The Predictive Value of 8-Hydroxy-Deoxyguanosine (8-OHdG) Serum Concentrations in Irradiated Non-Small Cell Lung Carcinoma (NSCLC) Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Treatment Schedule
2.3. Patient Assessment
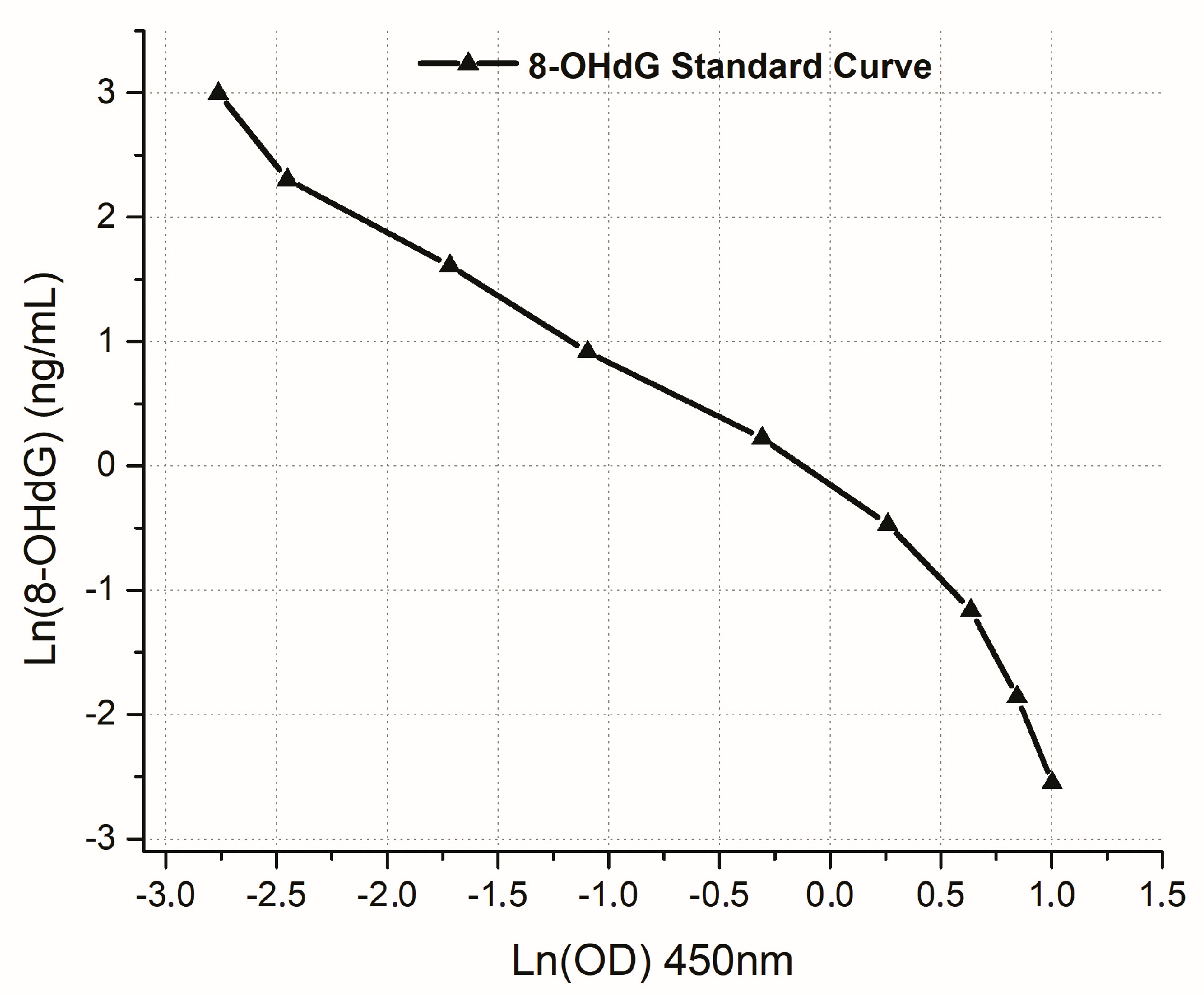
2.4. Sample Collection and Preparation
2.5. Statistical Analysis
3. Results
3.1. Patients
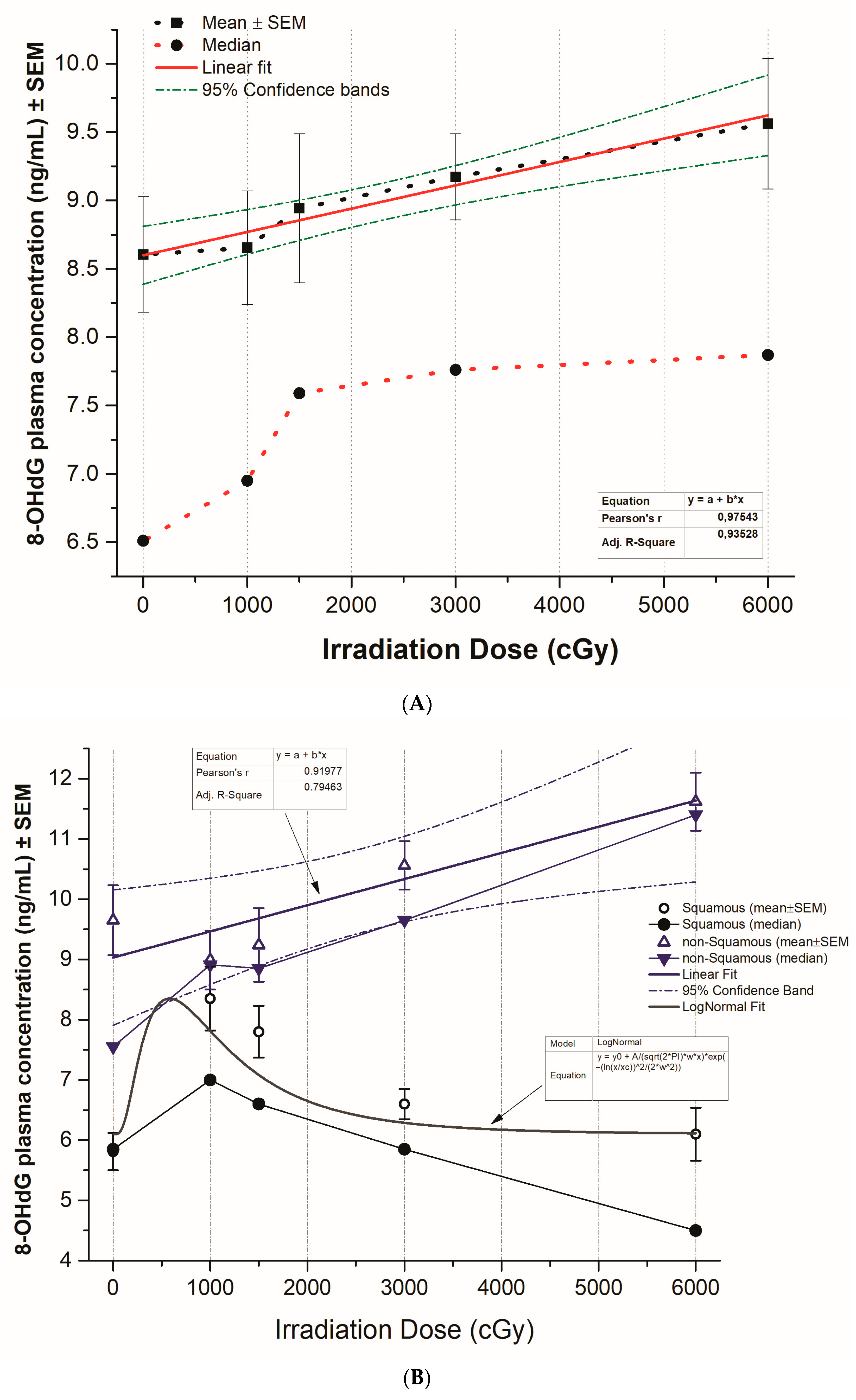
3.2. Plasma 8-OHdG Levels in Patients Receiving Radiotherapy
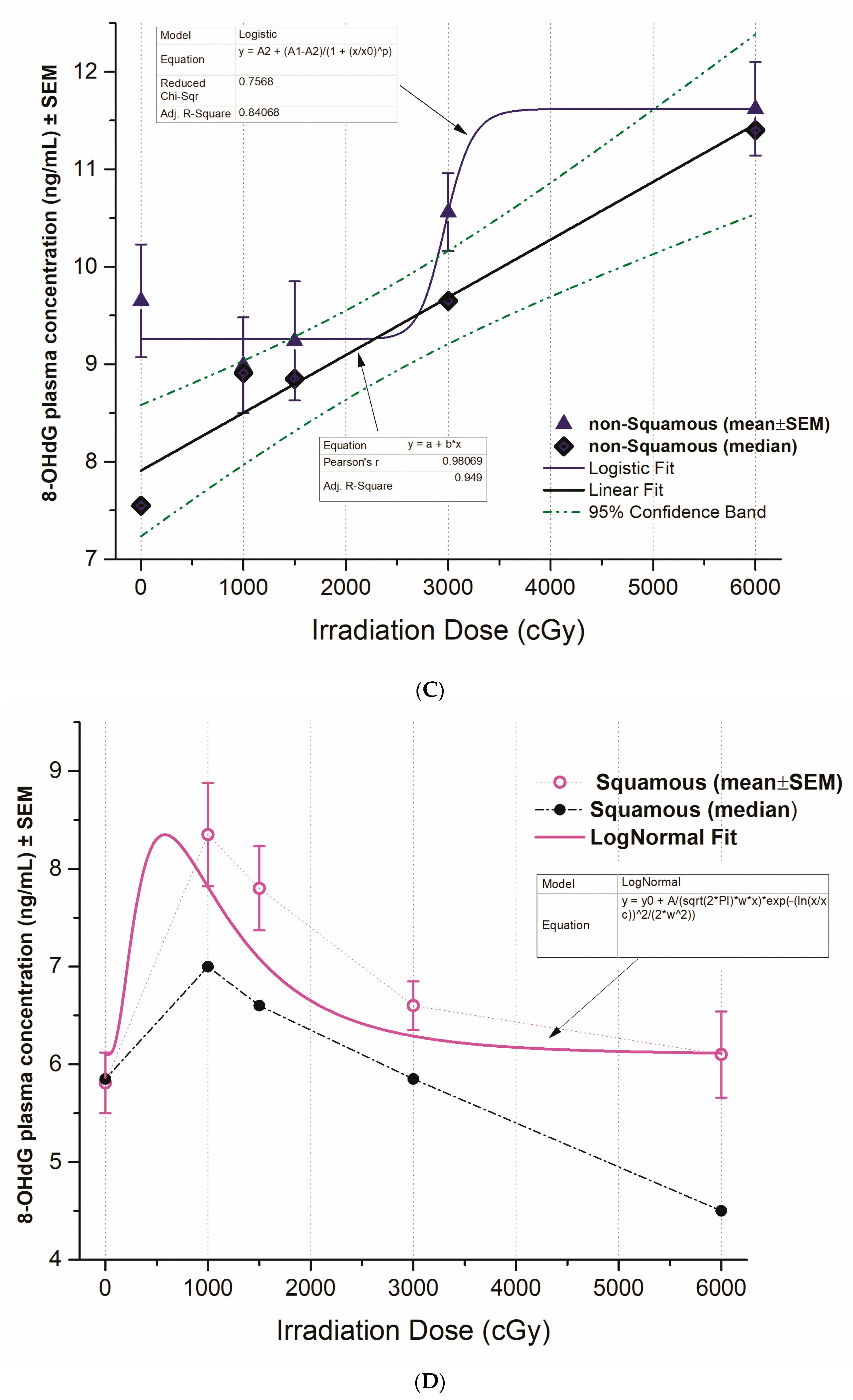
3.3. The Pattern of 8-OHdG Levels in Squamous versus Non-Squamous NSCLC
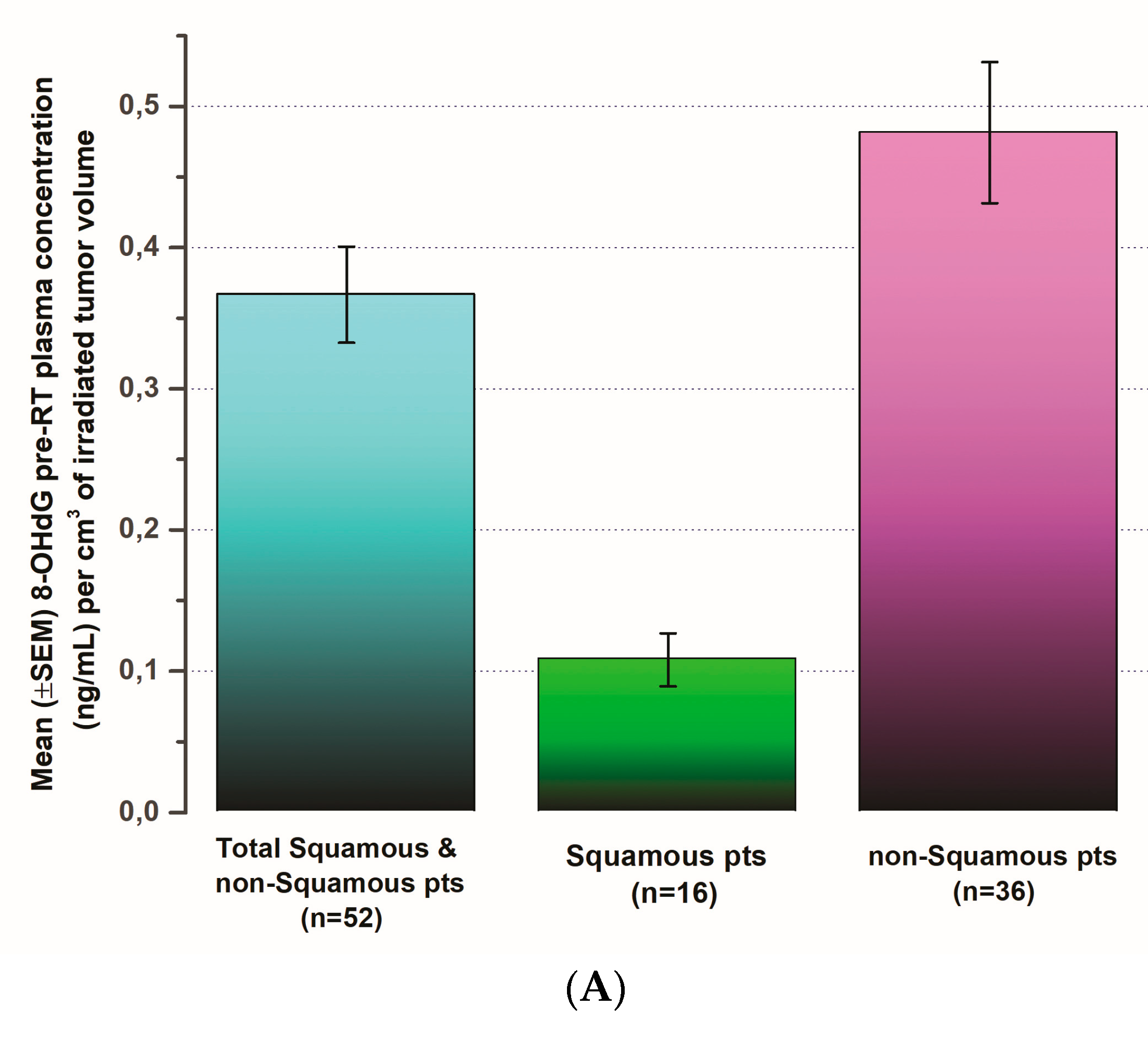
3.4. The Pattern of 8-OHdG Levels in Comparison to Pre-Operative Tumor Volume
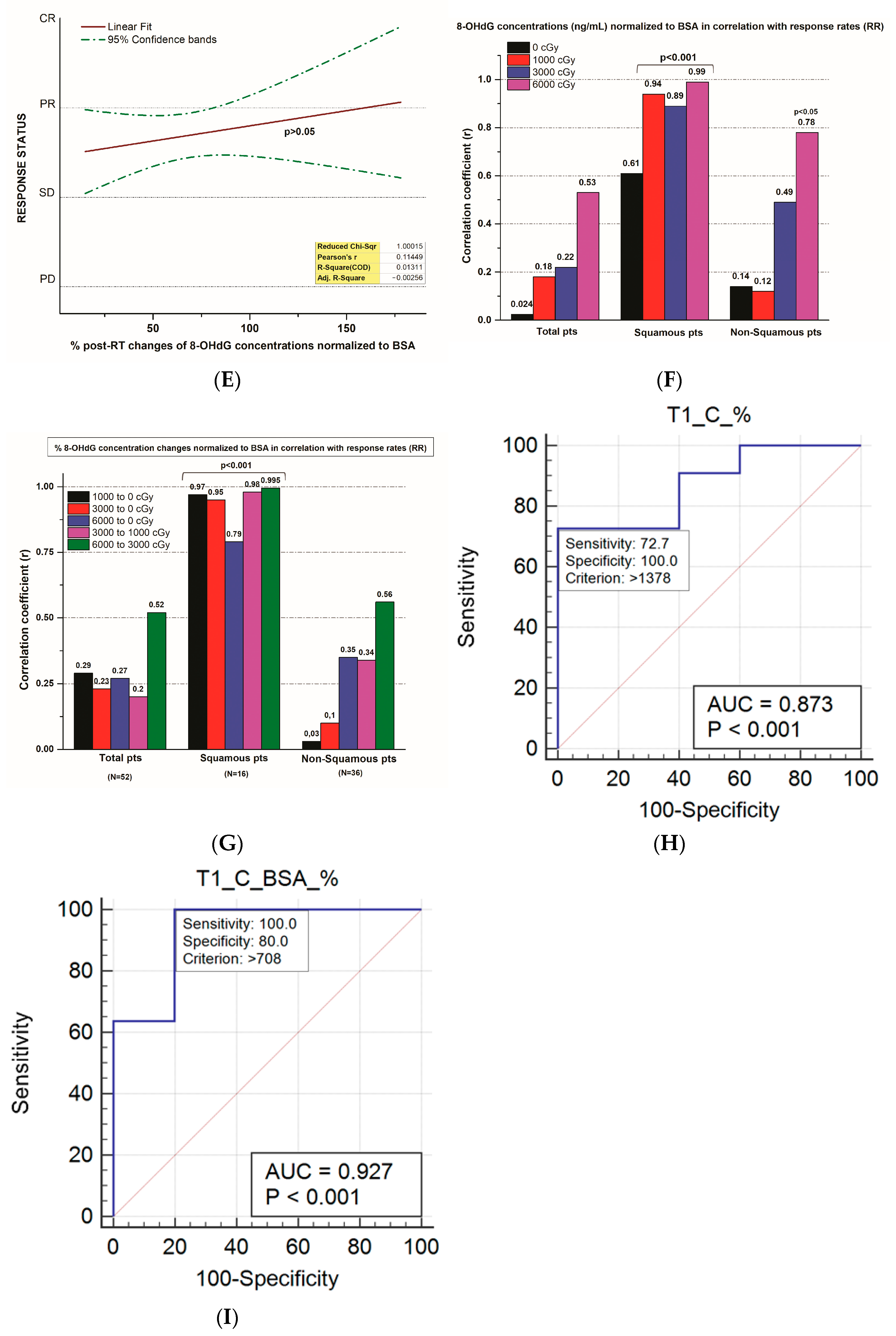
3.5. 8-OHdG Plasma Concentration Changes and the Tumor Response Rate
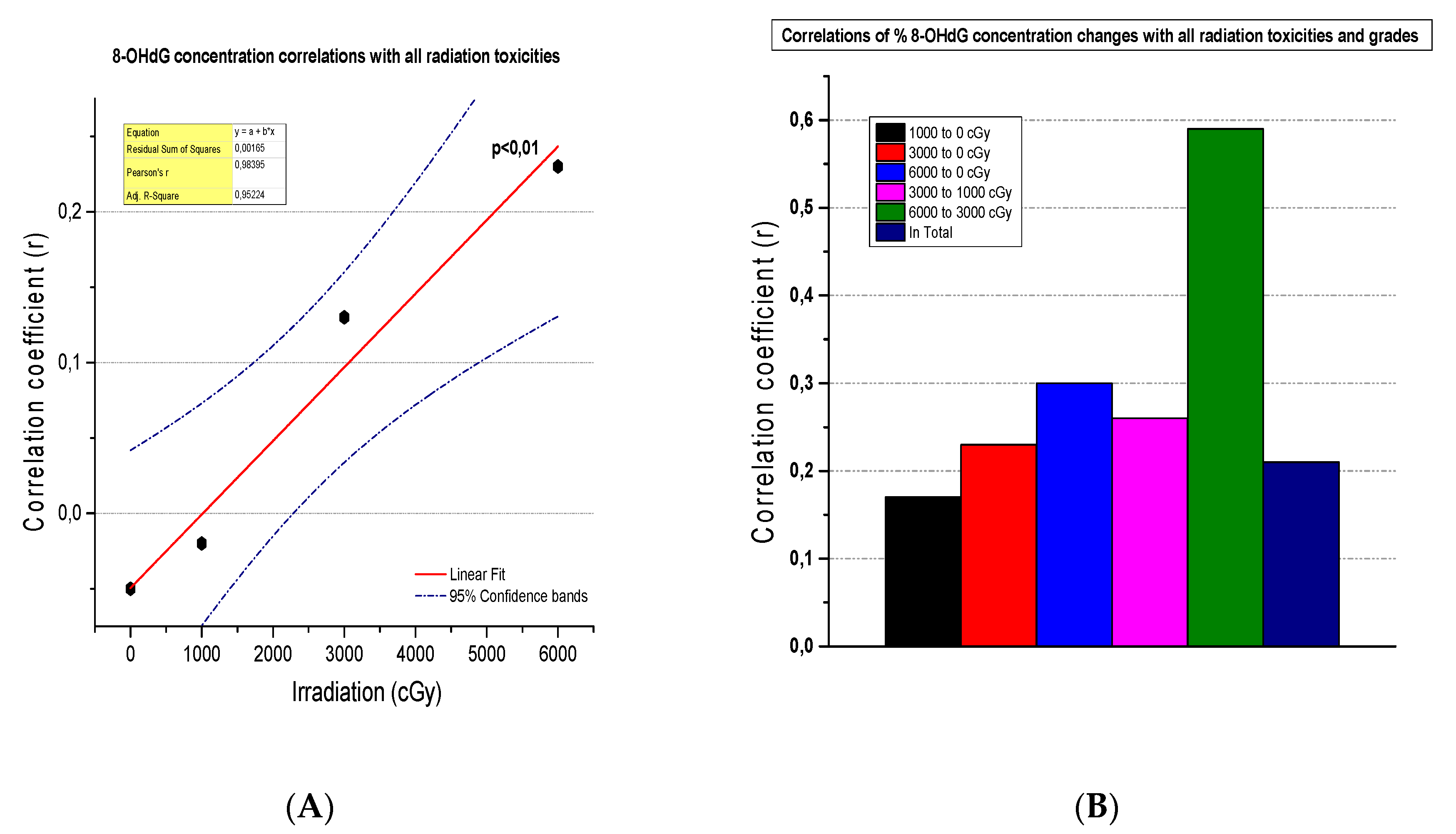
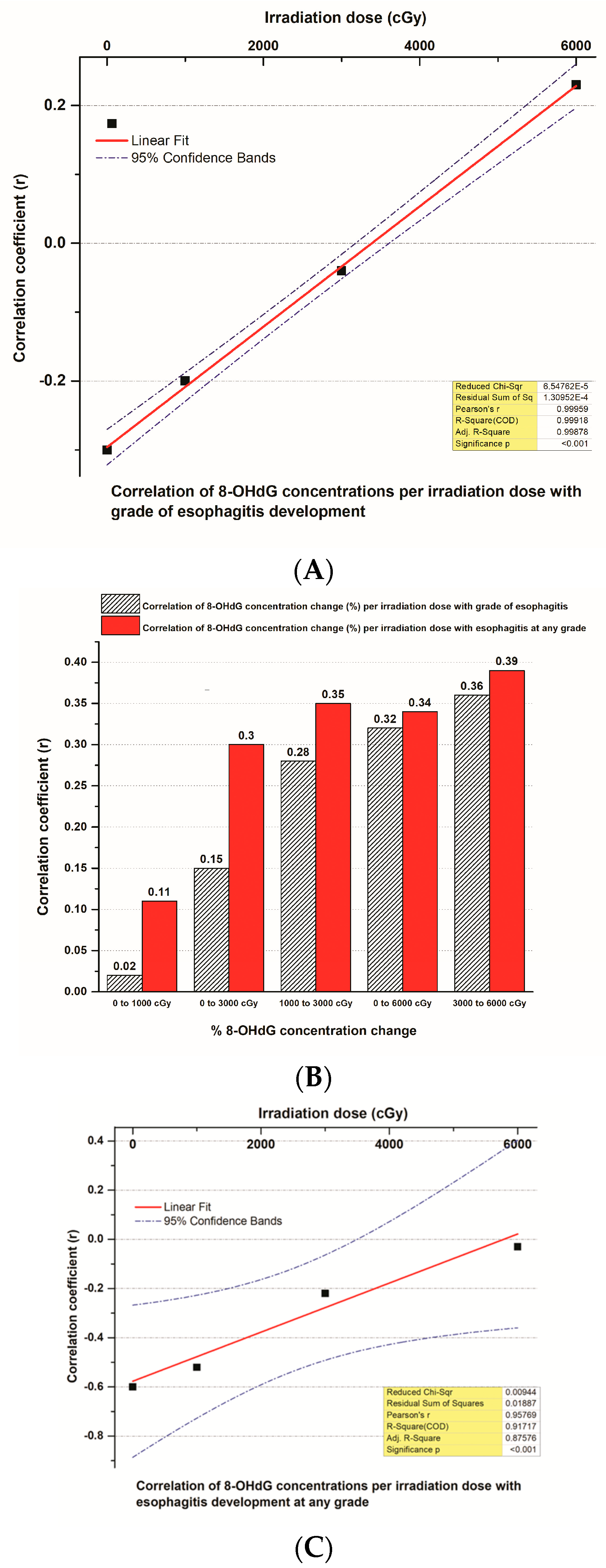
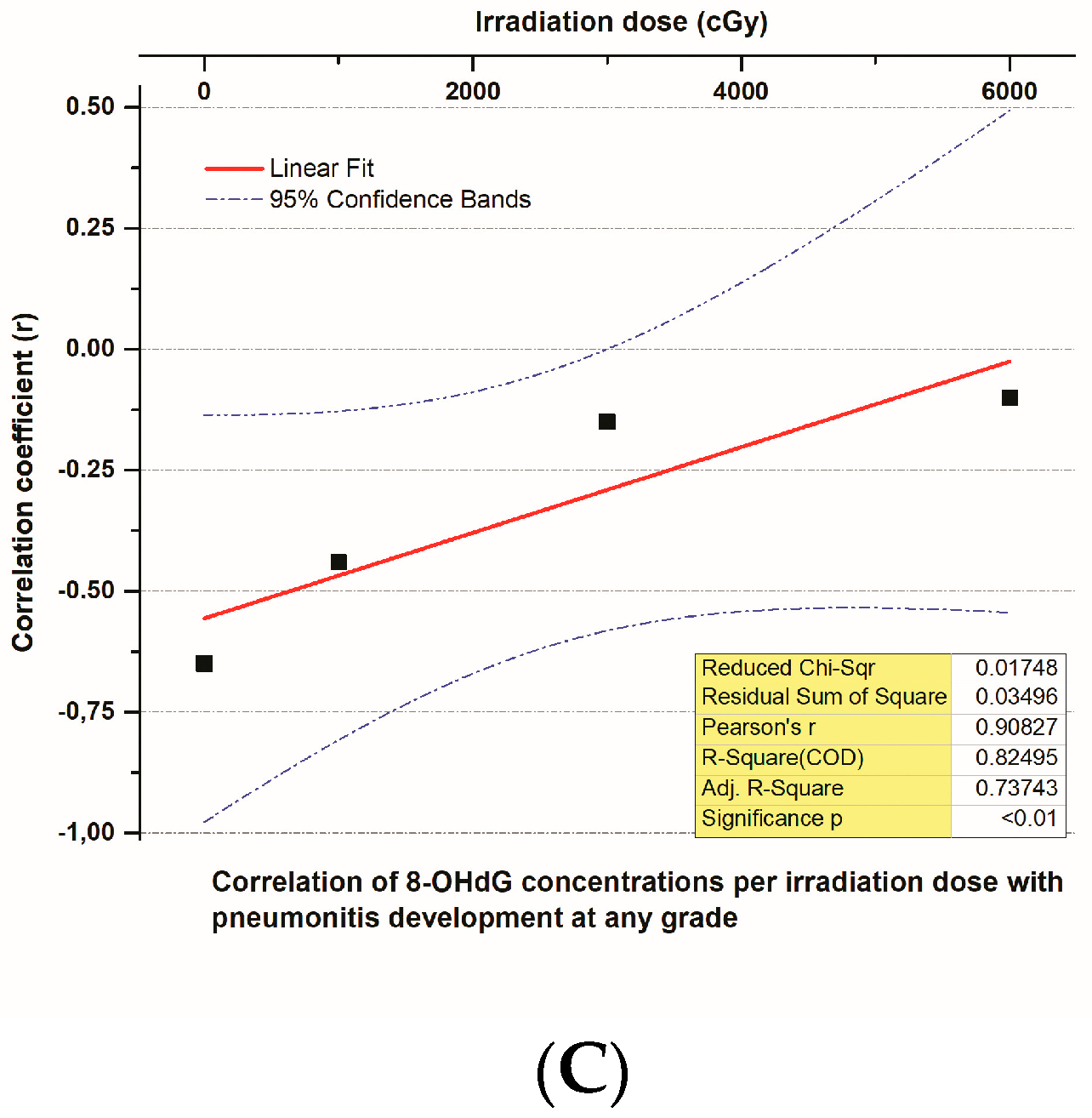
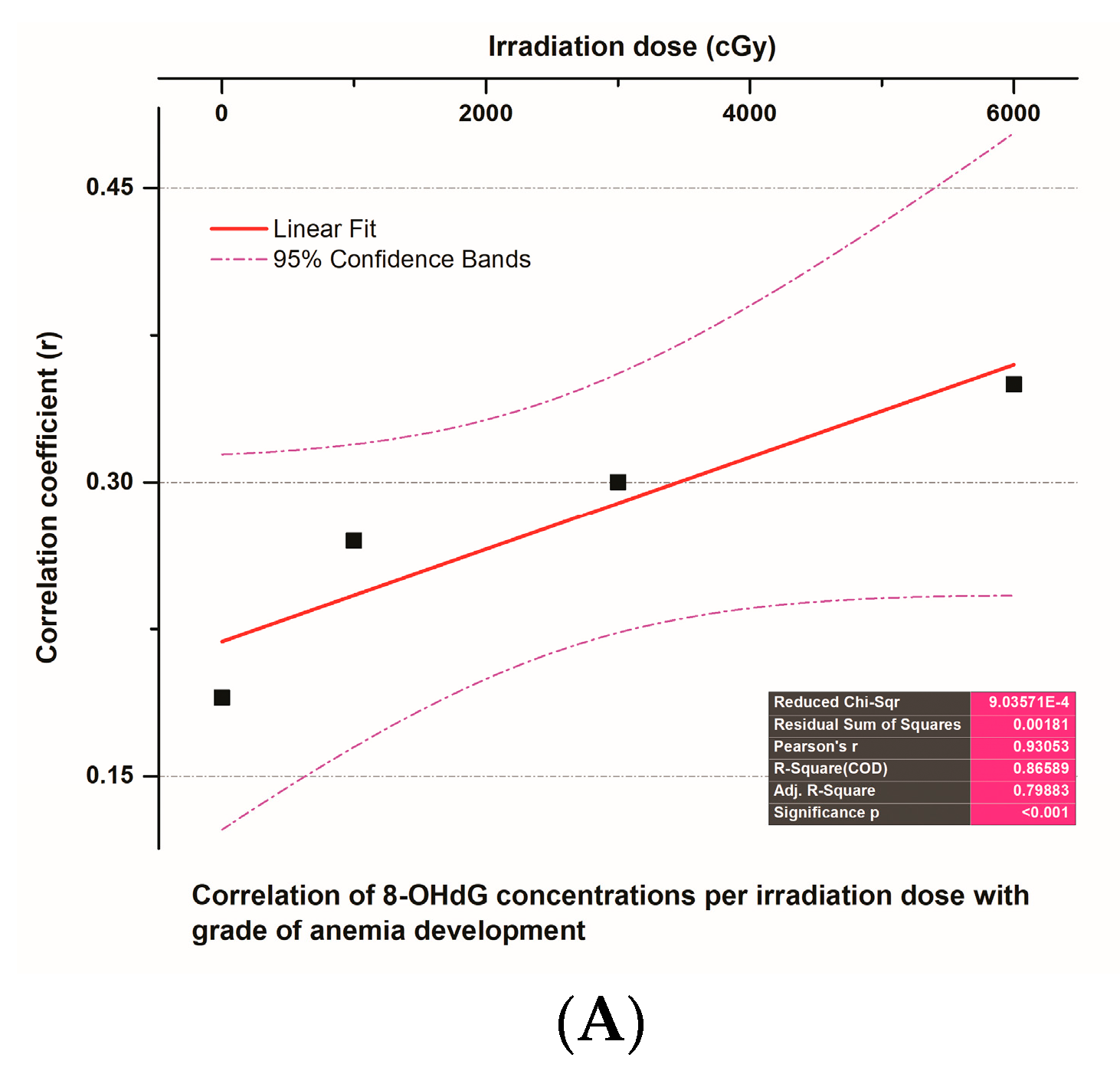
3.6. The Correlation of 8-OHdG Plasma Concentrations and Toxicities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2020. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 20 January 2023).
- SEER. Cancer Stat Facts: Lung and Bronchus Cancer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 20 January 2023).
- Trafalis, D.T.; Alifieris, C.; Stathopoulos, G.P.; Sitaras, N. Phase II Study of Bevacizumab plus Irinotecan on the Treatment of Relapsed Resistant Small Cell Lung Cancer. Cancer Chemother. Pharmacol. 2016, 77, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Kamiya, K. Molecular Nature of Radiation Injury and DNA Repair Disorders Associated with Radiosensitivity. Int. J. Hematol. 