A Preliminary Study Indicating Improvement in the Median Survival Time of Glioblastoma Multiforme Patients by the Application of Deuterium Depletion in Combination with Conventional Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Administration of DDW
2.3. Statistical Evaluation
3. Results
3.1. Evaluation of the Whole Study Population of 55 GBM Patients Consuming DDW
3.1.1. Characteristics of the Whole Study Population
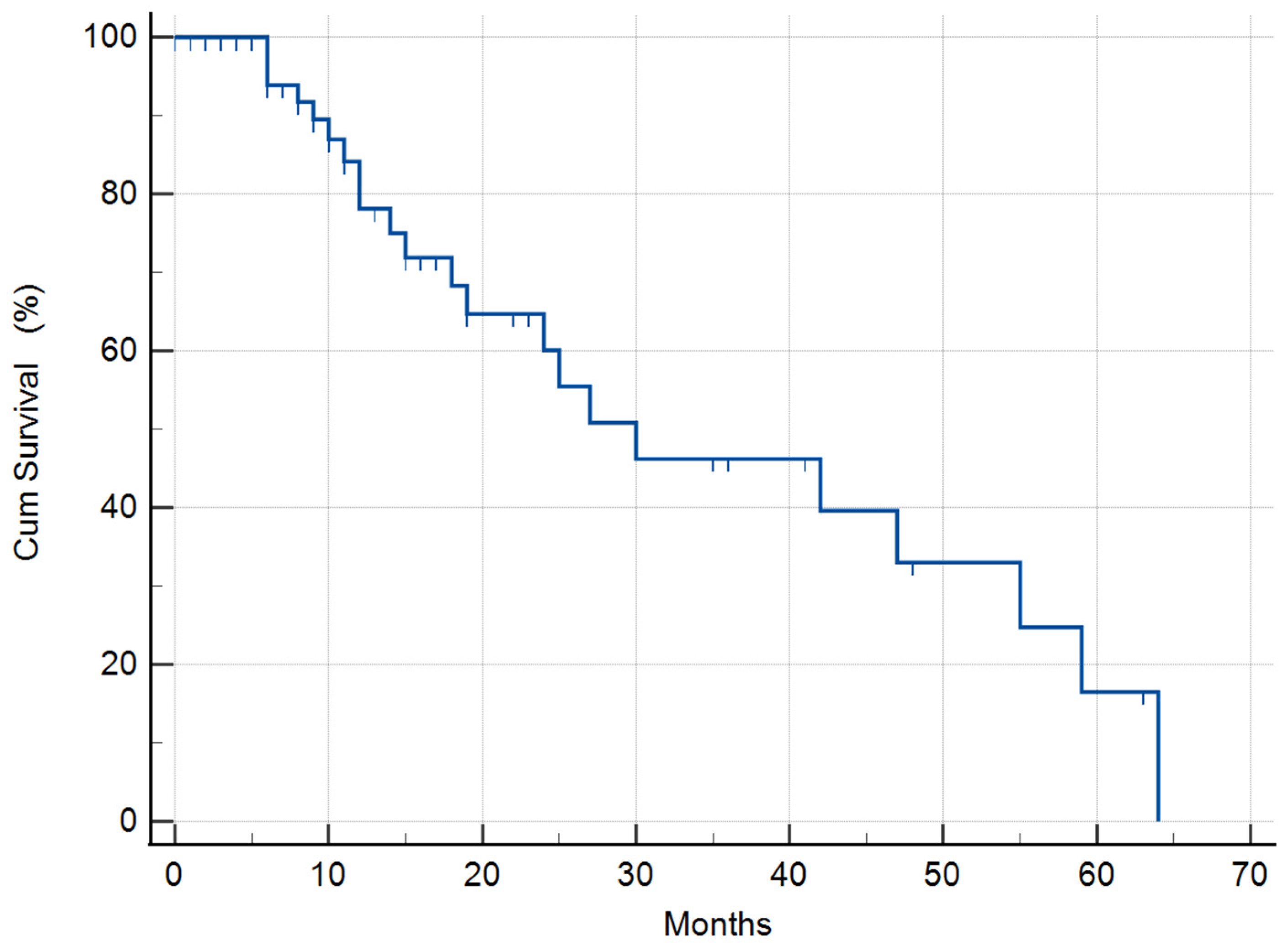
3.1.2. Median Survival Time (MST) of the Whole Study Population
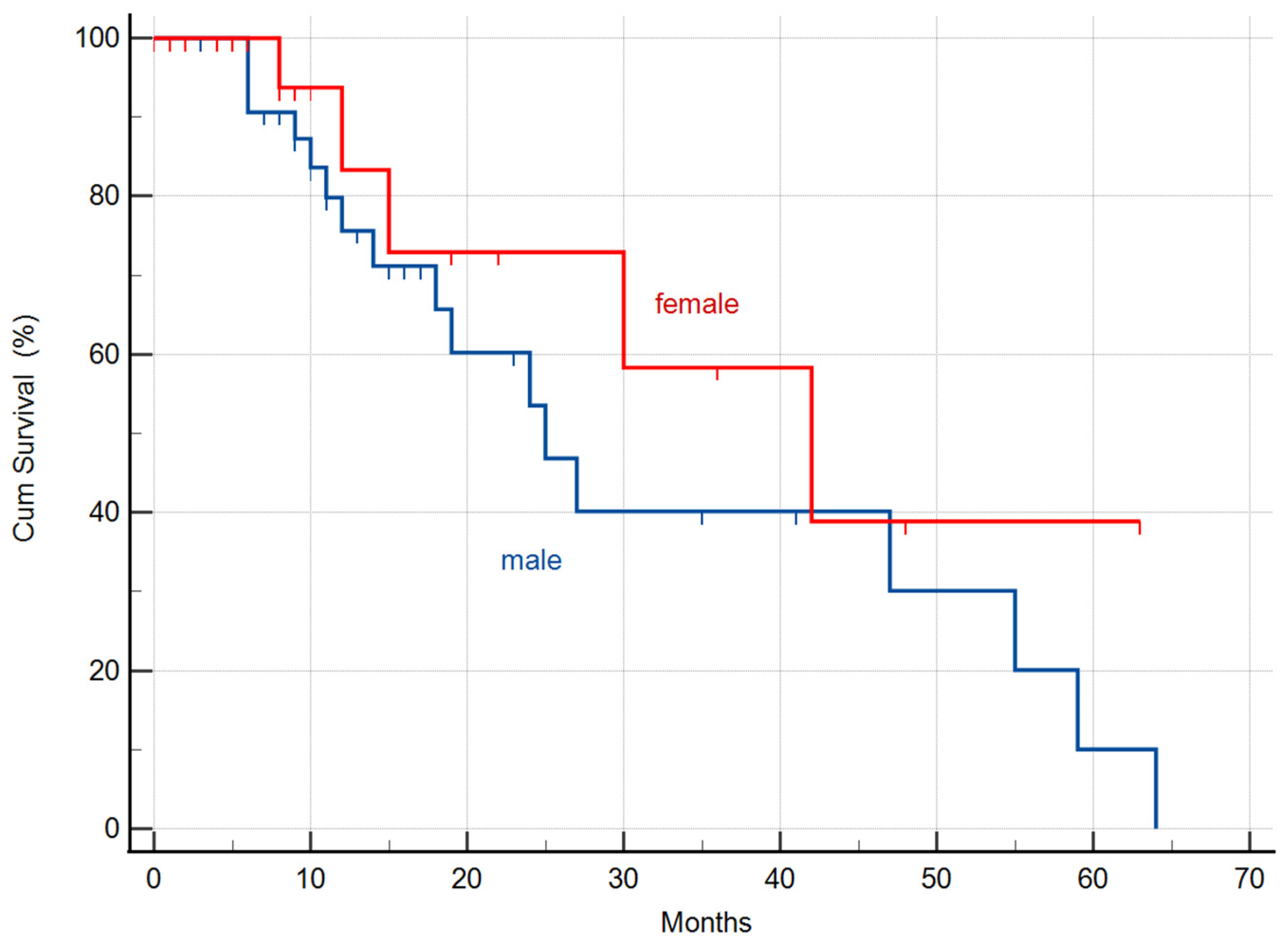
3.1.3. Differences of the MSTs in the Whole Cohort by Gender
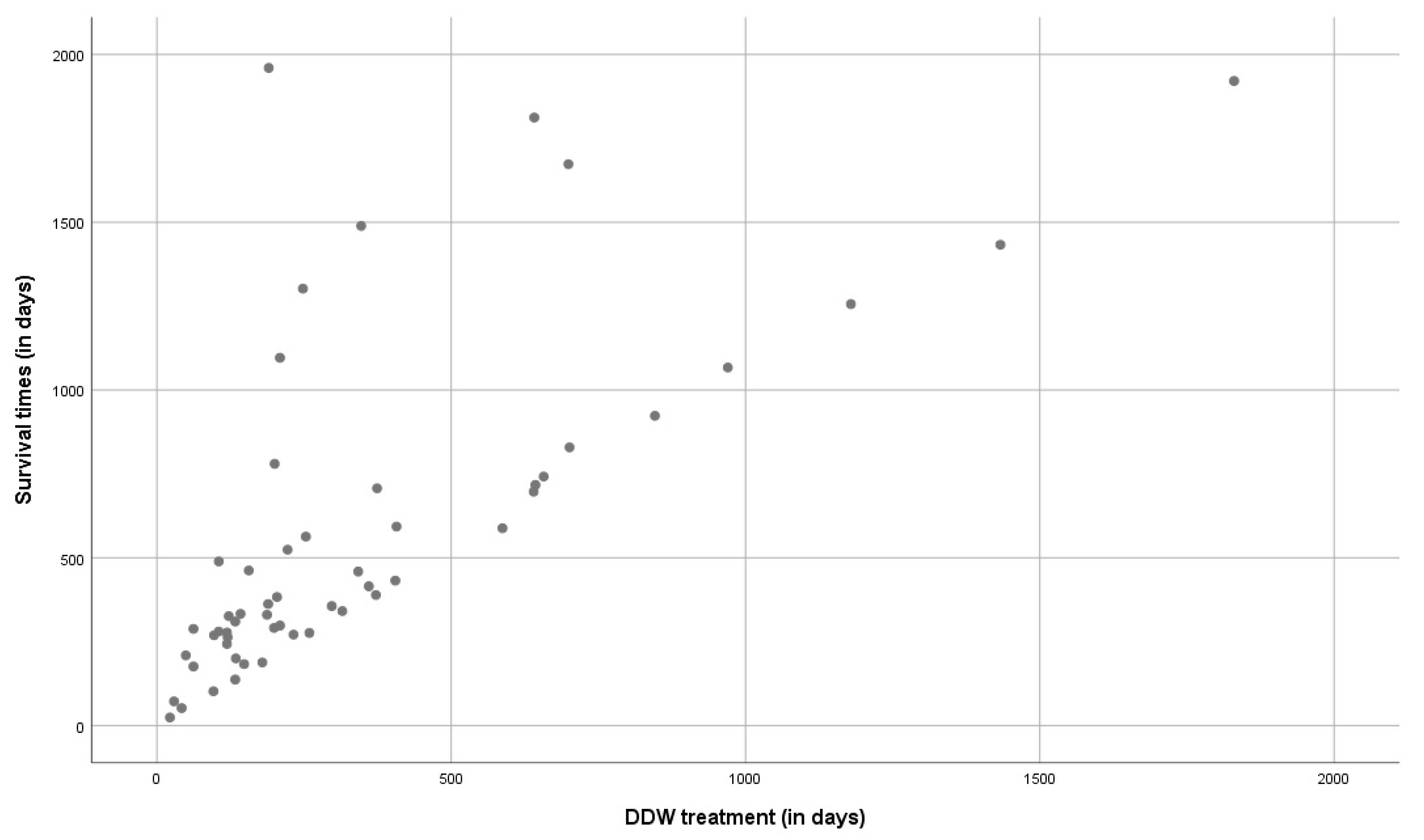
3.1.4. Correlation between the Survival Times and the Duration of DDW Consumption in the Whole Study Population
3.1.5. MST in the Whole Study Population Stratified by Temozolomide Treatment
3.2. Detailed Evaluation of the Selected 31 GBM Patients
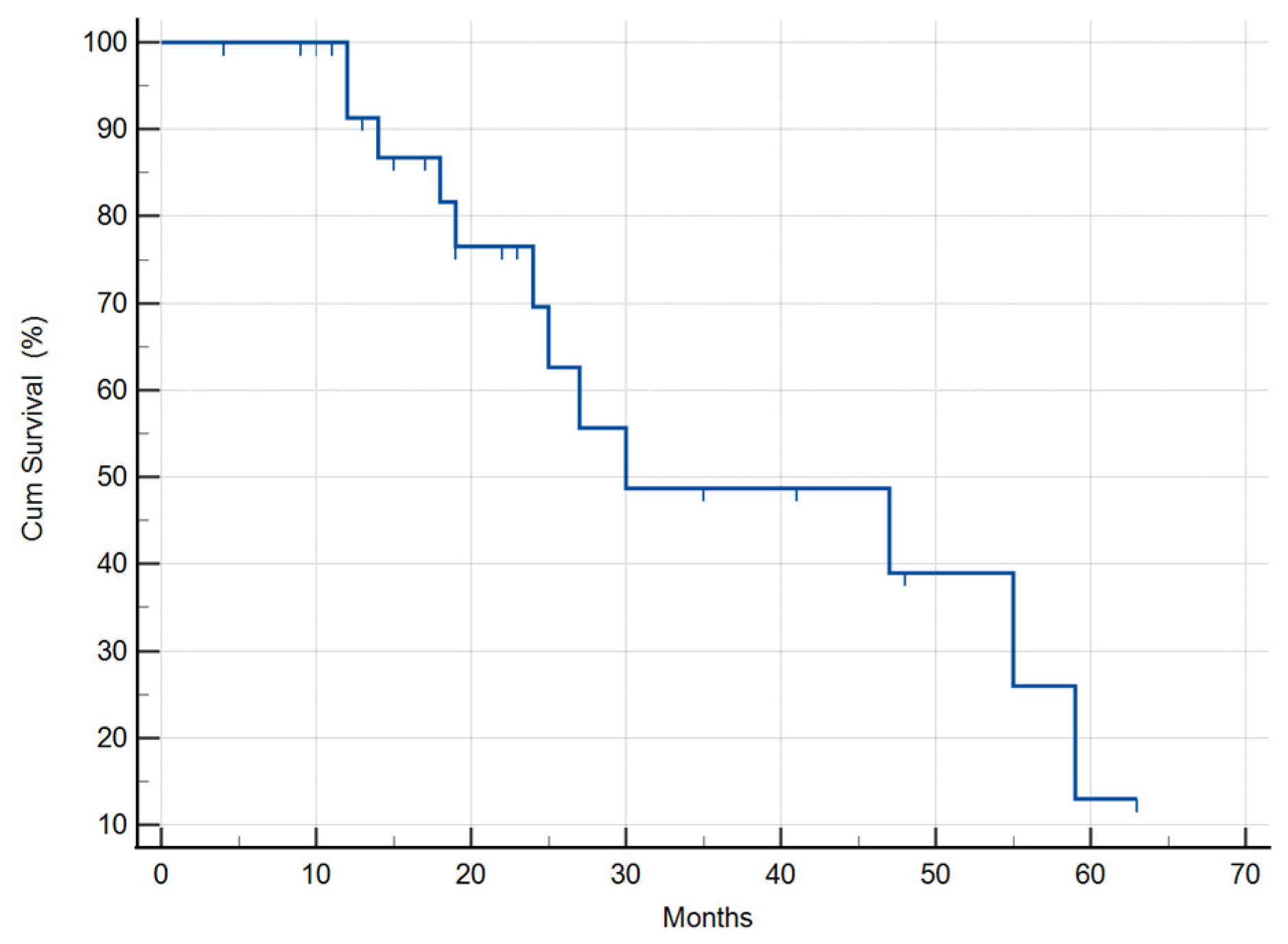
3.2.1. Median Survival Time of the Selected 31 GBM Patients
3.2.2. Calculation of the MST in the Selected 31 GBM Patients Stratified by Relapse Status
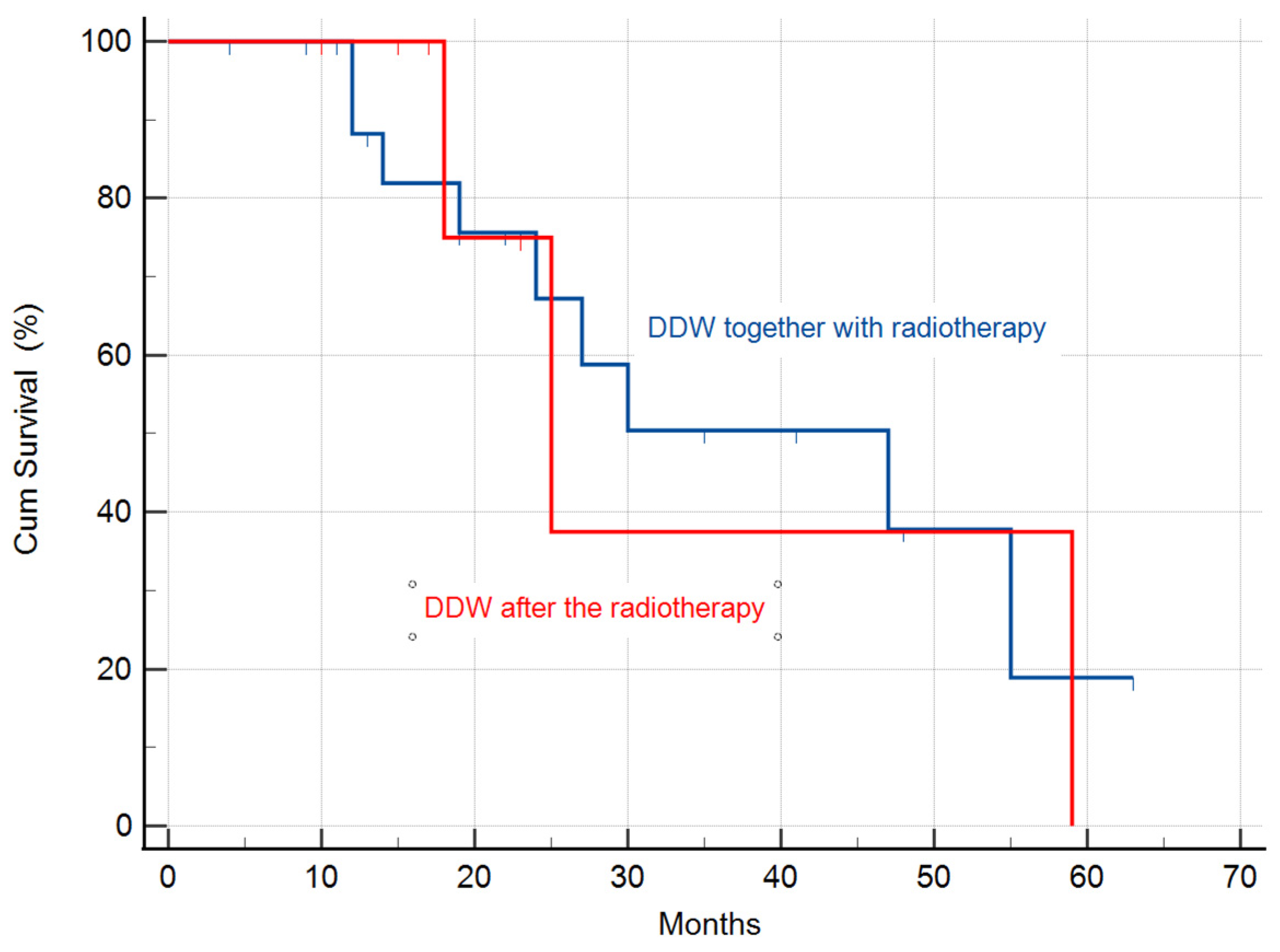
3.2.3. Calculation of the MST in the Study Population Stratified by Radiotherapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacy, J.; Saadati, H.; Yu, J. Complications of brain tumors and their treatment. Hemat. Oncol. Clin. N. Am. 2012, 26, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Jacques, G.; Cormac, O. Central Nervous System Tumors. Handb. Clin. Neurol. 2013, 112, 931–958. [Google Scholar] [PubMed]
