Breaking through Multiple Myeloma: A Paradigm for a Comprehensive Tumor Ecosystem Targeting
Abstract
1. Introduction
Overview on Multiple Myeloma Impact
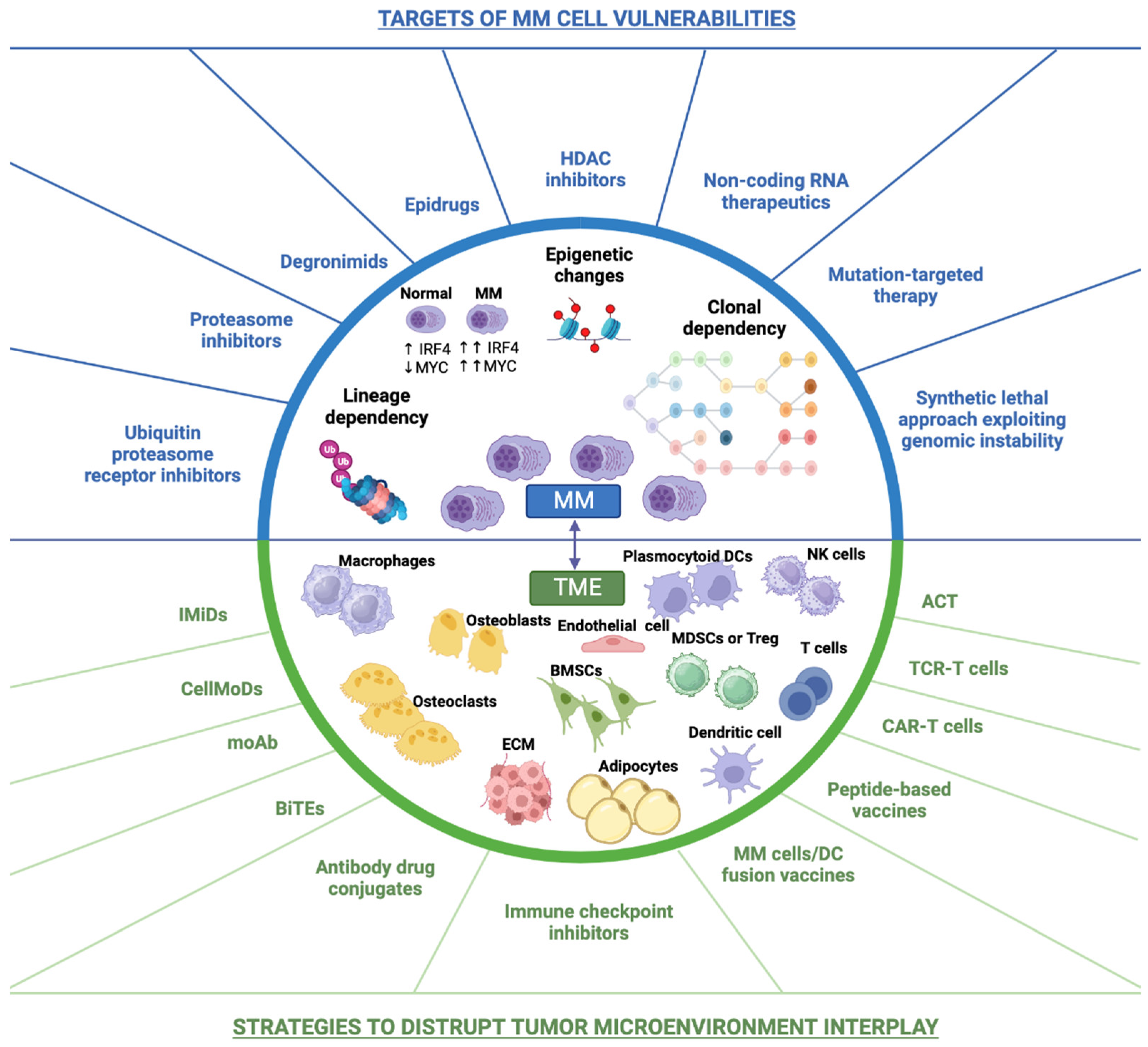
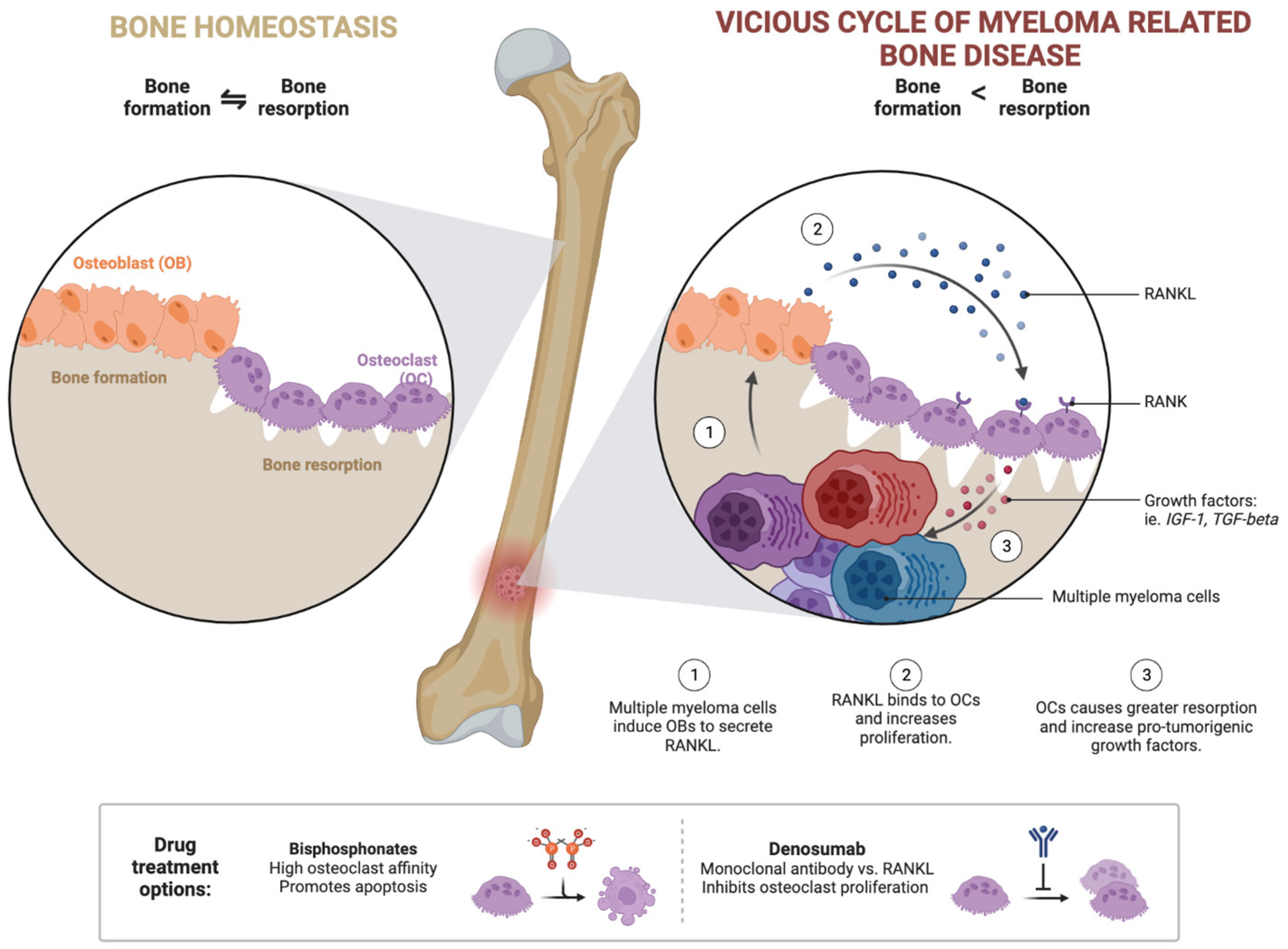
2. New Insights into the Biology of the Disease
3. Risk Stratification
4. Modern Therapeutic Approach to Multiple Myeloma
4.1. Newly Diagnosed MM: Autologous Transplant Candidate
4.2. Newly Diagnosed MM Not Eligible for Autologous Transplant
4.3. Therapeutic Approach to the Patient with Relapsed/Refractory Myeloma
4.4. Therapeutic Options for Patients Refractory to Immunomodulators, Proteasome Inhibitors and Anti-CD38 Monoclonal Antibodies
5. Current Status and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kristinsson, S.Y.; Landgren, O.; Dickman, P.W.; Derolf, Å.R.; Björkholm, M. Patterns of Survival in Multiple Myeloma: A Population-Based Study of Patients Diagnosed in Sweden from 1973 to 2003. J. Clin. Oncol. 2007, 25, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Anderson, K. Multiple Myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef]
- Leuraud, K.; Richardson, D.B.; Cardis, E.; Daniels, R.D.; Gillies, M.; O’Hagan, J.A.; Hamra, G.B.; Haylock, R.; Laurier, D.; Moissonnier, M.; et al. Ionising Radiation and Risk of Death from Leukaemia and Lymphoma in Radiation-Monitored Workers (INWORKS): An International Cohort Study. Lancet Haematol. 2015, 2, e276–e281. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Mitsiades, C.; Tonon, G.; Richardson, P.G.; Anderson, K.C. Understanding Multiple Myeloma Pathogenesis in the Bone Marrow to Identify New Therapeutic Targets. Nat. Rev. Cancer 2007, 7, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, W.M.; Bergsagel, P.L. Multiple Myeloma: Evolving Genetic Events and Host Interactions. Nat. Rev. Cancer 2002, 2, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Bergsagel, P.L.; Kuehl, W.M. Molecular Pathogenesis and a Consequent Classification of Multiple Myeloma. J. Clin. Oncol. 2005, 23, 6333–6338. [Google Scholar] [CrossRef]
- Da Vià, M.C.; Solimando, A.G.; Garitano-Trojaola, A.; Barrio, S.; Munawar, U.; Strifler, S.; Haertle, L.; Rhodes, N.; Teufel, E.; Vogt, C.; et al. CIC Mutation as a Molecular Mechanism of Acquired Resistance to Combined BRAF-MEK Inhibition in Extramedullary Multiple Myeloma with Central Nervous System Involvement. Oncologist 2019, 25, 112–118. [Google Scholar] [CrossRef]
- Seibold, M.; Stühmer, T.; Kremer, N.; Mottok, A.; Scholz, C.-J.; Schlosser, A.; Leich, E.; Holzgrabe, U.; Brünnert, D.; Barrio, S.; et al. RAL GTPases Mediate Multiple Myeloma Cell Survival and Are Activated Independently of Oncogenic RAS. Haematologica 2019, 105, 2316–2326. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Attal, M.; Moreau, P.; Charbonnel, C.; Garban, F.; Hulin, C.; Leyvraz, S.; Michallet, M.; Yakoub-Agha, I.; Garderet, L.; et al. Genetic Abnormalities and Survival in Multiple Myeloma: The Experience of the Intergroupe Francophone Du Myélome. Blood 2007, 109, 3489–3495. [Google Scholar] [CrossRef]
- Solimando, A.G.; Da Vià, M.C.; Cicco, S.; Leone, P.; Di Lernia, G.; Giannico, D.; Desantis, V.; Frassanito, M.A.; Morizio, A.; Delgado Tascon, J.; et al. High-Risk Multiple Myeloma: Integrated Clinical and Omics Approach Dissects the Neoplastic Clone and the Tumor Microenvironment. J. Clin. Med. 2019, 8, 997. [Google Scholar] [CrossRef]
- Solimando, A.G.; Krebs, M.; Bittrich, M.; Einsele, H. The Urgent Need for Precision Medicine in Cancer and Its Microenvironment: The Paradigmatic Case of Multiple Myeloma. J. Clin. Med. 2022, 11, 5461. [Google Scholar] [CrossRef]
- Bahlis, N.J. Darwinian Evolution and Tiding Clones in Multiple Myeloma. Blood 2012, 120, 927–928. [Google Scholar] [CrossRef] [PubMed]
- Desantis, V.; Frassanito, M.A.; Tamma, R.; Saltarella, I.; Di Marzo, L.; Lamanuzzi, A.; Solimando, A.G.; Ruggieri, S.; Annese, T.; Nico, B.; et al. Rhu-Epo down-Regulates pro-Tumorigenic Activity of Cancer-Associated Fibroblasts in Multiple Myeloma. Ann. Hematol. 2018, 97, 1251–1258. [Google Scholar] [CrossRef]
- Zavidij, O.; Haradhvala, N.J.; Mouhieddine, T.H.; Sklavenitis-Pistofidis, R.; Cai, S.; Reidy, M.; Rahmat, M.; Flaifel, A.; Ferland, B.; Su, N.K.; et al. Single-Cell RNA Sequencing Reveals Compromised Immune Microenvironment in Precursor Stages of Multiple Myeloma. Nat. Cancer 2020, 1, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Solimando, A.G.; Brandl, A.; Mattenheimer, K.; Graf, C.; Ritz, M.; Ruckdeschel, A.; Stühmer, T.; Mokhtari, Z.; Rudelius, M.; Dotterweich, J.; et al. JAM-A as a Prognostic Factor and New Therapeutic Target in Multiple Myeloma. Leukemia 2018, 32, 736–743. [Google Scholar] [CrossRef]
- Solimando, A.G.; Da Vià, M.C.; Leone, P.; Borrelli, P.; Croci, G.A.; Tabares, P.; Brandl, A.; Di Lernia, G.; Bianchi, F.P.; Tafuri, S.; et al. Halting the Vicious Cycle within the Multiple Myeloma Ecosystem: Blocking JAM-A on Bone Marrow Endothelial Cells Restores the Angiogenic Homeostasis and Suppresses Tumor Progression. Haematologica 2020, 106, 1943–1956. [Google Scholar] [CrossRef]
- Argentiero, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Pantano, F.; Iuliani, M.; Santini, D.; Silvestris, N.; Vacca, A. Skeletal Metastases of Unknown Primary: Biological Landscape and Clinical Overview. Cancers 2019, 11, 1270. [Google Scholar] [CrossRef]
- Solimando, A.G.; Melaccio, A.; Vacca, A.; Ria, R. The Bone Marrow Niche Landscape: A Journey through Aging, Extrinsic and Intrinsic Stressors in the Haemopoietic Milieu. J. Cancer Metastasis Treat. 2022, 8, 9. [Google Scholar] [CrossRef]
- Leich, E.; Weißbach, S.; Klein, H.-U.; Grieb, T.; Pischimarov, J.; Stühmer, T.; Chatterjee, M.; Steinbrunn, T.; Langer, C.; Eilers, M.; et al. Multiple Myeloma Is Affected by Multiple and Heterogeneous Somatic Mutations in Adhesion- and Receptor Tyrosine Kinase Signaling Molecules. Blood Cancer J. 2013, 3, e102. [Google Scholar] [CrossRef]
- Antonio, G.; Oronzo, B.; Vito, L.; Angela, C.; Antonel-la, A.; Roberto, C.; Giovanni, S.A.; Antonella, L. Immune System and Bone Microenvironment: Rationale for Targeted Cancer Therapies. Oncotarget 2020, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Lamanuzzi, A.; Saltarella, I.; Desantis, V.; Frassanito, M.A.; Leone, P.; Racanelli, V.; Nico, B.; Ribatti, D.; Ditonno, P.; Prete, M.; et al. Inhibition of MTOR Complex 2 Restrains Tumor Angiogenesis in Multiple Myeloma. Oncotarget 2018, 9, 20563–20577. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Di Lernia, G.; Solimando, A.G.; Cicco, S.; Saltarella, I.; Lamanuzzi, A.; Ria, R.; Frassanito, M.A.; Ponzoni, M.; Ditonno, P.; et al. Bone Marrow Endothelial Cells Sustain a Tumor-Specific CD8+ T Cell Subset with Suppressive Function in Myeloma Patients. Oncoimmunology 2019, 8, e1486949. [Google Scholar] [CrossRef]