2012, 95, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Verigos, K.E.; Sagredou, S.; Orfanakos, K.; Dalezis, P.; Trafalis, D.T. 8-Hydroxy-2′-Deoxyguanosine and 8-Nitroguanine Production and Detection in Blood Serum of Breast Cancer Patients in Response to Postoperative Complementary External Ionizing Irradiation of Normal Tissues. Dose. Res. 2020, 18, 1559325820982172. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Soto, G.; Ortiz-López, R.; Rojas-Martínez, A. Ionizing Radiation-Induced DNA Injury and Damage Detection in Patients with Breast Cancer. Genet. Mol. Biol. 2015, 38, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Arthur, D.W. The Emergence of Advanced Brachytherapy Techniques for Common Malignancies. Hematol. Oncol. Clin. N. Am. 2006, 20, 97–118. [Google Scholar] [CrossRef]
- Hall, E.J.; Astor, M.; Bedford, J.; Borek, C.; Curtis, S.B.; Fry, M.; Geard, C.; Hei, T.; Mitchell, J.; Oleinick, N.; et al. Basic Radiobiology. Am. J. Clin. Oncol. Cancer Clin. Trials 1988, 11, 220–252. [Google Scholar] [CrossRef]
- O’Neill, P.; Wardman, P. Radiation Chemistry Comes before Radiation Biology. Int. J. Radiat. Biol. 2009, 85, 9–25. [Google Scholar] [CrossRef]
- Ahmad, I.M.; Abdalla, M.Y.; Moore, T.A.; Bartenhagen, L.; Case, A.J.; Zimmerman, M.C. Healthcare Workers Occupationally Exposed to Ionizing Radiation Exhibit Altered Levels of Inflammatory Cytokines and Redox Parameters. Antioxidants 2019, 8, 12. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, P.; Wang, Z.; Han, L.; Li, J.; Tian, C.; Zhao, F.; Wang, J.; Zhao, F.; Zhang, Q.; et al. Serum 8-Hydroxy-2′-Deoxyguanosine Level as a Potential Biomarker of Oxidative DNA Damage Induced by Ionizing Radiation in Human Peripheral Blood. Dose. Res. 2019, 17, 1559325818820649. [Google Scholar] [CrossRef]