- DeRobles, P.; Fiest, K.M.; Frolkis, A.D.; Pringsheim, T.; Atta, C.; St. Germaine-Smith, C.; Day, L.; Lam, D.; Jette, N. The worldwide incidence and prevalence of primary brain tumors: A systematic review and meta-analysis. Neuro-Oncology 2015, 17, 776–783. [Google Scholar] [CrossRef]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. J. Am. Med. Assoc. 2013, 310, 1842–1850. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Eng. J. Med. 2015, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Young, W.K.; Albright, R.E.; Olson, J.; Frederics, R.; Fink, K.; Prados, M.D.; Brada, M.; Spence, A.; Hohl, R.J.; Shapiro, W.; et al. A Phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first by relapse. Br. J. Cancer 2000, 83, 588–593. [Google Scholar] [CrossRef]
- Gyöngyi, Z.; Somlyai, G. Deuterium depletion can decrease the expression of c-myc, Ha-ras and p53 gene in carcinogen-treated mice. In Vivo 2000, 14, 437–440. [Google Scholar]
- Krempels, K.; Somlyai, I.; Somlyai, G. A retrospective evaluation of the effects of deuterium depleted water consumption on four patients with brain metastases from lung cancer. Integr. Cancer Ther. 2008, 7, 172–181. [Google Scholar] [CrossRef]
- Cong, F.S.; Zhang, Y.R.; Sheng, H.C.; Ao, Z.H.; Zhang, S.Y. Deuterium-depleted water inhibits human lung carcinoma cell growth by apoptosis. Exp. Ther. Med. 2010, 1, 277–283. [Google Scholar] [CrossRef]
- Kovács, A.; Guller, I.; Krempels, K.; Somlyai, I.; Jánosi, I.; Gyöngyi, Z.; Szabó, I.; Ember, I.; Somlyai, G. Deuterium Depletion May Delay the Progression of Prostate Cancer. J. Cancer Ther. 2011, 2, 548–556. [Google Scholar] [CrossRef]