- Roodman, G.D. Pathogenesis of Myeloma Bone Disease. Leukemia 2009, 23, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Javadrashid, D.; Baghbanzadeh, A.; Derakhshani, A.; Leone, P.; Silvestris, N.; Racanelli, V.; Solimando, A.G.; Baradaran, B. Pancreatic Cancer Signaling Pathways, Genetic Alterations, and Tumor Microenvironment: The Barriers Affecting the Method of Treatment. Biomedicines 2021, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Solimando, A.G.; Annese, T.; Tamma, R.; Ingravallo, G.; Maiorano, E.; Vacca, A.; Specchia, G.; Ribatti, D. New Insights into Diffuse Large B-Cell Lymphoma Pathobiology. Cancers 2020, 12, 1869. [Google Scholar] [CrossRef]
- Leone, P.; Solimando, A.G.; Malerba, E.; Fasano, R.; Buonavoglia, A.; Pappagallo, F.; De Re, V.; Argentiero, A.; Silvestris, N.; Vacca, A.; et al. Actors on the Scene: Immune Cells in the Myeloma Niche. Front. Oncol. 2020, 10, 599098. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Giannico, D.; Leone, P.; Solimando, A.G.; Maiorano, E.; Caporusso, C.; Duda, L.; Tamma, R.; Mallamaci, R.; Susca, N.; et al. HB-EGF-EGFR Signaling in Bone Marrow Endothelial Cells Mediates Angiogenesis Associated with Multiple Myeloma. Cancers 2020, 12, 173. [Google Scholar] [CrossRef]
- De Jong, M.M.E.; Kellermayer, Z.; Papazian, N.; Tahri, S.; Hofste op Bruinink, D.; Hoogenboezem, R.; Sanders, M.A.; van de Woestijne, P.C.; Bos, P.K.; Khandanpour, C.; et al. The Multiple Myeloma Microenvironment Is Defined by an Inflammatory Stromal Cell Landscape. Nat. Immunol. 2021, 22, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Vacca, A.; Melaccio, A.; Sportelli, A.; Solimando, A.G.; Dammacco, F.; Ria, R. Subcutaneous Immunoglobulins in Patients with Multiple Myeloma and Secondary Hypogammaglobulinemia: A Randomized Trial. Clin. Immunol. 2018, 191, 110–115. [Google Scholar] [CrossRef]
- Solimando, A.G.; Malerba, E.; Leone, P.; Prete, M.; Terragna, C.; Cavo, M.; Racanelli, V. Drug Resistance in Multiple Myeloma: Soldiers and Weapons in the Bone Marrow Niche. Front. Oncol. 2022, 12, 973836. [Google Scholar] [CrossRef]
- Cavo, M.; Gay, F.; Beksac, M.; Pantani, L.; Petrucci, M.T.; Dimopoulos, M.A.; Dozza, L.; van der Holt, B.; Zweegman, S.; Oliva, S.; et al. Autologous Haematopoietic Stem-Cell Transplantation versus Bortezomib–Melphalan–Prednisone, with or without Bortezomib–Lenalidomide–Dexamethasone Consolidation Therapy, and Lenalidomide Maintenance for Newly Diagnosed Multiple Myeloma (EMN02/HO95): A Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Haematol. 2020, 7, e456–e468. [Google Scholar] [CrossRef] [PubMed]
- Ria, R.; Reale, A.; Solimando, A.G.; Mangialardi, G.; Moschetta, M.; Gelao, L.; Iodice, G.; Vacca, A. Induction Therapy and Stem Cell Mobilization in Patients with Newly Diagnosed Multiple Myeloma. Stem Cells Int. 2012, 2012, 607260. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal Gammopathy of Undetermined Significance (MGUS) Consistently Precedes Multiple Myeloma: A Prospective Study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.M.; Abadie, J.; Verma, P.; Howard, R.S.; Kuehl, W.M. A Monoclonal Gammopathy Precedes Multiple Myeloma in Most Patients. Blood 2009, 113, 5418–5422. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Blimark, C.H.; Turesson, I.; Genell, A.; Ahlberg, L.; Björkstrand, B.; Carlson, K.; Forsberg, K.; Juliusson, G.; Linder, O.; Mellqvist, U.-H.; et al. Outcome and Survival of Myeloma Patients Diagnosed 2008-2015. Real-World Data on 4904 Patients from the Swedish Myeloma Registry. Haematologica 2018, 103, 506–513. [Google Scholar] [CrossRef]
- Terpos, E.; Dimopoulos, M.-A. Myeloma Bone Disease: Pathophysiology and Management. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2005, 16, 1223–1231. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Larson, D.; Kyle, R.A. Diagnosis of Smoldering Multiple Myeloma. N. Engl. J. Med. 2011, 365, 474–475. [Google Scholar] [CrossRef]
- Kastritis, E.; Terpos, E.; Moulopoulos, L.; Spyropoulou-Vlachou, M.; Kanellias, N.; Eleftherakis-Papaiakovou, E.; Gkotzamanidou, M.; Migkou, M.; Gavriatopoulou, M.; Roussou, M.; et al. Extensive Bone Marrow Infiltration and Abnormal Free Light Chain Ratio Identifies Patients with Asymptomatic Myeloma at High Risk for Progression to Symptomatic Disease. Leukemia 2013, 27, 947–953. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Kyle, R.A.; Katzmann, J.A.; Therneau, T.M.; Larson, D.; Benson, J.; Clark, R.J.; Melton, L.J.; Gertz, M.A.; Kumar, S.K.; et al. Immunoglobulin Free Light Chain Ratio Is an Independent Risk Factor for Progression of Smoldering (Asymptomatic) Multiple Myeloma. Blood 2008, 111, 785–789. [Google Scholar] [CrossRef]
- Larsen, J.T.; Kumar, S.K.; Dispenzieri, A.; Kyle, R.A.; Katzmann, J.A.; Rajkumar, S.V. Serum Free Light Chain Ratio as a Biomarker for High-Risk Smoldering Multiple Myeloma. Leukemia 2013, 27, 941–946. [Google Scholar] [CrossRef]
- Hillengass, J.; Fechtner, K.; Weber, M.-A.; Bäuerle, T.; Ayyaz, S.; Heiss, C.; Hielscher, T.; Moehler, T.M.; Egerer, G.; Neben, K.; et al. Prognostic Significance of Focal Lesions in Whole-Body Magnetic Resonance Imaging in Patients With Asymptomatic Multiple Myeloma. J. Clin. Oncol. 2010, 28, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Moulopoulos, L.A.; Terpos, E.; Koutoulidis, V.; Dimopoulos, M.A. The Prognostic Importance of the Presence of More than One Focal Lesion in Spine MRI of Patients with Asymptomatic (Smoldering) Multiple Myeloma. Leukemia 2014, 28, 2402–2403. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Rajkumar, S.V. Criteria for Diagnosis, Staging, Risk Stratification and Response Assessment of Multiple Myeloma. Leukemia 2009, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 Patients With Newly Diagnosed Multiple Myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Kyle, R.; Merlini, G.; Miguel, J.S.; Ludwig, H.; Hajek, R.; Palumbo, A.; Jagannath, S.; Blade, J.; Lonial, S.; et al. International Myeloma Working Group Guidelines for Serum-Free Light Chain Analysis in Multiple Myeloma and Related Disorders. Leukemia 2009, 23, 215–224. [Google Scholar] [CrossRef]
- Avet-Loiseau, H. Role of Genetics in Prognostication in Myeloma. Best Pract. Res. Clin. Haematol. 2007, 20, 625–635. [Google Scholar] [CrossRef]
