Abstract
Poor quality of sleep leads to an increase in severity of the symptoms associated with fibromyalgia (FM) syndrome and vice versa. The aim of this study was to determine if the poor perceived sleep quality in FM patients could be corroborated by objective physiological determinations. Perceived sleep quality was evaluated (through the Pittsburgh Sleep Quality Index) in 68 FM patients compared to an age-matched reference group of 68 women without FM. Objective sleep quality (measured using accelerometry), and systemic concentrations of sleep-related hormones (catecholamines, oxytocin, serotonin, and melatonin) were evaluated in two representative groups from the reference control group (n = 11) and FM patients (n = 11). FM patients reported poorer subjective sleep quality compared to the reference group. However, no significant differences were found in accelerometry parameters, except for a delay in getting in and out of bed. In addition, FM patients showed no significant differences in oxytocin concentration and adrenaline/noradrenaline ratio, as well as a lower serotonin/melatonin ratio. Poor perception of sleep quality in FM patients does not correspond to objective determinations. A dysregulation of the stress response could be associated with the delay in their resting circadian rhythm and difficulty falling asleep. This would be the cause that justifies the perceived lack of rest and the fatigue they feel when waking up.
1. Introduction
While pain and fatigue are the most frequent symptoms associated with fibromyalgia (FM), most patients with this syndrome also suffer from sleep disturbances and cognitive and mood alterations [1,2]. Although numerous precedents have highlighted the role of sleep in this syndrome [3], the substantial importance of sleep disturbances and sleep quality has only recently been recognised in the aetiology of FM. In fact, the American College of Rheumatology (ACR) proposed a modification to the diagnostic criteria for FM in 2010, which, in addition to removing trigger points, emphasised the assessment of the severity of sleep problems and fatigue [4].
Sleep problems are known to be related to nonspecific pain: pain leads to decreased sleep, and sleep deprivation leads to pain, producing a repetitive circle of decreased sleep and increased pain [5,6]. It has been suggested that two thirds of FM patients have sleep disturbances, but this is not considered an underlying component in its aetiopathogenesis. However, it is accepted that there is a bidirectional relationship in which poor sleep quality leads to increased pain severity and poor cognitive performance in patients with FM [7], clearly constituting a reciprocal relationship [8,9]. Furthermore, this poor sleep quality reported in FM patients, generally assessed only subjectively, leads to worsening symptoms such as depression [10], emotional distress [11,12,13], and difficulties with memory and attention [8,14] that usually develop throughout the course of this disease [15].
Sleep is a complex physiological process related to the preservation of homeostasis and neuroplasticity, regulated globally and locally both by cellular and molecular mechanisms [16]. Most sleep researchers agree with the fact that sleep having a single function is not a realistic view, because sleep is involved in numerous vital physiological functions, such as the development and conservation of energy, modulation of immune responses, cognition, and psychological conditions [17,18], all of which have been reported to be deteriorated in FM syndrome [15,19]. Many neurotransmitters and hormones are involved in sleep regulation [20], including catecholamines (adrenaline, noradrenaline), serotonin [21,22], oxytocin [23,24], and melatonin [25,26].
In this context, as a continuation of our previous study in the present special issue on “Advanced Research on Fibromyalgia” [27], the main objective of the present investigation was to determine if the poor perceived sleep quality in FM patients could be corroborated by objective determinations, such as objective accelerometry tests and ratios of the systemic concentrations of hormones and neuromediators related to good or bad sleep quality, particularly focused on the adrenaline/noradrenaline and serotonin/melatonin ratios.
2. Materials and Methods
2.1. Participants and Experimental Design
This study was carried out on 68 women diagnosed with FM (FM patient group) belonging to the FM association EXISTIMOS ® (Badajoz, Extremadura, Spain; Extremadura is a reference region in the FM investigation due to the homogeneous population in terms of lifestyle [28,29]). All participants included in the study were within the age range of 40 to 65 years. Sixty-eight women of the same age range were used as a reference group (RG) of “healthy” women, not diagnosed with FM, CFS, any other inflammatory or rheumatic pathology, or any pathology that could potentially affect sleep quality. All volunteers from the FM association who met the inclusion criteria were selected: (a) diagnosis of FM by rheumatologists or internal medicine professionals according to ACR diagnostic criteria [30]; (b) aged between 40 and 65 years; (c) not having a diagnosis of depression; (d) not suffering from multiple chemical sensitivity; and (e) not taking corticosteroids; all of them in accordance with our previous investigations [15,27]. In the first part of the study, participants filled in the Pittsburgh Sleep Quality Index (PSQI) under supervision and with the corresponding instructions in due time and proper course.