- Somlyai, G.; Kovács, B.Z.; Somlyai, I.; Papp, A.; Nagy, L.I.; Puskás, L.G. Deuterium depletion inhibits lung cancer cell growth and migration in vitro and results in severalfold increase of median survival time of non-small cell lung cancer patients receiving conventional therapy. J. Cancer Res. Ther. 2021, 9, 12–19. [Google Scholar] [CrossRef]
- Somlyai, G.; Jancsó, G.; Jákli, G.; Vass, K.; Barna, B.; Lakics, V.; Gaál, T. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett. 1993, 317, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.J.; Crespi, H.L. Isotope effects in biological systems. In Isotope Effects in Chemical Reactions; Collins, C.J., Bowman, N.S., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1971; pp. 286–363. [Google Scholar]
- Jancsó, G. Isotope effects. In Handbook of Nuclear Chemistry; Vértes, A., Nagy, S., Klencsár, Z., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 85–116. [Google Scholar]
- Enright, J. Heavy water slows biological timing processes. Z. Für Vgl. Physiol. 1971, 72, 1–16. [Google Scholar] [CrossRef]
- Harvey, E.N. Biological effects of heavy water. Biol. Bull. 1934, 66, 91–96. [Google Scholar] [CrossRef]
- Somlyai, G.; Laskay, G.; Berkényi, T.; Jákli, G.; Jancsó, G. Naturally occurring deuterium may have a central role in cell signaling. In Synthesis and Applications of Isotopically Labelled Compounds; Heys, J.R., Melillo, D., Eds.; John Wiley and Sons Ltd.: New York, NY, USA, 1988; pp. 137–141. [Google Scholar]
- Yavari, K.; Kooshesh, L. Deuterium Depleted Water Inhibits the Proliferation of Human MCF7 Breast Cancer Cell Lines by Inducing Cell Cycle Arrest. Nutr. Cancer 2019, 71, 1019–1029. [Google Scholar] [CrossRef]