- Anderson, K.C.; Alsina, M.; Atanackovic, D.; Biermann, J.S.; Chandler, J.C.; Costello, C.; Djulbegovic, B.; Fung, H.C.; Gasparetto, C.; Godby, K.; et al. NCCN Guidelines Insights: Multiple Myeloma, Version 3.2016. J. Natl. Compr. Cancer Netw. 2016, 14, 389–400. [Google Scholar] [CrossRef]
- Terpos, E.; Moulopoulos, L.A.; Dimopoulos, M.A. Advances in Imaging and the Management of Myeloma Bone Disease. J. Clin. Oncol. 2011, 29, 1907–1915. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Terpos, E.; Comenzo, R.L.; Tosi, P.; Beksac, M.; Sezer, O.; Siegel, D.; Lokhorst, H.; Kumar, S.; Rajkumar, S.V.; et al. International Myeloma Working Group Consensus Statement and Guidelines Regarding the Current Role of Imaging Techniques in the Diagnosis and Monitoring of Multiple Myeloma. Leukemia 2009, 23, 1545–1556. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Hillengass, J.; Usmani, S.; Zamagni, E.; Lentzsch, S.; Davies, F.E.; Raje, N.; Sezer, O.; Zweegman, S.; Shah, J.; et al. Role of Magnetic Resonance Imaging in the Management of Patients With Multiple Myeloma: A Consensus Statement. J. Clin. Oncol. 2015, 33, 657–664. [Google Scholar] [CrossRef]
- Song, M.-K.; Chung, J.-S.; Lee, J.-J.; Min, C.-K.; Ahn, J.-S.; Lee, S.-M.; Shin, D.-Y.; Bae, S.-H.; Hong, J.; Lee, G.; et al. Magnetic Resonance Imaging Pattern of Bone Marrow Involvement as a New Predictive Parameter of Disease Progression in Newly Diagnosed Patients with Multiple Myeloma Eligible for Autologous Stem Cell Transplantation. Br. J. Haematol. 2014, 165, 777–785. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Mitchell, A.; Waheed, S.; Crowley, J.; Hoering, A.; Petty, N.; Brown, T.; Bartel, T.; Anaissie, E.; van Rhee, F.; et al. Prognostic Implications of Serial 18-Fluoro-Deoxyglucose Emission Tomography in Multiple Myeloma Treated with Total Therapy 3. Blood 2013, 121, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Tirumani, S.H.; Sakellis, C.; Jacene, H.; Shinagare, A.B.; Munshi, N.C.; Ramaiya, N.H.; Van den Abbeele, A.D. Role of FDG-PET/CT in Extramedullary Multiple Myeloma: Correlation of FDG-PET/CT Findings With Clinical Outcome. Clin. Nucl. Med. 2016, 41, e7–e13. [Google Scholar] [CrossRef] [PubMed]
- Siontis, B.; Kumar, S.; Dispenzieri, A.; Drake, M.T.; Lacy, M.Q.; Buadi, F.; Dingli, D.; Kapoor, P.; Gonsalves, W.; Gertz, M.A.; et al. Positron Emission Tomography-Computed Tomography in the Diagnostic Evaluation of Smoldering Multiple Myeloma: Identification of Patients Needing Therapy. Blood Cancer J. 2015, 5, e364. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, E.; Nanni, C.; Gay, F.; Pezzi, A.; Patriarca, F.; Bellò, M.; Rambaldi, I.; Tacchetti, P.; Hillengass, J.; Gamberi, B.; et al. 18F-FDG PET/CT Focal, but Not Osteolytic, Lesions Predict the Progression of Smoldering Myeloma to Active Disease. Leukemia 2016, 30, 417–422. [Google Scholar] [CrossRef]
- Zamagni, E.; Patriarca, F.; Nanni, C.; Zannetti, B.; Englaro, E.; Pezzi, A.; Tacchetti, P.; Buttignol, S.; Perrone, G.; Brioli, A.; et al. Prognostic Relevance of 18-F FDG PET/CT in Newly Diagnosed Multiple Myeloma Patients Treated with up-Front Autologous Transplantation. Blood 2011, 118, 5989–5995. [Google Scholar] [CrossRef]
- Zamagni, E.; Nanni, C.; Patriarca, F.; Englaro, E.; Castellucci, P.; Geatti, O.; Tosi, P.; Tacchetti, P.; Cangini, D.; Perrone, G.; et al. A Prospective Comparison of 18F-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography, Magnetic Resonance Imaging and Whole-Body Planar Radiographs in the Assessment of Bone Disease in Newly Diagnosed Multiple Myeloma. Haematologica 2007, 92, 50–55. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Nanni, C.; Versari, A.; Chauvie, S.; Bertone, E.; Bianchi, A.; Rensi, M.; Bellò, M.; Gallamini, A.; Patriarca, F.; Gay, F.; et al. Interpretation Criteria for FDG PET/CT in Multiple Myeloma (IMPeTUs): Final Results. IMPeTUs (Italian Myeloma Criteria for PET USe). Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Durie, B.G.M.; Salmon, S.E. A Clinical Staging System for Multiple Myeloma Correlation of Measured Myeloma Cell Mass with Presenting Clinical Features, Response to Treatment, and Survival. Cancer 1975, 36, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.M.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Cairns, D.A.; Lahuerta, J.J.; Wester, R.; Bertsch, U.; Waage, A.; Zamagni, E.; Mateos, M.-V.; Dall’Olio, D.; van de Donk, N.W.C.J.; et al. Second Revision of the International Staging System (R2-ISS) for Overall Survival in Multiple Myeloma: A European Myeloma Network (EMN) Report Within the HARMONY Project. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 3406–3418. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Gertz, M.A. Clinical Implications of t(11;14)(Q13;Q32), t(4;14)(P16.3;Q32), and -17p13 in Myeloma Patients Treated with High-Dose Therapy. Blood 2005, 106, 2837–2840. [Google Scholar] [CrossRef]
- Zhou, Y.; Barlogie, B.; Shaughnessy, J.D. The Molecular Characterization and Clinical Management of Multiple Myeloma in the Post-Genome Era. Leukemia 2009, 23, 1941–1956. [Google Scholar] [CrossRef]
- Walker, B.A.; Mavrommatis, K.; Wardell, C.P.; Ashby, T.C.; Bauer, M.; Davies, F.; Rosenthal, A.; Wang, H.; Qu, P.; Hoering, A.; et al. A High-Risk, Double-Hit, Group of Newly Diagnosed Myeloma Identified by Genomic Analysis. Leukemia 2019, 33, 159–170. [Google Scholar] [CrossRef]
- Walker, B.A.; Wardell, C.P.; Chiecchio, L.; Smith, E.M.; Boyd, K.D.; Neri, A.; Davies, F.E.; Ross, F.M.; Morgan, G.J. Aberrant Global Methylation Patterns Affect the Molecular Pathogenesis and Prognosis of Multiple Myeloma. Blood 2011, 117, 553–562. [Google Scholar] [CrossRef]
- Engelhardt, M.; Dold, S.M.; Ihorst, G.; Zober, A.; Moller, M.; Reinhardt, H.; Hieke, S.; Schumacher, M.; Wasch, R. Geriatric Assessment in Multiple Myeloma Patients: Validation of the International Myeloma Working Group (IMWG) Score and Comparison with Other Common Comorbidity Scores. Haematologica 2016, 101, 1110–1119. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Mateos, M.-V.; Larocca, A.; Facon, T.; Kumar, S.K.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric Assessment Predicts Survival and Toxicities in Elderly Myeloma Patients: An International Myeloma Working Group Report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef]