In the second part of the study (objective determinations), a representative group from the RG and “FM patient” group were selected (RRG = 11 and RFM = 11, respectively). This reduced number of representative volunteers from each group (all volunteers were in the range of the same anthropometric characteristics and PSQI score than the RG and FM patient groups) allowed us to objectively determine the sleep quality and hormones related to sleep and stress, at the same time with the same devices, and in the same neuroendocrine assays, without interassay variations.
In order to obtain more information and given that pace of life could differ depending on whether it was a weekend or a weekday, the accelerometry study was carried out over a period of seven days. Serum and saliva samples were collected at 08:00 a.m. after the last accelerometry test to determine the systemic concentration of serotonin, oxytocin, adrenaline, noradrenaline, and melatonin using ELISA. It is important to note that on the night of saliva collection, participants were monitored with an accelerometer to ensure that no other sleep disturbances occurred that night.
Written informed consent was also requested from all participants before participating in the study. The research had been previously approved by the Bioethics Committee of the University of Extremadura by the Directives of the Council of Europe and the Declaration of Helsinki (registration number 13/2020). This study was registered with ClinicalTests.gov (identifier: NCT05323838—available on the website).
Table 1 shows the main characteristics of the participants: anthropometric data and PSQI score. At the time of the present study, all of patients were prescribed with different types of anti-inflammatory drugs (e.g., tramadol, acetaminophen, ibuprofen). Prescription drugs related to improving sleep quality were restricted.

Table 1.
Anthropometric characteristics and subjective sleep quality of the entire and representative groups.
2.2. Subjective Determination of Sleep Quality: Pittsburgh Sleep Quality Index (PSQI)
The PSQI is one of the most frequently used instruments for subjective sleep assessment. It has appropriate internal consistency, sensitivity, and specificity for the assessment of sleep in primary insomnia [31]. The Spanish version of the PSQI used by Hita-Contreras and co-workers [32] provides a robust instrument with good cross-validity for measuring sleep quality among Spanish patients with FM.
2.3. Objective Determination of Sleep Quality: Accelerometry
Objective levels of sleep quality were evaluated following previous studies from our laboratory [27]. Briefly, the Actigraph wGT3X-BT accelerometer was used to measure different objective parameters related to sleep quality: in-bed and out-bed times, latency, efficiency, wake after sleep onset (WASO), number of awakenings, and daily lux average counts. Participants wore the accelerometer on the nondominant wrist for seven consecutive days without interruption, except during showers or any water-related activity, which could disrupt proper functioning. Subsequently, the files generated by the accelerometer were analysed using a specific software called Actilifie 6 (ActiGraph, LLC, Pensacola, FL, USA) using the Cole–Kripke algorithm [33].
2.4. Saliva and Blood Samples
Blood samples were collected from fasting subjects at 08:00 a.m. on the same day of actigraphic device collection and placed in collection tubes for serum isolation, where they were kept for 15–20 min at room temperature. The serum was centrifuged at 1800× g for 15 min. Serum samples were coded and gradually refrigerated at −20 °C as they were obtained. Finally, the samples were stored at −80 °C until further analysis.
Saliva samples were extracted using a non-invasive method (collection methods: SalivaBio Oral Swab, Salimetrics, Carlsbad, CA, USA) at 08:00 a.m. Participants were required not to ingest any food or drink containing sugars, alcohol and/or caffeine, or tobacco at least 12 h before testing. Volunteers were requested to open the container and remove the sterile swab and place it correctly in the mouth, under the tongue, and were recommended to hold it for at least 2 min to ensure that there were no fluctuations in the sample volume. Immediately following this, the samples were refrigerated at −20 °C and finally stored at −80 °C until further analysis.