- Syroeshkin, A.; Levitskaya, O.; Uspenskaya, E.; Pleteneva, T.; Romaykina, D.; Ermakova, D. Deuterium Depleted Water as an Adjuvant in Treatment of Cancer. Sys. Rev. Pharm. 2019, 10, 112–117. [Google Scholar]
- Zlatskiy, I.A.; Zlatska, A.V.; Antipova, N.V.; Syroeshkin, A.V. Effect of deuterium on the morpho-functional characteristics of normal and cancer cells in vitro. Trace Elem. Electrolytes 2018, 35, 211–214. [Google Scholar] [CrossRef]
- Somlyai, G.; Laskay, G.; Berkényi, T.; Galbács, Z.; Galbács, G.; Kiss, S.A.; Jákli, G.; Jancsó, G. The biological effects of deuterium-depleted water a possible new tool in cancer therapy. J. Oncol. 1998, 30, 91–94. [Google Scholar]
- Zhang, X.; Gaetani, M.; Chernobrovkin, A.; Zubarev, R.A. Anticancer Effect of Deuterium Depleted Water—Redox Disbalance Leads to Oxidative Stress. Mol. Cell. Proteom. 2019, 18, 2373–2387. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zubarev, R.A. Slight deuterium enrichment in water acts as an antioxidant: Is deuterium a cell growth regulator? Mol. Cell. Proteom. 2020, 19, 1790–1804. [Google Scholar] [CrossRef]
- Boros, L.G.; D’Agostino, D.P.; Katz, H.E.; Roth, J.P.; Meuillet, E.J.; Somlyai, G. Submolecular regulation of cell transformation by deuterium depleting water exchange reactions in the tricarboxylic acid substrate cycle. Med. Hypothesis 2016, 87, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Krempels, K.; Somlyai, I.; Gyöngyi, Z.; Ember, I.; Balog, K.; Abonyi, O.; Somlyai, G. A retrospective study of survival in breast cancer patients undergoing deuterium depletion in addition to conventional therapies. J. Cancer Res. Ther. 2013, 194–200. [Google Scholar] [CrossRef]