- Mina, R.; Lonial, S. Is There Still a Role for Stem Cell Transplantation in Multiple Myeloma? Cancer 2019, 125, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Mina, R.; Musto, P.; Rota-Scalabrini, D.; Paris, L.; Gamberi, B.; Palmas, A.; Aquino, S.; de Fabritiis, P.; Giuliani, N.; De Rosa, L.; et al. Carfilzomib Induction, Consolidation, and Maintenance with or without Autologous Stem-Cell Transplantation in Patients with Newly Diagnosed Multiple Myeloma: Pre-Planned Cytogenetic Subgroup Analysis of the Randomised, Phase 2 FORTE Trial. Lancet Oncol. 2023, 24, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.H.; Al Hamed, R.; Malard, F.; Bazarbachi, A.; Harousseau, J.-L.; Mohty, M. Induction Therapy Prior to Autologous Stem Cell Transplantation (ASCT) in Newly Diagnosed Multiple Myeloma: An Update. Blood Cancer J. 2022, 12, 47. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, Thalidomide, and Dexamethasone with or without Daratumumab before and after Autologous Stem-Cell Transplantation for Newly Diagnosed Multiple Myeloma (CASSIOPEIA): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D.; et al. Daratumumab, Lenalidomide, Bortezomib, and Dexamethasone for Transplant-Eligible Newly Diagnosed Multiple Myeloma: The GRIFFIN Trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef]
- Roussel, M.; Moreau, P.; Hebraud, B.; Laribi, K.; Jaccard, A.; Dib, M.; Slama, B.; Dorvaux, V.; Royer, B.; Frenzel, L.; et al. Bortezomib, Thalidomide, and Dexamethasone with or without Daratumumab for Transplantation-Eligible Patients with Newly Diagnosed Multiple Myeloma (CASSIOPEIA): Health-Related Quality of Life Outcomes of a Randomised, Open-Label, Phase 3 Trial. Lancet Haematol. 2020, 7, e874–e883. [Google Scholar] [CrossRef] [PubMed]
- Harousseau, J.L.; Mohty, M. Daratumumab in Transplant Regimens for Myeloma? Blood 2020, 136, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Afifi, S.; Adel, N.G.; Devlin, S.; Duck, E.; Vanak, J.; Landau, H.; Chung, D.J.; Lendvai, N.; Lesokhin, A.; Korde, N.; et al. Upfront Plerixafor plus G-CSF versus Cyclophosphamide plus G-CSF for Stem Cell Mobilization in Multiple Myeloma: Efficacy and Cost Analysis Study. Bone Marrow Transplant. 2016, 51, 546–552. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Facon, T. Frontline Therapy of Multiple Myeloma. Blood 2015, 125, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Schmidt-Wolf, I.G.H.; van der Holt, B.; el Jarari, L.; Bertsch, U.; Salwender, H.; Zweegman, S.; Vellenga, E.; Broyl, A.; Blau, I.W.; et al. Bortezomib Induction and Maintenance Treatment in Patients With Newly Diagnosed Multiple Myeloma: Results of the Randomized Phase III HOVON-65/GMMG-HD4 Trial. J. Clin. Oncol. 2012, 30, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Stadtmauer, E.A.; Pasquini, M.C.; Blackwell, B.; Hari, P.; Bashey, A.; Devine, S.; Efebera, Y.; Ganguly, S.; Gasparetto, C.; Geller, N.; et al. Autologous Transplantation, Consolidation, and Maintenance Therapy in Multiple Myeloma: Results of the BMT CTN 0702 Trial. J. Clin. Oncol. 2019, 37, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Goldschmidt, H.; Rosinol, L.; Pantani, L.; Zweegman, S.; Salwender, H.J.; Lahuerta, J.J.; Lokhorst, H.M.; Petrucci, M.T.; Blau, I.; et al. Double Vs Single Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma: Long-Term Follow-up (10-Years) Analysis of Randomized Phase 3 Studies. Blood 2018, 132, 124. [Google Scholar] [CrossRef]
- Cavo, M.; Beksac, M.; Dimopoulos, M.A.; Pantani, L.; Gay, F.; Hájek, R.; Testoni, N.; Mellqvist, U.-H.; Patriarca, F.; Montefusco, V.; et al. Intensification Therapy with Bortezomib-Melphalan-Prednisone Versus Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma: An Intergroup, Multicenter, Phase III Study of the European Myeloma Network (EMN02/HO95 MM Trial). Blood 2016, 128, 673. [Google Scholar] [CrossRef]
- Cavo, M.; Pantani, L.; Petrucci, M.T.; Patriarca, F.; Zamagni, E.; Donnarumma, D.; Crippa, C.; Boccadoro, M.; Perrone, G.; Falcone, A.; et al. Bortezomib-Thalidomide-Dexamethasone Is Superior to Thalidomide-Dexamethasone as Consolidation Therapy after Autologous Hematopoietic Stem Cell Transplantation in Patients with Newly Diagnosed Multiple Myeloma. Blood 2012, 120, 9–19. [Google Scholar] [CrossRef]
- Cavo, M.; Tacchetti, P.; Patriarca, F.; Petrucci, M.T.; Pantani, L.; Galli, M.; Di Raimondo, F.; Crippa, C.; Zamagni, E.; Palumbo, A.; et al. Bortezomib with Thalidomide plus Dexamethasone Compared with Thalidomide plus Dexamethasone as Induction Therapy before, and Consolidation Therapy after, Double Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Randomised Phase 3 Study. Lancet 2010, 376, 2075–2085. [Google Scholar] [CrossRef]
- Gay, F.; Cerrato, C.; Petrucci, M.T.; Zambello, R.; Gamberi, B.; Ballanti, S.; Omedè, P.; Palmieri, S.; Troia, R.; Spada, S.; et al. Efficacy of Carfilzomib Lenalidomide Dexamethasone (KRd) with or without Transplantation in Newly Diagnosed Myeloma According to Risk Status: Results from the FORTE Trial. J. Clin. Oncol. 2019, 37, 8002. [Google Scholar] [CrossRef]
- Barlogie, B.; van Rhee, F.; Shaughnessy, J.D.; Epstein, J.; Yaccoby, S.; Pineda-Roman, M.; Hollmig, K.; Alsayed, Y.; Hoering, A.; Szymonifka, J.; et al. Seven-Year Median Time to Progression with Thalidomide for Smoldering Myeloma: Partial Response Identifies Subset Requiring Earlier Salvage Therapy for Symptomatic Disease. Blood 2008, 112, 3122–3125. [Google Scholar] [CrossRef]
- Palumbo, A.; Cavallo, F.; Gay, F.; Di Raimondo, F.; Ben Yehuda, D.; Petrucci, M.T.; Pezzatti, S.; Caravita, T.; Cerrato, C.; Ribakovsky, E.; et al. Autologous Transplantation and Maintenance Therapy in Multiple Myeloma. N. Engl. J. Med. 2014, 371, 895–905. [Google Scholar] [CrossRef]
- Gay, F.; Oliva, S.; Petrucci, M.T.; Conticello, C.; Catalano, L.; Corradini, P.; Siniscalchi, A.; Magarotto, V.; Pour, L.; Carella, A.; et al. Chemotherapy plus Lenalidomide versus Autologous Transplantation, Followed by Lenalidomide plus Prednisone versus Lenalidomide Maintenance, in Patients with Multiple Myeloma: A Randomised, Multicentre, Phase 3 Trial. Lancet Oncol. 2015, 16, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Lauwers-Cances, V.; Marit, G.; Caillot, D.; Moreau, P.; Facon, T.; Stoppa, A.M.; Hulin, C.; Benboubker, L.; Garderet, L.; et al. Lenalidomide Maintenance after Stem-Cell Transplantation for Multiple Myeloma. N. Engl. J. Med. 2012, 366, 1782–1791. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Owzar, K.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Richardson, P.G.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Lenalidomide after Stem-Cell Transplantation for Multiple Myeloma. N. Engl. J. Med. 2012, 366, 1770–1781. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Gay, F.; Musto, P.; Rota Scalabrini, D.; Galli, M.; Belotti, A.; Zamagni, E.; Bertamini, L.; Zambello, R.; Quaresima, M.; De Sabbata, G.; et al. Survival Analysis of Newly Diagnosed Transplant-Eligible Multiple Myeloma Patients in the Randomized Forte Trial. Blood 2020, 136, 35–37. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Gay, F.; Schjesvold, F.; Beksac, M.; Hajek, R.; Weisel, K.C.; Goldschmidt, H.; Maisnar, V.; Moreau, P.; Min, C.K.; et al. Oral Ixazomib Maintenance Following Autologous Stem Cell Transplantation (TOURMALINE-MM3): A Double-Blind, Randomised, Placebo-Controlled Phase 3 Trial. Lancet 2019, 393, 253–264. [Google Scholar] [CrossRef]