2.5. Determination of Neuroendocrine Markers
Serum concentrations of oxytocin (CloudClone Corp., Katy, TX, USA), serotonin (Reddot Biotech. Inc., Katy, TX, USA), adrenaline, and noradrenaline (Demeditec Diagnostic GmbH, Kiel, Germany) were measured using commercial ELISA kits. Salivary melatonin concentrations were also measured using commercial ELISA kits (Salimetrics, Carlsbad, CA, USA). Melatonin levels in serum parallel the corresponding variations in saliva, where salivary concentrations are approximately 30% of those found in serum, with a high correlation coefficient (R2 = 0.8) [34]. The measurement of salivary melatonin is advantageous, especially to avoid invasive procedures [35].
2.6. Statistical Analysis
The values are expressed as mean ± SEM. The normality of the variables was checked using the Shapiro–Wilk test, followed by Student’s t-test for normally distributed samples or Mann–Whitney test for nonparametric samples. The minimum level of significance was set at p < 0.05. Statistical analysis was performed with the SPSS® Statistics v.27.0 package.
3. Results
All participants were Caucasian women. FM patients had been diagnosed with FM for more than two years. No significant differences were found between the groups in age and BMI. FM patients showed worse subjective sleep quality (p < 0.001) with respect to the RG. The representative groups, RRG and RFM, presented age, BMI, and PSQI score in the same range than all volunteers from RG and FM patient groups. Thus, PSQI was also higher (p < 0.001) in the RFM group with respect to RRG (Table 1).
3.1. Objective Sleep Quality: Accelerometry Parameters
Table 2 shows actigraphy sleep outcomes. No significant differences were found between the two experimental groups in terms of latency (time it takes a person to fall asleep after turning the lights out), sleep efficiency (ratio of total sleep time and time in bed), WASO (wake after sleep onset), average nightly awakenings, and daily lux average counts (average lux value during scored and non-scored time), measured using accelerometry. Notably, the FM patients went to bed and woke up later than the RG women.

Table 2.
Objective sleep-related parameters measured by accelerometry.
3.2. Concentration of Hormones and Neuromediators Related to Stress and Sleep
No significant differences between FM patients and the control group (in accordance with data from our previous study [27]) were found in the systemic levels of oxytocin (1229 ± 171 µg/mL vs. 1248 ± 120 µg/mL, respectively), serotonin (135 ± 14 ng/mL vs. 271 ± 79 ng/mL, respectively), melatonin (13 ± 1 µg/mL vs. 17 ± 2 µg/mL, respectively), adrenaline (31 ± 2 µg/mL vs. 32 ± 4 µg/mL, respectively), and noradrenaline (185 ± 9 µg/mL vs. 168 ± 8 µg/mL, respectively), in this last case with a clear tendency to be higher (p < 0.07). Figure 1 represents the adrenaline/noradrenaline ratio (Figure 1A) and serotonin/melatonin ratio (Figure 1B) of their systemic concentrations. As expected, when evaluating the adrenaline/noradrenaline ratio, no significant differences were observed between the groups. However, FM patients (RFM) showed lower (p < 0.05) serotonin/melatonin values than those obtained the reference group (RRG).
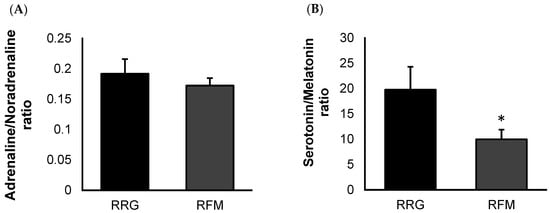
Figure 1.
Ratio of systemic concentrations of sleep-related neuromediators from representative FM patient group (RFM; n = 11) compared to an age-matched reference group of “healthy” women (RRG; n = 11): adrenaline/noradrenaline ratio (A), and serotonin/melatonin ratio (B). Values are expressed by the mean ± SEM in each group. * p < 0.05 with respect to RRG.
4. Discussion
The relevance of sleep problems in the pathophysiology of FM has generated numerous articles in recent years. Despite this, the causes of this disorder and how it might influence the many symptoms associated with the disease are still being investigated. It is well established that there is a bidirectional neuroimmunoendocrine communication that can affect the different physiological systems, so sleep dysregulation could be exacerbating the alteration of a multitude of disorders such as pain, fatigue, or low mood [36]. In addition, an altered inflammatory state could also be altering the stress response [37] and sleep [38]. Then, it seems very clear that sleep disorders can negatively affect FM symptoms and vice versa [6].