- Pilger, A.; Rüdiger, H.W. 8-Hydroxy-2′-Deoxyguanosine as a Marker of Oxidative DNA Damage Related to Occupational and Environmental Exposures. Int. Arch. Occup. Environ. Health 2006, 80, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Petkau, A. Role of Superoxide Dismutase in Modification of Radiation Injury. Br. J. Cancer 1987, 55, 87–95. [Google Scholar]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Schilsky, R.L.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. American Joint Committee on Cancer (AJCC). In AJCC Cancer Staging Manual; Springer: Cham, Switzerland, 2017; ISBN 9783319406176. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Reeve, B.B.; Mitchell, S.A.; Clauser, S.B.; Minasian, L.M.; Dueck, A.C.; Mendoza, T.R.; Hay, J.; Atkinson, T.M.; Abernethy, A.P.; et al. Development of the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). J. Natl. Cancer Inst. 2014, 106, dju244. [Google Scholar] [CrossRef] [PubMed]
- Erhola, M.; Toyokuni, S.; Okada, K.; Tanaka, T.; Hiai, H.; Ochi, H.; Uchida, K.; Osawa, T.; Nieminen, M.M.; Alho, H.; et al. Biomarker Evidence of DNA Oxidation in Lung Cancer Patients: Association of Urinary 8-Hydroxy-2′-Deoxyguanosine Excretion with Radiotherapy, Chemotherapy, and Response to Treatment. FEBS Lett. 1997, 409, 287–291. [Google Scholar] [CrossRef]
- Yamazaki, H.; Inoue, T.; Koizumi, M.; Tanaka, E.; Yoshioka, Y.; Nakamura, H.; Shuo, X.; Inoue, T. Urinary 8-Hydroxy-2′-Deoxyguanosine Excretion as a Biomarker for Estimating DNA Oxidation in Patients Undergoing External Radiotherapy and/or Brachytherapy. Oncol. Rep. 2005, 13, 847–851. [Google Scholar] [CrossRef]