- Moreau, P.; Hulin, C.; Perrot, A.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Zweegman, S.; Caillon, H.; Caillot, D.; et al. Maintenance with Daratumumab or Observation Following Treatment with Bortezomib, Thalidomide, and Dexamethasone with or without Daratumumab and Autologous Stem-Cell Transplant in Patients with Newly Diagnosed Multiple Myeloma (CASSIOPEIA): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 1378–1390. [Google Scholar] [CrossRef]
- Bruno, B.; Rotta, M.; Patriarca, F.; Mordini, N.; Allione, B.; Carnevale-Schianca, F.; Giaccone, L.; Sorasio, R.; Omedè, P.; Baldi, I.; et al. A Comparison of Allografting with Autografting for Newly Diagnosed Myeloma. N. Engl. J. Med. 2007, 356, 1110–1120. [Google Scholar] [CrossRef]
- Giaccone, L.; Storer, B.; Patriarca, F.; Rotta, M.; Sorasio, R.; Allione, B.; Carnevale-Schianca, F.; Festuccia, M.; Brunello, L.; Omedè, P.; et al. Long-Term Follow-up of a Comparison of Nonmyeloablative Allografting with Autografting for Newly Diagnosed Myeloma. Blood 2011, 117, 6721–6727. [Google Scholar] [CrossRef]
- Gay, F.; Engelhardt, M.; Terpos, E.; Wäsch, R.; Giaccone, L.; Auner, H.W.; Caers, J.; Gramatzki, M.; van de Donk, N.; Oliva, S.; et al. From Transplant to Novel Cellular Therapies in Multiple Myeloma: European Myeloma Network Guidelines and Future Perspectives. Haematologica 2018, 103, 197–211. [Google Scholar] [CrossRef]
- Larocca, A.; Bonello, F.; Gaidano, G.; D’Agostino, M.; Offidani, M.; Cascavilla, N.; Capra, A.; Benevolo, G.; Tosi, P.; Galli, M.; et al. Dose/Schedule-Adjusted Rd-R vs Continuous Rd for Elderly, Intermediate-Fit Patients with Newly Diagnosed Multiple Myeloma. Blood 2021, 137, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Liberati, A.M.; Caravita, T.; Falcone, A.; Callea, V.; Montanaro, M.; Ria, R.; Capaldi, A.; Zambello, R.; et al. Oral Melphalan, Prednisone, and Thalidomide in Elderly Patients with Multiple Myeloma: Updated Results of a Randomized Controlled Trial. Blood 2008, 112, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Caravita, T.; Merla, E.; Capparella, V.; Callea, V.; Cangialosi, C.; Grasso, M.; Rossini, F.; Galli, M.; et al. Oral Melphalan and Prednisone Chemotherapy plus Thalidomide Compared with Melphalan and Prednisone Alone in Elderly Patients with Multiple Myeloma: Randomised Controlled Trial. Lancet 2006, 367, 825–831. [Google Scholar] [CrossRef]
- Facon, T.; Mary, J.Y.; Hulin, C.; Benboubker, L.; Attal, M.; Pegourie, B.; Renaud, M.; Harousseau, J.L.; Guillerm, G.; Chaleteix, C.; et al. Melphalan and Prednisone plus Thalidomide versus Melphalan and Prednisone Alone or Reduced-Intensity Autologous Stem Cell Transplantation in Elderly Patients with Multiple Myeloma (IFM 99–06): A Randomised Trial. Lancet 2007, 370, 1209–1218. [Google Scholar] [CrossRef]
- Hulin, C.; Facon, T.; Rodon, P.; Pegourie, B.; Benboubker, L.; Doyen, C.; Dib, M.; Guillerm, G.; Salles, B.; Eschard, J.-P.; et al. Efficacy of Melphalan and Prednisone Plus Thalidomide in Patients Older Than 75 Years With Newly Diagnosed Multiple Myeloma: IFM 01/01 Trial. J. Clin. Oncol. 2009, 27, 3664–3670. [Google Scholar] [CrossRef]
- Kapoor, P.; Rajkumar, S.V.; Dispenzieri, A.; Gertz, M.A.; Lacy, M.Q.; Dingli, D.; Mikhael, J.R.; Roy, V.; Kyle, R.A.; Greipp, P.R.; et al. Melphalan and Prednisone versus Melphalan, Prednisone and Thalidomide for Elderly and/or Transplant Ineligible Patients with Multiple Myeloma: A Meta-Analysis. Leukemia 2011, 25, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Wijermans, P.; Schaafsma, M.; Termorshuizen, F.; Ammerlaan, R.; Wittebol, S.; Sinnige, H.; Zweegman, S.; van Marwijk Kooy, M.; van der Griend, R.; Lokhorst, H.; et al. Phase III Study of the Value of Thalidomide Added to Melphalan Plus Prednisone in Elderly Patients With Newly Diagnosed Multiple Myeloma: The HOVON 49 Study. J. Clin. Oncol. 2010, 28, 3160–3166. [Google Scholar] [CrossRef]
- Fayers, P.M.; Palumbo, A.; Hulin, C.; Waage, A.; Wijermans, P.; Beksaç, M.; Bringhen, S.; Mary, J.-Y.; Gimsing, P.; Termorshuizen, F.; et al. Thalidomide for Previously Untreated Elderly Patients with Multiple Myeloma: Meta-Analysis of 1685 Individual Patient Data from 6 Randomized Clinical Trials. Blood 2011, 118, 1239–1247. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Schlag, R.; Khuageva, N.K.; Dimopoulos, M.A.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, M.T.; Palumbo, A.; Samoilova, O.S.; et al. Persistent Overall Survival Benefit and No Increased Risk of Second Malignancies With Bortezomib-Melphalan-Prednisone Versus Melphalan-Prednisone in Patients With Previously Untreated Multiple Myeloma. J. Clin. Oncol. 2013, 31, 448–455. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Schlag, R.; Khuageva, N.K.; Dimopoulos, M.A.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, M.T.; Palumbo, A.; Samoilova, O.S.; et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N. Engl. J. Med. 2008, 359, 906–917. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Cavo, M.; Blade, J.; Dimopoulos, M.A.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; et al. Overall Survival with Daratumumab, Bortezomib, Melphalan, and Prednisone in Newly Diagnosed Multiple Myeloma (ALCYONE): A Randomised, Open-Label, Phase 3 Trial. Lancet 2020, 395, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Stadtmauer, E.A.; Weber, D.M.; Niesvizky, R.; Belch, A.; Prince, M.H.; San Miguel, J.F.; Facon, T.; Olesnyckyj, M.; Yu, Z.; Zeldis, J.B.; et al. Lenalidomide in Combination with Dexamethasone at First Relapse in Comparison with Its Use as Later Salvage Therapy in Relapsed or Refractory Multiple Myeloma. Eur. J. Haematol. 2009, 82, 426–432. [Google Scholar] [CrossRef]