Among the different ways of assessing these sleep problems, subjective measures through questionnaires have great clinical utility due to their simplicity and low cost, but may provide results that do not exactly match those obtained through objective measurements [39]. Very few studies exist in the literature on the objective assessment of sleep in FM patients, particularly through accelerometry. In this context, sleep difficulties in FM appear to be increased when reported subjectively, using sleep quality questionnaires, but tend to have modest correlations when assessed objectively with actigraphic devices [40] or polysomnography [41]. Then, these sleep difficulties may be more a consequence of perception. In this context, our results are consistent with those of other authors who used accelerometry as an objective measure of sleep in women with FM, finding no major differences between them and healthy women [42]. Nevertheless, other authors did find that abnormal nocturnal activity patterns collected using accelerometry were associated with poorer sleep quality and greater FM symptomatology [43,44,45]. This dissonance raises the question of whether psychological disturbances influence the perception of sleep quality, or whether it is really poor sleep quality that contributes to poor mood and prevents emotional recovery from stressful experiences [8,12].
The absence of significant differences with respect to the reference control group in the objective sleep quality of FM patients is also confirmed by the results obtained in the assessment of the main neuroendocrine mediators involved in sleep. For example, the levels of oxytocin, a hormone that is clearly related to sleep quality [46] and also related to pain and depressive states in FM, were similar to those observed in the control group, in agreement with previous research in FM patients not diagnosed with depression [47]. Nevertheless, to the best of our knowledge, there are no studies evaluating the relationship between oxytocin levels and sleep quality in FM patients, which indicates the novelty of the results of the present investigation in this respect.
Regarding catecholamines, results of the present investigation agree with the few studies carried out in this context. It is known that, while adrenaline is related to high levels of stress [46] that can impair sleep, noradrenaline is strongly associated with sleep quality and maintenance [48], and people with sleep disorders have been found to have low plasma noradrenaline levels [46]. The absence of differences in the ratio of adrenaline/noradrenaline concentration seems to be in agreement with the results of accelerometry tests showing that FM patients do not have an objective sleep disturbance, at least related to these neurohormones. In fact, these results are consistent with those from Rus and co-workers [49], who found no differences in adrenaline levels in FM patients compared to healthy people, although they did have increased noradrenaline levels. These results also align with previous results from our research group where higher levels of noradrenaline were observed in FM patients compared with healthy women [19,37]. Nonetheless, the present study is the first to show a link between catecholamines and objective sleep levels in FM patients.
Even more paradoxical were the results obtained for serotonin and melatonin concentrations, which further support the absence of an objectively worse sleep quality in our FM patients, although they seem to be compatible with elevated noradrenaline levels and dysregulation of the stress response [27]. Serotonin and melatonin are two hormones that play a key role in the sleep/wake cycle [50]. In contrast to melatonin, a hormone that signals the body that it is time to sleep when it is dark, serotonin levels tend to peak in the presence of light, and are therefore associated with waking states, while during deep sleep they fall to their minimum. Stress is one of the most common causes of low serotonin levels and this causes a cascade of events: insomnia, depression, daytime fatigue, and anxiety, which in turn causes sleep problems [51]. In fact, there is strong evidence that deficiency in normal serotonergic functioning may be related to the pathophysiology of FM [52]. On the other hand, melatonin has been measured in FM patients in numerous studies, finding different results, ranging from normal [53] to decreased [54] and even increased [55] melatonin levels. It has been suggested that altered melatonin levels may disrupt night-time sleep in FM patients, which could lead to altered pain perception in the early morning [56]. One of the major postulated hypotheses suggests that alterations in melatonin secretion could also cause changes in the daytime secretion of hormones related to the HPA axis [57], and this dysregulation, as mentioned above, could promote specific symptoms of this disease. Furthermore, numerous studies have reported that the potential dysregulation described on the secretion of cortisol, serotonin, cytokines [37,58], and melatonin [54,55] would lead to alterations in the circadian rhythm in these patients, which could contribute to sleep disturbance at night, fatigue during the day, and altered pain perception [56]. Thus, in this context, the serotonin/melatonin ratio is also important in order to evaluate objective sleep quality, and a lower ratio in FM patients compared with the reference group without FM does not seem to be consistent with an impaired sleep quality, reinforcing accelerometry and catecholamine ratio results.