- Gyöngyi, Z.; Budán, F.; Szabó, I.; Ember, I.; Kiss, I.; Krempels, K.; Somlyai, I.; Somlyai, G. Deuterium Depleted Water Effects on Survival of Lung Cancer Patients and Expression of Kras, Bcl2, and Myc Genes in Mouse Lung. Nutr. Cancer 2003, 65, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 DD. and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Teugels, E.; Sadones, J.; Quartier, E.; Huylebrouck, M.; Du Four, S.; Le Mercier, M.; De Witte, O.; Salmon, I.; Michotte, A.; et al. Correlation between IDH1 gene mutation status and survival of patients treated for recurrent glioma. Anticancer Res. 2011, 31, 4457–4463. [Google Scholar] [CrossRef]
- Chesnelong, C.; Chaumeil, M.M.; Blough, M.D.; Al-Najjar, M.; Stechishin, O.D.; Chan, J.A.; O Pieper, R.; Ronen, S.M.; Weiss, S.; Luchman, H.A.; et al. Lactate dehydrogenase. A silencing in IDH mutant gliomas. Neuro Oncol. 2014, 16, 686–695. [Google Scholar] [CrossRef]
- Liu, F.M.; Gao, Y.F.; Kong, Y.; Guan, Y.; Zhang, J.; Li, S.H.; Ye, D.; Wen, W.; Zuo, C.; Hua, W. The diagnostic value of lower glucose consumption for IDH1 mutated gliomas on FDG-PET. BMC Cancer 2021, 21, 83. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis 2014, 35, 515–527. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Mukherjee, P.; Iyikesici, M.S.; Slocum, A.; Kalamian, M.; Spinosa, J.P.; Chinopoulos, C. Consideration of Ketogenic Metabolic Therapy as a Complementary or Alternative Approach for Managing Breast Cancer. Front. Nutr. 2020, 7, 21. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Shivane, A.G.; Kalamian, M.; Maroon, J.C.; Mukherjee, P.; Zuccoli, G. Ketogenic Metabolic Therapy, Without Chemo or Radiation, for the Long-Term Management of IDH1-Mutant Glioblastoma: An 80-Month Follow-Up Case Report. Front. Nutr. 2021, 8, 281. [Google Scholar] [CrossRef]