- Wang, M.; Dimopoulos, M.A.; Chen, C.; Cibeira, M.T.; Attal, M.; Spencer, A.; Rajkumar, S.V.; Yu, Z.; Olesnyckyj, M.; Zeldis, J.B.; et al. Lenalidomide plus Dexamethasone Is More Effective than Dexamethasone Alone in Patients with Relapsed or Refractory Multiple Myeloma Regardless of Prior Thalidomide Exposure. Blood 2008, 112, 4445–4451. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Jacobus, S.; Callander, N.S.; Fonseca, R.; Vesole, D.H.; Williams, M.E.; Abonour, R.; Siegel, D.S.; Katz, M.; Greipp, P.R.; et al. Lenalidomide plus High-Dose Dexamethasone versus Lenalidomide plus Low-Dose Dexamethasone as Initial Therapy for Newly Diagnosed Multiple Myeloma: An Open-Label Randomised Controlled Trial. Lancet Oncol. 2010, 11, 29–37. [Google Scholar] [CrossRef]
- Benboubker, L.; Dimopoulos, M.A.; Dispenzieri, A.; Catalano, J.; Belch, A.R.; Cavo, M.; Pinto, A.; Weisel, K.; Ludwig, H.; Bahlis, N.; et al. Lenalidomide and Dexamethasone in Transplant-Ineligible Patients with Myeloma. N. Engl. J. Med. 2014, 371, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with Lenalidomide and Dexamethasone versus Lenalidomide and Dexamethasone Alone in Patients with Newly Diagnosed Myeloma without Intent for Immediate Autologous Stem-Cell Transplant (SWOG S0777): A Randomised, Open-Label, Phase 3 Trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Sexton, R.; Abidi, M.H.; Epstein, J.; Rajkumar, S.V.; Dispenzieri, A.; Kahanic, S.P.; Thakuri, M.C.; Reu, F.J.; et al. Longer Term Follow-up of the Randomized Phase III Trial SWOG S0777: Bortezomib, Lenalidomide and Dexamethasone vs. Lenalidomide and Dexamethasone in Patients (Pts) with Previously Untreated Multiple Myeloma without an Intent for Immediate Autologous Stem Cell Transplant (ASCT). Blood Cancer J. 2020, 10, 53. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- San-Miguel, J.; Avet-Loiseau, H.; Paiva, B.; Kumar, S.; Dimopoulos, M.A.; Facon, T.; Mateos, M.-V.; Touzeau, C.; Jakubowiak, A.; Usmani, S.Z.; et al. Sustained Minimal Residual Disease Negativity in Newly Diagnosed Multiple Myeloma and the Impact of Daratumumab in MAIA and ALCYONE. Blood 2022, 139, 492–501. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Larocca, A.; Rossi, D.; Di Raimondo, F.; Magarotto, V.; Patriarca, F.; Levi, A.; Benevolo, G.; Vincelli, I.D.; et al. Bortezomib-Melphalan-Prednisone-Thalidomide Followed by Maintenance With Bortezomib-Thalidomide Compared With Bortezomib-Melphalan-Prednisone for Initial Treatment of Multiple Myeloma: Updated Follow-Up and Improved Survival. J. Clin. Oncol. 2014, 32, 634–640. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Oriol, A.; Martínez-López, J.; Gutiérrez, N.; Teruel, A.-I.; López de la Guía, A.; López, J.; Bengoechea, E.; Pérez, M.; Polo, M.; et al. Maintenance Therapy with Bortezomib plus Thalidomide or Bortezomib plus Prednisone in Elderly Multiple Myeloma Patients Included in the GEM2005MAS65 Trial. Blood 2012, 120, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Špička, I.; Quach, H.; Oriol, A.; Hájek, R.; Garg, M.; Beksac, M.; Bringhen, S.; Katodritou, E.; Chng, W.-J.; et al. Ixazomib as Postinduction Maintenance for Patients With Newly Diagnosed Multiple Myeloma Not Undergoing Autologous Stem Cell Transplantation: The Phase III TOURMALINE-MM4 Trial. J. Clin. Oncol. 2020, 38, 4030–4041. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Pour, L.; Grosicki, S.; et al. Daratumumab Plus Bortezomib, Melphalan, and Prednisone Versus Bortezomib, Melphalan, and Prednisone in Transplant-Ineligible Newly Diagnosed Multiple Myeloma: Frailty Subgroup Analysis of ALCYONE. Clin. Lymphoma Myeloma Leuk. 2021, 21, 785–798. [Google Scholar] [CrossRef]
- Facon, T.; Cook, G.; Usmani, S.Z.; Hulin, C.; Kumar, S.; Plesner, T.; Touzeau, C.; Bahlis, N.J.; Basu, S.; Nahi, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone in Transplant-Ineligible Newly Diagnosed Multiple Myeloma: Frailty Subgroup Analysis of MAIA. Leukemia 2022, 36, 1066–1077. [Google Scholar] [CrossRef]
- Bonello, F.; Boccadoro, M.; Larocca, A. Diagnostic and Therapeutic Challenges in the Management of Intermediate and Frail Elderly Multiple Myeloma Patients. Cancers 2020, 12, 3106. [Google Scholar] [CrossRef]
- D’Agostino, M.; Larocca, A.; Offidani, M.; Liberati, A.M.; Gaidano, G.; Petrucci, M.T.; Derudas, D.; Capra, A.; Zambello, R.; Cascavilla, N.; et al. Octogenarian Newly Diagnosed Multiple Myeloma Patients without Geriatric Impairments: The Role of Age >80 in the IMWG Frailty Score. Blood Cancer J. 2021, 11, 73. [Google Scholar] [CrossRef]
- Facon, T.; Leleu, X.; Manier, S. How I Treat Multiple Myeloma in the Geriatric Patient. Blood 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.; Pawlyn, C.; Cairns, D.A.; Jackson, G.H. Defining FiTNEss for Treatment for Multiple Myeloma. Lancet Healthy Longev. 2022, 3, e729–e730. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Chen, C.; Spencer, A.; Niesvizky, R.; Attal, M.; Stadtmauer, E.A.; Petrucci, M.T.; Yu, Z.; Olesnyckyj, M.; Zeldis, J.B.; et al. Long-Term Follow-up on Overall Survival from the MM-009 and MM-010 Phase III Trials of Lenalidomide plus Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma. Leukemia 2009, 23, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- San-Miguel, J.F.; Dimopoulos, M.A.; Stadtmauer, E.A.; Rajkumar, S.V.; Siegel, D.; Bravo, M.-L.; Olesnyckyj, M.; Knight, R.D.; Zeldis, J.B.; Harousseau, J.-L.; et al. Effects of Lenalidomide and Dexamethasone Treatment Duration on Survival in Patients With Relapsed or Refractory Multiple Myeloma Treated With Lenalidomide and Dexamethasone. Clin. Lymphoma Myeloma Leuk. 2011, 11, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Richardson, P.G.; Sonneveld, P.; Schuster, M.W.; Irwin, D.; San Miguel, J.-F.; Crawford, B.; Massaro, J.; Dhawan, R.; Gupta, S.; et al. Bortezomib Is Associated with Better Health-Related Quality of Life than High-Dose Dexamethasone in Patients with Relapsed Multiple Myeloma: Results from the APEX Study. Br. J. Haematol. 2008, 143, 511–519. [Google Scholar] [CrossRef]
- Richardson, P.G.; Sonneveld, P.; Schuster, M.W.; Irwin, D.; Stadtmauer, E.A.; Facon, T.; Harousseau, J.-L.; Ben-Yehuda, D.; Lonial, S.; San Miguel, J.-F.; et al. Safety and Efficacy of Bortezomib in High-Risk and Elderly Patients with Relapsed Multiple Myeloma. Br. J. Haematol. 2007, 137, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Sonneveld, P.; Schuster, M.; Irwin, D.; Stadtmauer, E.; Facon, T.; Harousseau, J.-L.; Ben-Yehuda, D.; Lonial, S.; Goldschmidt, H.; et al. Extended Follow-up of a Phase 3 Trial in Relapsed Multiple Myeloma: Final Time-to-Event Results of the APEX Trial. Blood 2007, 110, 3557–3560. [Google Scholar] [CrossRef] [PubMed]