So, the question is: why do FM patients perceive that they sleep poorly? This subjective perception of poor sleep quality could be related to a reduced physiological and psychological capacity to regulate stress responses [19], which could also influence a delay and dysregulation of the rest–activity circadian rhythm that is objectively manifested by delayed bedtime and wake-up times and decreased serotonin/melatonin ratio, more compatible with nocturnal levels in healthy people (according to manufacturing information).
5. Conclusions
Considering the results obtained in the present investigation, it cannot be concluded that patients with FM present objectively worse sleep quality than the reference group of women of the same age range without FM. This is corroborated both by accelerometry determinations and by the absence of impaired ratios of the main neuroendocrine biomarkers involved in sleep.
The results of this pilot study in a group of patients diagnosed with FM may contribute to a better understanding of the pathophysiology and aetiology of this syndrome, particularly in relation to sleep and, therefore, to perceived fatigue. The present research also has a number of limitations that will have to be addressed in the future. One of these may be the limited sample size in the second part of the study, related to objective determinations. This will be need to be carried out mainly in clinical tests by increasing the number of patients and assessing the levels of all the evaluated objective biomarkers, but particularly those of melatonin (but also serotonin and catecholamines), over a greater number of hours throughout the day and particularly during night.
Author Contributions
Conceptualization, E.O. (Eduardo Ortega) and M.D.H.; methodology, E.O. (Eduardo Otero), M.D.H., I.G., and L.M.-C.; formal analysis, E.O. (Eduardo Otero), M.d.C.N. and M.D.H.; investigation, M.D.H., E.O. (Eduardo Ortega) and E.O. (Eduardo Otero); writing—original draft preparation, M.D.H. and E.O. (Eduardo Otero); writing—review and editing, M.D.H., I.G. and E.O. (Eduardo Ortega); supervision, E.O. (Eduardo Ortega); project administration, E.O. (Eduardo Ortega); funding acquisition, E.O. (Eduardo Ortega). All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Gobierno de Extremadura—Fondo Europeo de Desarrollo Regional, Spain (GR21079) and by aid to promote the hiring of research support staff in the Autonomous Community of Extremadura (TE-0027-19).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of University of Extremadura, Spain (N Reg. 73/2021, 9 June 2021). This study was registered on ClinicalTrials.gov (identifier: NCT05323838).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The raw data supporting the conclusions of the manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Acknowledgments
We are grateful to the associations of Fibromyalgia and Chronic Fatigue Syndrome belonging to the community of Extremadura: EXISTIMOS (Badajoz, Spain).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Choy, E.H.S. The Role of Sleep in Pain and Fibromyalgia. Nat. Rev. Rheumatol. 2015, 11, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann. Intern. Med. 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Moldofsky, H. The Significance, Assessment, and Management of Nonrestorative Sleep in Fibromyalgia Syndrome. CNS Spectr. 2008, 13, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Affleck, G.; Urrows, S.; Tennen, H.; Higgins, P.; Abeles, M. Sequential Daily Relations of Sleep, Pain Intensity, and Attention to Pain among Women with Fibromyalgia. Pain 1996, 68, 363–368. [Google Scholar] [CrossRef]
- Nicassio, P.M.; Moxham, E.G.; Schuman, C.E.; Gevirtz, R.N. The Contribution of Pain, Reported Sleep Quality, and Depressive Symptoms to Fatigue in Fibromyalgia. Pain 2002, 100, 271–279. [Google Scholar] [CrossRef]
