleep-Disordered Breathing after Acute Myocardial Infarction. Biomedicines, 12(1), 154. https://doi.org/10.3390/biomedicines12010154