- Di Lernia, G.; Leone, P.; Solimando, A.G.; Buonavoglia, A.; Saltarella, I.; Ria, R.; Ditonno, P.; Silvestris, N.; Crudele, L.; Vacca, A.; et al. Bortezomib Treatment Modulates Autophagy in Multiple Myeloma. J. Clin. Med. 2020, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; De Veirman, K.; Giannico, D.; Saltarella, I.; Desantis, V.; Frassanito, M.A.; Solimando, A.G.; Ribatti, D.; Prete, M.; Harstrick, A.; et al. Targeting Angiogenesis in Multiple Myeloma by the VEGF and HGF Blocking DARPin® Protein MP0250: A Preclinical Study. Oncotarget 2018, 9, 13366–13381. [Google Scholar] [CrossRef]
- Desantis, V.; Saltarella, I.; Lamanuzzi, A.; Melaccio, A.; Solimando, A.G.; Mariggiò, M.A.; Racanelli, V.; Paradiso, A.; Vacca, A.; Frassanito, M.A. MicroRNAs-Based Nano-Strategies as New Therapeutic Approach in Multiple Myeloma to Overcome Disease Progression and Drug Resistance. Int. J. Mol. Sci. 2020, 21, 3084. [Google Scholar] [CrossRef]
- Desantis, V.; Solimando, A.G.; Saltarella, I.; Sacco, A.; Giustini, V.; Bento, M.; Lamanuzzi, A.; Melaccio, A.; Frassanito, M.A.; Paradiso, A.; et al. MicroRNAs as a Potential New Preventive Approach in the Transition from Asymptomatic to Symptomatic Multiple Myeloma Disease. Cancers 2021, 13, 3650. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Richardson, P.G.; Kumar, S.K.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; et al. Final Overall Survival Analysis of the TOURMALINE-MM1 Phase III Trial of Ixazomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. 2021, 39, 2430–2442. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Weisel, K.; San-Miguel, J.; Shpilberg, O.; Grosicki, S.; Špička, I.; Walter-Croneck, A.; et al. Elotuzumab, Lenalidomide, and Dexamethasone in RRMM: Final Overall Survival Results from the Phase 3 Randomized ELOQUENT-2 Study. Blood Cancer J. 2020, 10, 91. [Google Scholar] [CrossRef]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.-V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef]
- Lokhorst, H.M.; Plesner, T.; Laubach, J.P.; Nahi, H.; Gimsing, P.; Hansson, M.; Minnema, M.C.; Lassen, U.; Krejcik, J.; Palumbo, A.; et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N. Engl. J. Med. 2015, 373, 1207–1219. [Google Scholar] [CrossRef]
- Lonial, S.; Weiss, B.M.; Usmani, S.Z.; Singhal, S.; Chari, A.; Bahlis, N.J.; Belch, A.; Krishnan, A.; Vescio, R.A.; Mateos, M.V.; et al. Daratumumab Monotherapy in Patients with Treatment-Refractory Multiple Myeloma (SIRIUS): An Open-Label, Randomised, Phase 2 Trial. Lancet 2016, 387, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Weiss, B.M.; Plesner, T.; Bahlis, N.J.; Belch, A.; Lonial, S.; Lokhorst, H.M.; Voorhees, P.M.; Richardson, P.G.; Chari, A.; et al. Clinical Efficacy of Daratumumab Monotherapy in Patients with Heavily Pretreated Relapsed or Refractory Multiple Myeloma. Blood 2016, 128, 37–44. [Google Scholar] [CrossRef]
- Bahlis, N.J.; Dimopoulos, M.A.; White, D.J.; Benboubker, L.; Cook, G.; Leiba, M.; Ho, P.J.; Kim, K.; Takezako, N.; Moreau, P.; et al. Daratumumab plus Lenalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma: Extended Follow-up of POLLUX, a Randomized, Open-Label, Phase 3 Study. Leukemia 2020, 34, 1875–1884. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Palumbo, A.; Joshua, D.; Pour, L.; Hájek, R.; Facon, T.; Ludwig, H.; Oriol, A.; Goldschmidt, H.; et al. Carfilzomib and Dexamethasone versus Bortezomib and Dexamethasone for Patients with Relapsed or Refractory Multiple Myeloma (ENDEAVOR): A Randomised, Phase 3, Open-Label, Multicentre Study. Lancet Oncol. 2016, 17, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Goldschmidt, H.; Niesvizky, R.; Joshua, D.; Chng, W.-J.; Oriol, A.; Orlowski, R.Z.; Ludwig, H.; Facon, T.; Hajek, R.; et al. Carfilzomib or Bortezomib in Relapsed or Refractory Multiple Myeloma (ENDEAVOR): An Interim Overall Survival Analysis of an Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2017, 18, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Dimopoulos, M.-A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Špička, I.; Baker, R.; Kim, K.; et al. Isatuximab, Carfilzomib, and Dexamethasone in Relapsed Multiple Myeloma (IKEMA): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet 2021, 397, 2361–2371. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Quach, H.; Mateos, M.-V.; Landgren, O.; Leleu, X.; Siegel, D.S.; Weisel, K.; Yang, H.; Klippel, Z.K.; Zahlten-Kumeli, A.; et al. Carfilzomib, Dexamethasone, and Daratumumab Versus Carfilzomib and Dexamethasone for the Treatment of Patients with Relapsed or Refractory Multiple Myeloma (RRMM): Primary Analysis Results from the Randomized, Open-Label, Phase 3 Study Candor (NCT03158688). Blood 2019, 134, LBA-6. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef]
- Spencer, A.; Lentzsch, S.; Weisel, K.; Avet-Loiseau, H.; Mark, T.M.; Spicka, I.; Masszi, T.; Lauri, B.; Levin, M.-D.; Bosi, A.; et al. Daratumumab plus Bortezomib and Dexamethasone versus Bortezomib and Dexamethasone in Relapsed or Refractory Multiple Myeloma: Updated Analysis of CASTOR. Haematologica 2018, 103, 2079–2087. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Hungria, V.T.M.; Yoon, S.-S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Günther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Panobinostat plus Bortezomib and Dexamethasone versus Placebo plus Bortezomib and Dexamethasone in Patients with Relapsed or Relapsed and Refractory Multiple Myeloma: A Multicentre, Randomised, Double-Blind Phase 3 Trial. Lancet Oncol. 2014, 15, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Miguel, J.S.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus Low-Dose Dexamethasone versus High-Dose Dexamethasone Alone for Patients with Relapsed and Refractory Multiple Myeloma (MM-003): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus Pomalidomide and Low-Dose Dexamethasone versus Pomalidomide and Low-Dose Dexamethasone in Patients with Relapsed and Refractory Multiple Myeloma (ICARIA-MM): A Randomised, Multicentre, Open-Label, Phase 3 Study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Richardson, P.G.; Oriol, A.; Beksac, M.; Liberati, A.M.; Galli, M.; Schjesvold, F.; Lindsay, J.; Weisel, K.; White, D.; Facon, T.; et al. Pomalidomide, Bortezomib, and Dexamethasone for Patients with Relapsed or Refractory Multiple Myeloma Previously Treated with Lenalidomide (OPTIMISMM): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2019, 20, 781–794. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab Mafodotin for Relapsed or Refractory Multiple Myeloma (DREAMM-2): A Two-Arm, Randomised, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor–Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Harousseau, J.-L.; Miguel, J.S.; Bladé, J.; Barlogie, B.; Anderson, K.; Gertz, M.; Dimopoulos, M.; Westin, J.; Sonneveld, P.; et al. International Uniform Response Criteria for Multiple Myeloma. Leukemia 2006, 20, 1467–1473. [Google Scholar] [CrossRef]