- Andrade, A.; Vilarino, G.T.; Sieczkowska, S.M.; Coimbra, D.R.; Bevilacqua, G.G.; Steffens, R.A.K. The Relationship between Sleep Quality and Fibromyalgia Symptoms. J. Health Psychol. 2020, 25, 1176–1186. [Google Scholar] [CrossRef]
- Miró, E.; Lupiáñez, J.; Hita, E.; Martínez, M.P.; Sánchez, A.I.; Buela-Casal, G. Attentional Deficits in Fibromyalgia and Its Relationships with Pain, Emotional Distress and Sleep Dysfunction Complaints. Psychol. Health 2011, 26, 765–780. [Google Scholar] [CrossRef]
- Fang, S.; Wu, Y.; Chen, S.; Teng, H.; Tsai, P. Subjective Sleep Quality as a Mediator in the Relationship between Pain Severity and Sustained Attention Performance in Patients with Fibromyalgia. J. Sleep Res. 2019, 28, e12843. [Google Scholar] [CrossRef]
- Diaz-Piedra, C.; Catena, A.; Sánchez, A.I.; Miró, E.; Martínez, M.P.; Buela-Casal, G. Sleep Disturbances in Fibromyalgia Syndrome: The Role of Clinical and Polysomnographic Variables Explaining Poor Sleep Quality in Patients. Sleep Med. 2015, 16, 917–925. [Google Scholar] [CrossRef]
- Máñez, I.; Fenollosa, P.; Martínez-Azucena, A.; Salazar, A. Sleep Quality, Pain and Depression in Fibromyalgia. Rev. Esp. DOLOR 2005, 12, 491. [Google Scholar]
- Hamilton, N.A.; Catley, D.; Karlson, C. Sleep and the Affective Response to Stress and Pain. Health Psychol. 2007, 26, 288. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.A.; Affleck, G.; Tennen, H.; Karlson, C.; Luxton, D.; Preacher, K.J.; Templin, J.L. Fibromyalgia: The Role of Sleep in Affect and in Negative Event Reactivity and Recovery. Health Psychol. 2008, 27, 490. [Google Scholar] [CrossRef] [PubMed]
- Coté, K.; Moldofsky, H. Sleep, Daytime Symptoms, and Cognitive Performance in Patients with Fibromyalgia. J. Rheumatol. 1997, 24, 2014–2023. [Google Scholar]
- Hinchado, M.D.; Otero, E.; del Navarro, M.C.; Martín-Cordero, L.; Gálvez, I.; Ortega, E. Influence of Codiagnosis of Chronic Fatigue Syndrome and Habitual Physical Exercise on the Psychological Status and Quality of Life of Patients with Fibromyalgia. J. Clin. Med. 2022, 11, 5735. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.R.; McKenna, J.T.; McCarley, R.W. Functions and Mechanisms of Sleep. AIMS Neurosci. 2016, 3, 67. [Google Scholar] [CrossRef]
- Siegel, J.M. Do All Animals Sleep? Trends Neurosci. 2008, 31, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.R.; Krueger, J.M. Sleep and Innate Immunity. Front. Biosci. (Schol. Ed.) 2011, 3, 632. [Google Scholar]
- Hinchado, M.D.; Quero-Calero, C.D.; Otero, E.; Gálvez, I.; Ortega, E. Synbiotic Supplementation Improves Quality of Life and Inmunoneuroendocrine Response in Patients with Fibromyalgia: Influence of Codiagnosis with Chronic Fatigue Syndrome. Nutrients 2023, 15, 1591. [Google Scholar] [CrossRef]
- Mong, J.A.; Cusmano, D.M. Sex Differences in Sleep: Impact of Biological Sex and Sex Steroids. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150110. [Google Scholar] [CrossRef]
- Melancon, M.O.; Lorrain, D.; Dionne, I.J. Exercise and Sleep in Aging: Emphasis on Serotonin. Pathol. Biol. 2014, 62, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Tadi, P. Biochemistry, Serotonin. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2021. [Google Scholar]