- AbuArrah, M.; Yuli Setianto, B.; Faisal, A.; Hamim Sadewa, A. 8-Hydroxy-2-Deoxyguanosine as Oxidative DNA Damage Biomarker of Medical Ionizing Radiation: A Scoping Review. J. Biomed. Phys. Eng. 2021, 11, 389–402. [Google Scholar] [CrossRef]
- Mrdjanovic, J.; Sudji, J.; Srdjenovic, B.; Dojcinovic, S.; Bogdanovic, V.; Jakovljevic, D.K.; Jurisic, V. Accidental Use of Milk With an Increased Concentration of Aflatoxins Causes Significant DNA Damage in Hospital Workers Exposed to Ionizing Radiation. Front. Public Health 2020, 8, 323. [Google Scholar] [CrossRef]
- Salehi, A.; Ebrahimpour, K.; Forouharmajd, F.; Zarean, M. The Relationship between Collective Effective Doses of Radiation and Urinary Concentration of 8-Dihydroxy-2′-Deoxyguanosine among Radiography Staff. Int. J. Radiat. Res. 2020, 18, 587–592. [Google Scholar]
- Kato, S.; Yoshimura, K.; Kimata, T.; Mine, K.; Uchiyama, T.; Kaneko, K. Urinary 8-Hydroxy-2′-Deoxyguanosine: A Biomarker for Radiation-Induced Oxidative DNA Damage in Pediatric Cardiac Catheterization. J. Pediatr. 2015, 167, 1369–1374.e1. [Google Scholar] [CrossRef]
- Bubici, C.; Papa, S.; Dean, K.; Franzoso, G. Mutual Cross-Talk between Reactive Oxygen Species and Nuclear Factor-Kappa B: Molecular Basis and Biological Significance. Oncogene 2006, 25, 6731–6748. [Google Scholar] [CrossRef]