- Mukherjee, P.; Augur, Z.M.; Li, M.; Hill, C.; Greenwood, B.; Domin, M.A.; Kondakci, G.; Narain, N.R.; Kiebish, M.A.; Bronson, R.T.; et al. Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun. Biol. 2019, 2, 200. [Google Scholar] [CrossRef] [PubMed]
- Denny, C.A.; Heinecke, K.A.; Kim, Y.P.; Baek, R.C.; Loh, K.S.; Butters, T.D.; Bronson, R.T.; Platt, F.M.; Seyfried, T.N. Restricted ketogenic diet enhances the therapeutic action of N-butyldeoxynojirimycin towards brain GM2 accumulation in adult Sandhoff disease mice. J. Neurochem. 2010, 113, 1525–1535. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Ozpinar, A.; Seyfried, T.N. Caprylic (Octanoic) Acid as a potential fatty acid chemotherapeutic for glioblastoma. Prostagl Leukot. Essent. Fatty Acids 2020, 159, 102142. [Google Scholar] [CrossRef] [PubMed]
- Basov, A.A.; Bykov, I.M.; Baryshev, M.G.; Dzhimak, S.S.; Bykov, M.I. Determination of deuterium concentration in foods and influence of water with modified isotopic composition on oxidation parameters and heavy hydrogen isotopes content in experimental animals. Vopr. Pitan. 2014, 83, 43–50. [Google Scholar] [PubMed]
- Rundel, P.W.; Ehleringer, J.R.; Nagy, K.A. Stable Isotopes in Ecological Research; Springer: New York, NY, USA, 1988. [Google Scholar]
- Siniak, I.E.; Turusov, V.S.; Grigoriev, A.I.; Zaridze, D.G.; Gaidadymov, V.B.; Gus’kova, E.I.; Antoshina, E.E.; Gor’kova, T.G.; Trukhanova, L.S. Consideration of the Deuterium-Free Water Supply to an Expedition to Mars. Aerosp. Environ. Med. 2003, 37, 60. [Google Scholar]