- Schuh-Hofer, S.; Eichhorn, N.; Grinevich, V.; Treede, R.-D. Sleep Deprivation Related Changes of Plasma Oxytocin in Males and Female Contraceptive Users Depend on Sex and Correlate Differentially with Anxiety and Pain Hypersensitivity. Front. Behav. Neurosci. 2018, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, S.C.; Liu, X.; Jia, S.; Wang, X.; Li, T.; Yu, J.; Parpura, V.; Wang, Y.-F. Neural Functions of Hypothalamic Oxytocin and Its Regulation. ASN Neuro 2022, 14, 17590914221100706. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New Perspectives on the Role of Melatonin in Human Sleep, Circadian Rhythms and Their Regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Poza, J.J.; Pujol, M.; Ortega-Albás, J.J.; Romero, O. Melatonin in Sleep Disorders. Neurologia 2022, 37, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Otero, E.; Gálvez, I.; Ortega, E.; Hinchado, M.D. Influence of Chronic Fatigue Syndrome Codiagnosis on the Relationship between Perceived and Objective Psychoneuro-Immunoendocrine Disorders in Women with Fibromyalgia. Biomedicines 2023, 11, 1488. [Google Scholar] [CrossRef]
- Aleixandre Benavent, R.; Alonso Arroyo, A.; Anguita Sánchez, M.; Bolaños Pizarro, M.; Heras, M.; González Alcalde, G.; Macaya Miguel, C.; Navarro Molina, C.; Castelló Cogollos, L.; Valderrama Zurián, J.C.; et al. Evolution and Scientific Impact of Research Grants from the Spanish Society of Cardiology and Spanish Heart Foundation (2000–2006). Rev. Esp. Cardiol. (Engl. Ed.) 2011, 64, 904–915. [Google Scholar] [CrossRef]
- Félix-Redondo, F.J.; Fernández-Bergés, D.; Fernando Pérez, J.; Zaro, M.J.; García, A.; Lozano, L.; Sanz, H.; Grau, M.; Álvarez-Palacios, P.; Tejero, V. Prevalence, Awareness, Treatment and Control of Cardiovascular Risk Factors in the Extremadura Population (Spain). HERMEX Study. Aten. Primaria 2011, 43, 426–434. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria. In Proceedings of the Seminars in Arthritis and Rheumatism; Elsevier: Amsterdam, The Netherlands, 2016; Volume 46, pp. 319–329. [Google Scholar]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Test–Retest Reliability and Validity of the Pittsburgh Sleep Quality Index in Primary Insomnia. J. Psychosom. Res. 2002, 53, 737–740. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Martínez-López, E.; Latorre-Román, P.A.; Garrido, F.; Santos, M.A.; Martínez-Amat, A. Reliability and Validity of the Spanish Version of the Pittsburgh Sleep Quality Index (PSQI) in Patients with Fibromyalgia. Rheumatol. Int. 2014, 34, 929–936. [Google Scholar] [CrossRef]
- Cole, R.J.; Kripke, D.F.; Gruen, W.; Mullaney, D.J.; Gillin, J.C. Automatic Sleep/Wake Identification from Wrist Activity. Sleep 1992, 15, 461–469. [Google Scholar] [CrossRef]
- Nowak, R.; Mcmillen, I.C.; Redman, J.; Short, R.V. The correlation between serum and salivary melatonin concentrations and urinary 6-hydroxymelatonin sulphate excretion rates: Two non-invasive techniques for monitoring human circadian rhythmicity. Clin. Endocrinol. 1987, 27, 445–452. [Google Scholar] [CrossRef]
- de Almeida, E.A.; Di Mascio, P.; Harumi, T.; Warren Spence, D.; Moscovitch, A.; Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Measurement of Melatonin in Body Fluids: Standards, Protocols and Procedures. Child’s Nerv. Syst. 2011, 27, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Prados, G.; Miró, E. Fibromialgia y Sueño: Una Revisión. Rev. Neurol. 2012, 54, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; García, J.J.; Bote, M.E.; Martín-Cordero, L.; Escalante, Y.; Saavedra, J.M.; Northoff, H.; Giraldo, E. Exercise in Fibromyalgia and Related Inflammatory Disorders: Known Effects and Unknown Chances. Exerc. Immunol. Rev. 2009, 15, 42–65. [Google Scholar]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Çetin, B.; Güleç, H.; Toktaş, H.E.; Ulutaş, Ö.; Yılmaz, S.G.; İsbir, T. Objective Measures of Sleep in Fibromyalgia Syndrome: Relationship to Clinical, Psychiatric, and Immunological Variables. Psychiatry Res. 2018, 263, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Chang, L.-Y.; Lee, H.-C.; Fang, S.-C.; Tsai, P.-S. Sleep Disturbances in Fibromyalgia: A Meta-Analysis of Case-Control Studies. J. Psychosom. Res. 2017, 96, 89–97. [Google Scholar] [CrossRef]