- Crohns, M.; Saarelainen, S.; Erhola, M.; Alho, H.; Kellokumpu-Lehtinen, P. Impact of Radiotherapy and Chemotherapy on Biomarkers of Oxidative DNA Damage in Lung Cancer Patients. Clin. Biochem. 2009, 42, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Shi, D.; Lv, X.; Wang, B.; Chen, S.; Shao, Z. Prognostic Significance of 8-Hydroxy-2′-Deoxyguanosine in Solid Tumors: A Meta-Analysis. BMC Cancer 2019, 19, 997. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, X.; Wang, R.; Yu, J.; Ye, M.; Mao, L.; Zhang, S.; Zheng, S. Association between Oxidative DNA Damage and Risk of Colorectal Cancer: Sensitive Determination of Urinary 8-Hydroxy-2′-Deoxyguanosine by UPLC-MS/MS Analysis. Sci. Rep. 2016, 6, 32581. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.W.; Chou, S.Y.; Hu, T.W.; Wu, F.Y.; Chen, D.J. Urinary 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) and Genetic Polymorphisms in Breast Cancer Patients. Mutat. Res. 2007, 631, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Karihtala, P.; Soini, Y.; Vaskivuo, L.; Bloigu, R.; Puistola, U. DNA Adduct 8-Hydroxydeoxyguanosine, a Novel Putative Marker of Prognostic Significance in Ovarian Carcinoma. Int. J. Gynecol. Cancer 2009, 19, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Pylväs-Eerola, M.; Karihtala, P.; Puistola, U. Preoperative Serum 8-Hydroxydeoxyguanosine Is Associated with Chemoresistance and Is a Powerful Prognostic Factor in Endometrioid-Type Epithelial Ovarian Cancer. BMC Cancer 2015, 15, 493. [Google Scholar] [CrossRef][Green Version]
- Loft, S.; Vistisen, K.; Ewertz, M.; Tjønneland, A.; Overvad, K.; Poulsen, H.E. Oxidative DNA Damage Estimated by 8-Hydroxydeoxyguanosine Excretion in Humans: Influence of Smoking, Gender and Body Mass Index. Carcinogenesis 1992, 13, 2241–2247. [Google Scholar] [CrossRef]