- Török, G.; Csík, M.; Pintér, A. Effects of Different Deuterium Concentrations of the Media on the Bacterial Growth and Mutagenesis. Egészségtudomány/Health Sci. 2000, 44, 331. [Google Scholar]
- Somlyai, G.; Molnár, M.; Laskay, G.; Szabó, M.; Berkényi, T.; Guller, I.; Kovács, A. Biological significance of naturally occurring deuterium: The antitumor effect of deuterium depletion. Orv. Hetil. 2010, 151, 1455. [Google Scholar] [CrossRef]
- Cuezva, J.M.; Chen, G.; Alonso, A.M.; Isidoro, A.; Misek, D.E.; Hanash, S.M.; Beer, D.G. The bioenergetic signature of lung adenocarcinomas is a molecular marker of cancer diagnosis and prognosis. Carcinogenesis 2004, 25, 1157–1163. [Google Scholar] [CrossRef]
- Zhou, W.; Mukherjee, P.; Kiebish, M.A.; Markis, W.T.; Mantis, J.G.; Seyfried, T.N. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr. Metab. 2007, 4, 5. [Google Scholar] [CrossRef]
- Khodadadi, S.; Sobhani, N.; Mirshekar, S.; Ghiasvand, R.; Pourmasoumi, M.; Miraghajani, M.; Dehsoukhteh, S.S. Tumor Cells Growth and Survival Time with the Ketogenic Diet in Animal Models: A Systematic Review. Int. J. Prev. Med. 2017, 8, 35. [Google Scholar] [CrossRef]
| Group | Subgroups | Group Size | MST (Months) |
|---|---|---|---|
| Historical control | Treated with radiotherapy | 12.1 | |
| Treated with radiotherapy + TMZ | 14.6 | ||
| All 55 patients | 55 | 30 (95% CI: 9.4–50.5) | |
| Treated without TMZ | 38 | 27 (95% CI: 18.8–35.1) | |
| Treated with TMZ | 17 | 42 (95% CI: 14.6–69.3) | |
| Males | 33 | 25 (95% CI: 15.9–34.0) | |
| Females | 22 | 42 (95% CI: 18.3–65.6) | |
| Selected 31 patients | 30 (95% CI: 0.5–59.4) | ||