- Chanan-Khan, A.A.; Giralt, S. Importance of Achieving a Complete Response in Multiple Myeloma, and the Impact of Novel Agents. J. Clin. Oncol. 2010, 28, 2612–2624. [Google Scholar] [CrossRef] [PubMed]
- Gay, F.; Larocca, A.; Wijermans, P.; Cavallo, F.; Rossi, D.; Schaafsma, R.; Genuardi, M.; Romano, A.; Liberati, A.M.; Siniscalchi, A.; et al. Complete Response Correlates with Long-Term Progression-Free and Overall Survival in Elderly Myeloma Treated with Novel Agents: Analysis of 1175 Patients. Blood 2011, 117, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Buadi, F.; Dingli, D.; Russell, S.J.; Hayman, S.R.; Witzig, T.E.; Lust, J.A.; et al. Importance of Achieving Stringent Complete Response After Autologous Stem-Cell Transplantation in Multiple Myeloma. J. Clin. Oncol. 2013, 31, 4529–4535. [Google Scholar] [CrossRef]
- Van de Velde, H.J.K.; Liu, X.; Chen, G.; Cakana, A.; Deraedt, W.; Bayssas, M. Complete Response Correlates with Long-Term Survival and Progression-Free Survival in High-Dose Therapy in Multiple Myeloma. Haematologica 2007, 92, 1399–1406. [Google Scholar] [CrossRef]
- Jakubowiak, A.J.; Dytfeld, D.; Griffith, K.A.; Lebovic, D.; Vesole, D.H.; Jagannath, S.; Al-Zoubi, A.; Anderson, T.; Nordgren, B.; Detweiler-Short, K.; et al. A Phase 1/2 Study of Carfilzomib in Combination with Lenalidomide and Low-Dose Dexamethasone as a Frontline Treatment for Multiple Myeloma. Blood 2012, 120, 1801–1809. [Google Scholar] [CrossRef]
- Paiva, B.; Gutiérrez, N.C.; Rosiñol, L.; Vídriales, M.-B.; Montalbán, M.-Á.; Martínez-López, J.; Mateos, M.-V.; Cibeira, M.-T.; Cordón, L.; Oriol, A.; et al. High-Risk Cytogenetics and Persistent Minimal Residual Disease by Multiparameter Flow Cytometry Predict Unsustained Complete Response after Autologous Stem Cell Transplantation in Multiple Myeloma. Blood 2012, 119, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Paiva, B.; Vidriales, M.-B.; Cerveró, J.; Mateo, G.; Pérez, J.J.; Montalbán, M.A.; Sureda, A.; Montejano, L.; Gutiérrez, N.C.; de Coca, A.G.; et al. Multiparameter Flow Cytometric Remission Is the Most Relevant Prognostic Factor for Multiple Myeloma Patients Who Undergo Autologous Stem Cell Transplantation. Blood 2008, 112, 4017–4023. [Google Scholar] [CrossRef]
- Martinez-Lopez, J.; Lahuerta, J.J.; Pepin, F.; González, M.; Barrio, S.; Ayala, R.; Puig, N.; Montalban, M.A.; Paiva, B.; Weng, L.; et al. Prognostic Value of Deep Sequencing Method for Minimal Residual Disease Detection in Multiple Myeloma. Blood 2014, 123, 3073–3079. [Google Scholar] [CrossRef]
- Puig, N.; Sarasquete, M.E.; Balanzategui, A.; Martínez, J.; Paiva, B.; García, H.; Fumero, S.; Jiménez, C.; Alcoceba, M.; Chillón, M.C.; et al. Critical Evaluation of ASO RQ-PCR for Minimal Residual Disease Evaluation in Multiple Myeloma. A Comparative Analysis with Flow Cytometry. Leukemia 2014, 28, 391–397. [Google Scholar] [CrossRef]
- Rawstron, A.C.; Child, J.A.; de Tute, R.M.; Davies, F.E.; Gregory, W.M.; Bell, S.E.; Szubert, A.J.; Navarro-Coy, N.; Drayson, M.T.; Feyler, S.; et al. Minimal Residual Disease Assessed by Multiparameter Flow Cytometry in Multiple Myeloma: Impact on Outcome in the Medical Research Council Myeloma IX Study. J. Clin. Oncol. 2013, 31, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Terpos, E.; Nanni, C.; Moreau, P.; Lentzsch, S.; Zweegman, S.; Hillengass, J.; Engelhardt, M.; Usmani, S.Z.; Vesole, D.H.; et al. Role of 18F-FDG PET/CT in the Diagnosis and Management of Multiple Myeloma and Other Plasma Cell Disorders: A Consensus Statement by the International Myeloma Working Group. Lancet Oncol. 2017, 18, e206–e217. [Google Scholar] [CrossRef]
- Charalampous, C.; Goel, U.; Broski, S.M.; Dingli, D.; Kapoor, P.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Hayman, S.R.; Buadi, F.; et al. Utility of PET/CT in Assessing Early Treatment Response in Patients with Newly Diagnosed Multiple Myeloma. Blood Adv. 2022, 6, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, T.; Paiva, B.; Hajek, R. Update on PD-1/PD-L1 Inhibitors in Multiple Myeloma. Front. Immunol. 2018, 9, 2431. [Google Scholar] [CrossRef] [PubMed]
- Solimando, A.G.; Crudele, L.; Leone, P.; Argentiero, A.; Guarascio, M.; Silvestris, N.; Vacca, A.; Racanelli, V. Immune Checkpoint Inhibitor-Related Myositis: From Biology to Bedside. Int. J. Mol. Sci. 2020, 21, 3054. [Google Scholar] [CrossRef] [PubMed]
- Argentiero, A.; Solimando, A.G.; Ungaro, V.; Laforgia, M.; Strippoli, S.; Pinto, D.; Negri, A.; Ferraiuolo, S.; Zito, A.; Guida, M. Case Report: Lymphocytosis Associated With Fatal Hepatitis in a Thymoma Patient Treated With Anti-PD1: New Insight Into the Immune-Related Storm. Front. Oncol. 2020, 10, 583781. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solimando, A.G.; Krebs, M.; Desantis, V.; Marziliano, D.; Caradonna, I.C.; Morizio, A.; Argentiero, A.; Shahini, E.; Bittrich, M. Breaking through Multiple Myeloma: A Paradigm for a Comprehensive Tumor Ecosystem Targeting. Biomedicines 2023, 11, 2087. https://doi.org/10.3390/biomedicines11072087
Solimando AG, Krebs M, Desantis V, Marziliano D, Caradonna IC, Morizio A, Argentiero A, Shahini E, Bittrich M. Breaking through Multiple Myeloma: A Paradigm for a Comprehensive Tumor Ecosystem Targeting. Biomedicines. 2023; 11(7):2087. https://doi.org/10.3390/biomedicines11072087
Chicago/Turabian StyleSolimando, Antonio G., Markus Krebs, Vanessa Desantis, Donatello Marziliano, Ingrid Catalina Caradonna, Arcangelo Morizio, Antonella Argentiero, Endrit Shahini, and Max Bittrich. 2023. "Breaking through Multiple Myeloma: A Paradigm for a Comprehensive Tumor Ecosystem Targeting" Biomedicines 11, no. 7: 2087. https://doi.org/10.3390/biomedicines11072087
APA StyleSolimando, A. G., Krebs, M., Desantis, V., Marziliano, D., Caradonna, I. C., Morizio, A., Argentiero, A., Shahini, E., & Bittrich, M. (2023). Breaking through Multiple Myeloma: A Paradigm for a Comprehensive Tumor Ecosystem Targeting. Biomedicines, 11(7), 2087. https://doi.org/10.3390/biomedicines11072087