- Akçay, T.; Saygili, I.; Andican, G.; Yalçin, V. Increased Formation of 8-Hydroxy-2′-Deoxyguanosine in Peripheral Blood Leukocytes in Bladder Cancer. Urol. Int. 2003, 71, 271–274. [Google Scholar] [CrossRef]
- Perdyan, A.; Jassem, J. Impact of Tobacco Smoking on Outcomes of Radiotherapy: A Narrative Review. Curr. Oncol. 2022, 29, 2284–2300. [Google Scholar] [CrossRef]
- Rades, D.; Setter, C.; Schild, S.E.; Dunst, J. Effect of Smoking during Radiotherapy, Respiratory Insufficiency, and Hemoglobin Levels on Outcome in Patients Irradiated for Non-Small-Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1134–1142. [Google Scholar] [CrossRef]
- Hernando, M.L.; Marks, L.B.; Bentel, G.C.; Zhou, S.M.; Hollis, D.; Das, S.K.; Fan, M.; Munley, M.T.; Shafman, T.D.; Anscher, M.S.; et al. Radiation-Induced Pulmonary Toxicity: A Dose-Volume Histogram Analysis in 201 Patients with Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, X.; Cao, Y.; Mao, Y.; Zhu, Y.; Zhang, Q.; Zhang, T.; Chang, L.; Wang, C. Dynamic Analysis of Predictive Biomarkers for Radiation Therapy Efficacy in Non-Small Cell Lung Cancer Patients by next-Generation Sequencing Based on Blood Specimens. Pathol. Res. Pract. 2023, 253, 154972. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, S.; Pang, J.; Yin, J.C.; Mu, D.; Wang, J.; Ge, H.; Ma, J.; Yang, Z.; Zheng, X.; et al. Genomic Profiling Reveals Novel Predictive Biomarkers for Chemo-Radiotherapy Efficacy and Thoracic Toxicity in Non-Small-Cell Lung Cancer. Front. Oncol. 2022, 12, 928605. [Google Scholar] [CrossRef] [PubMed]