| In relapse at the beginning of DDW treatment | 20 | 30 (95% CI: 22.6–37.3) | |
| In remission at the beginning of DDW treatment | 10 | 47 (95% CI: 0.0–0.0) | |
| DDW along with radiotherapy | 24 | 47 (95% CI: 18.8–75.1) | |
| DDW after radiotherapy | 7 | 25 (95% CI: 14.5–35.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somlyai, G.; Kovács, B.Z.; Papp, A.; Somlyai, I. A Preliminary Study Indicating Improvement in the Median Survival Time of Glioblastoma Multiforme Patients by the Application of Deuterium Depletion in Combination with Conventional Therapy. Biomedicines 2023, 11, 1989. https://doi.org/10.3390/biomedicines11071989
Somlyai G, Kovács BZ, Papp A, Somlyai I. A Preliminary Study Indicating Improvement in the Median Survival Time of Glioblastoma Multiforme Patients by the Application of Deuterium Depletion in Combination with Conventional Therapy. Biomedicines. 2023; 11(7):1989. https://doi.org/10.3390/biomedicines11071989
Chicago/Turabian StyleSomlyai, Gábor, Beáta Zsuzsanna Kovács, András Papp, and Ildikó Somlyai. 2023. "A Preliminary Study Indicating Improvement in the Median Survival Time of Glioblastoma Multiforme Patients by the Application of Deuterium Depletion in Combination with Conventional Therapy" Biomedicines 11, no. 7: 1989. https://doi.org/10.3390/biomedicines11071989
APA StyleSomlyai, G., Kovács, B. Z., Papp, A., & Somlyai, I. (2023). A Preliminary Study Indicating Improvement in the Median Survival Time of Glioblastoma Multiforme Patients by the Application of Deuterium Depletion in Combination with Conventional Therapy. Biomedicines, 11(7), 1989. https://doi.org/10.3390/biomedicines11071989