- Alge, O.; Soroushmehr, S.M.R.; Gryak, J.; Kratz, A.; Najarian, K. Predicting Poor Sleep Quality in Fibromyalgia with Wrist Sensors. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 4290–4293. [Google Scholar]
- Segura-Jiménez, V.; Camiletti-Moirón, D.; Munguía-Izquierdo, D.; Álvarez-Gallardo, I.C.; Ruiz, J.R.; Ortega, F.B.; Delgado-Fernández, M. Agreement between Self-Reported Sleep Patterns and Actigraphy in Fibromyalgia and Healthy Women. Clin Exp. Rheumatol. 2015, 33, S58–S67. [Google Scholar]
- Korszun, A.; Young, E.A.; Engleberg, N.C.; Brucksch, C.B.; Greden, J.F.; Crofford, L.A. Use of Actigraphy for Monitoring Sleep and Activity Levels in Patients with Fibromyalgia and Depression. J. Psychosom. Res. 2002, 52, 439–443. [Google Scholar] [CrossRef]
- Landis, C.A.; Frey, C.A.; Lentz, M.J.; Rothermel, J.; Buchwald, D.; Shaver, J.L.F. Self-Reported Sleep Quality and Fatigue Correlates with Actigraphy in Midlife Women with Fibromyalgia. Nurs. Res. 2003, 52, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Okifuji, A.; Hare, B.D. Nightly Analyses of Subjective and Objective (Actigraphy) Measures of Sleep in Fibromyalgia Syndrome: What Accounts for the Discrepancy? Clin. J. Pain 2011, 27, 289. [Google Scholar] [CrossRef] [PubMed]
- Cerro, D.-D.; Félix, J.; Tresguerres, J.A.F.; De la Fuente, M. Improvement of Several Stress Response and Sleep Quality Hormones in Men and Women after Sleeping in a Bed That Protects against Electromagnetic Fields. Environ. Health 2022, 21, 72. [Google Scholar] [CrossRef]
- Anderberg, U.M.; Uvnäs-Moberg, K. Plasma Oxytocin Levels in Female Fibromyalgia Syndrome Patients. Z. Rheumatol. 2000, 59, 373–379. [Google Scholar] [CrossRef]
- Gottesmann, C. The Involvement of Noradrenaline in Rapid Eye Movement Sleep Mentation. Front. Neurol. 2011, 2, 81. [Google Scholar] [CrossRef]
- Rus, A.; Molina, F.; Del Moral, M.L.; Ramírez-Expósito, M.J.; Martínez-Martos, J.M. Catecholamine and Indolamine Pathway: A Case-Control Study in Fibromyalgia. Biol. Res. Nurs. 2018, 20, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef]
- van den Buuse, M.; Hale, M.W. Chapter 10—Serotonin in Stress. In Stress: Physiology, Biochemistry, and Pathology; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 115–123. ISBN 978-0-12-813146-6. [Google Scholar]
- Neeck, G. Pathogenic Mechanisms of Fibromyalgia. Ageing Res. Rev. 2002, 1, 243–255. [Google Scholar] [CrossRef]
- Press, J.; Phillip, M.; Neumann, L.; Barak, R.; Segev, Y.; Abu-Shakra, M.; Buskila, D. Normal Melatonin Levels in Patients with Fibromyalgia Syndrome. J. Rheumatol. 1998, 25, 551–555. [Google Scholar]
- Wikner, J.; Hirsch, U.; Wetterberg, L.; Röjdmark, S. Fibromyalgia—A Syndrome Associated with Decreased Nocturnal Melatonin Secretion. Clin. Endocrinol. 1998, 49, 179–183. [Google Scholar] [CrossRef]
- Korszun, A.; Sackett-Lundeen, L.; Papadopoulos, E.; Brucksch, C.; Masterson, L.; Engelberg, N.C.; Haus, E.; Demitrack, M.A.; Crofford, L. Melatonin Levels in Women with Fibromyalgia and Chronic Fatigue Syndrome. J. Rheumatol. 1999, 26, 2675–2680. [Google Scholar] [PubMed]
- Mahdi, A.A.; Fatima, G.; Das, S.K.; Verma, N.S. Abnormality of Circadian Rhythm of Serum Melatonin and Other Biochemical Parameters in Fibromyalgia Syndrome. Indian J. Biochem. Biophys. 2011, 48, 82–87. [Google Scholar] [PubMed]
- Webb, S.M. Fibromyalgia and Melatonin: Are They Related? Clin. Endocrinol. 1998, 49, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Bote, M.E.; Garca, J.J.; Hinchado, M.D.; Ortega, E. Inflammatory/Stress Feedback Dysregulation in Women with Fibromyalgia. Neuroimmunomodulation 2012, 19, 343–351. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).