- Turnu, L.; Porro, B.; Alfieri, V.; Di Minno, A.; Russo, E.; Barbieri, S.; Bonomi, A.; Dello Russo, A.; Tondo, C.; D’Alessandra, Y.; et al. Does Fluoroscopy Induce DNA Oxidative Damage in Patients Undergoing Catheter Ablation? Antioxid. Redox Signal. 2018, 28, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Pirich, C.; Pilger, A.; Schwameis, E.; Germadnik, D.; Prüfert, U.; Havlik, E.; Lang, S.; Kvaternik, H.; Flores, J.A.; Angelberger, P.; et al. Radiation Synovectomy Using 165Dy Ferric-Hydroxide and Oxidative DNA Damage in Patients with Different Types of Arthritis. J. Nucl. Med. 2000, 41, 250–256. [Google Scholar]
- Graille, M.; Wild, P.; Sauvain, J.J.; Hemmendinger, M.; Canu, I.G.; Hopf, N.B. Urinary 8-OHdG as a Biomarker for Oxidative Stress: A Systematic Literature Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3743. [Google Scholar] [CrossRef]
- Kroese, L.J.; Scheffer, P.G. 8-Hydroxy-2′-Deoxyguanosine and Cardiovascular Disease: A Systematic Review. Curr. Atheroscler. Rep. 2014, 16, 452. [Google Scholar] [CrossRef]





| Characteristic | Value |
|---|---|
| Total number (N) of patients | 52 |
| Sex (N) | |
| Male | 37 |
| Female | 15 |
| Age (y) in average (range) | 66.8 (53–76) |
| Smoking n, (%) | 52 (100%) |
| Histology (N) | |
| Squamous (SQ) | 16 |
| Non-squamous (NSQ) | 36 |
| Stage (N) | |
| IIIA | 4 |
| IIIB | 17 |
| IIIC | 31 |
| Chemotherapy 1 (N) | |
| Before radiotherapy | 20 |
| Post radiotherapy | 11 |
| Pre- and Post-radiotherapy | 14 |
| Concurrently | 0 |
| No chemotherapy | 7 |
| Overall Response Rate, n (%) | |
| Squamous | 11 (68.8%) |
| Non-squamous | 21 (58.3%) |
| Body Surface Area (BSA) (m2), mean (range) | 1.80 (1.45–2.02) |
| Body Mass Index (BMI) (kg/m2), mean (range) | 26.76 (20.16–30.42) |
| Irradiated Tumor Volume (TV) (cm3), mean (range) | |
| Squamous (SQ) | 163.56 (30.5–376.8) |
| Non-squamous (NSQ) | 178.14 (18.1–811.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orfanakos, K.; Alifieris, C.E.; Verigos, E.K.; Deligiorgi, M.V.; Verigos, K.E.; Panayiotidis, M.I.; Nikolaou, M.; Trafalis, D.T. The Predictive Value of 8-Hydroxy-Deoxyguanosine (8-OHdG) Serum Concentrations in Irradiated Non-Small Cell Lung Carcinoma (NSCLC) Patients. Biomedicines 2024, 12, 134. https://doi.org/10.3390/biomedicines12010134
Orfanakos K, Alifieris CE, Verigos EK, Deligiorgi MV, Verigos KE, Panayiotidis MI, Nikolaou M, Trafalis DT. The Predictive Value of 8-Hydroxy-Deoxyguanosine (8-OHdG) Serum Concentrations in Irradiated Non-Small Cell Lung Carcinoma (NSCLC) Patients. Biomedicines. 2024; 12(1):134. https://doi.org/10.3390/biomedicines12010134
Chicago/Turabian StyleOrfanakos, Kyriakos, Constantinos E. Alifieris, Emmanouil K. Verigos, Maria V. Deligiorgi, Kosmas E. Verigos, Mihalis I. Panayiotidis, Michail Nikolaou, and Dimitrios T. Trafalis. 2024. "The Predictive Value of 8-Hydroxy-Deoxyguanosine (8-OHdG) Serum Concentrations in Irradiated Non-Small Cell Lung Carcinoma (NSCLC) Patients" Biomedicines 12, no. 1: 134. https://doi.org/10.3390/biomedicines12010134
APA StyleOrfanakos, K., Alifieris, C. E., Verigos, E. K., Deligiorgi, M. V., Verigos, K. E., Panayiotidis, M. I., Nikolaou, M., & Trafalis, D. T. (2024). The Predictive Value of 8-Hydroxy-Deoxyguanosine (8-OHdG) Serum Concentrations in Irradiated Non-Small Cell Lung Carcinoma (NSCLC) Patients. Biomedicines, 12(1), 134. https://doi.org/10.3390/biomedicines12010134








