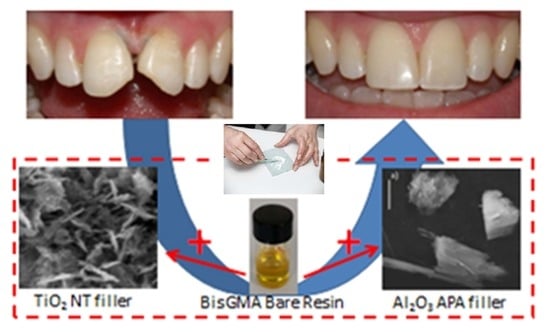
Innovative Nanostructured Fillers for Dental Resins: Nanoporous Alumina and Titania Nanotubes
Abstract
1. Introduction
2. Materials and Methods
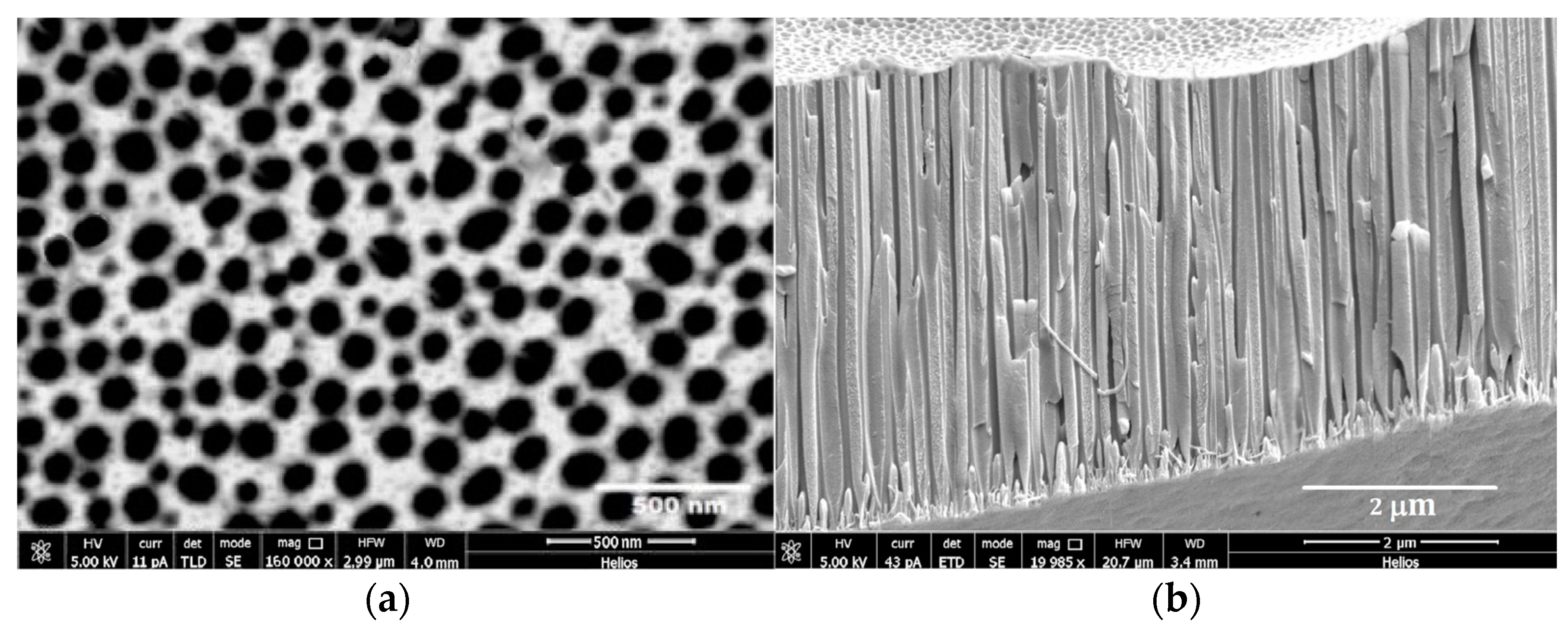
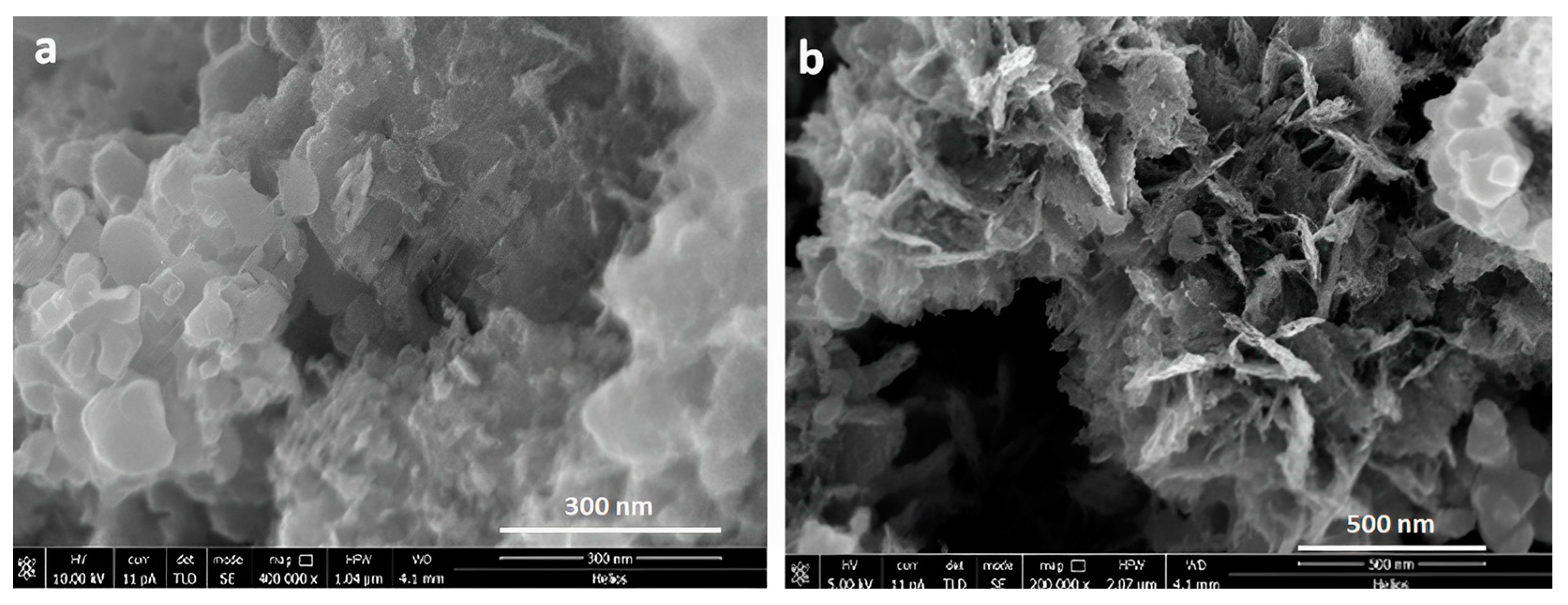
2.1. APA Preparation
2.2. TiO2 Nanotube Preparation
2.3. Preparation of Resin Composite Samples
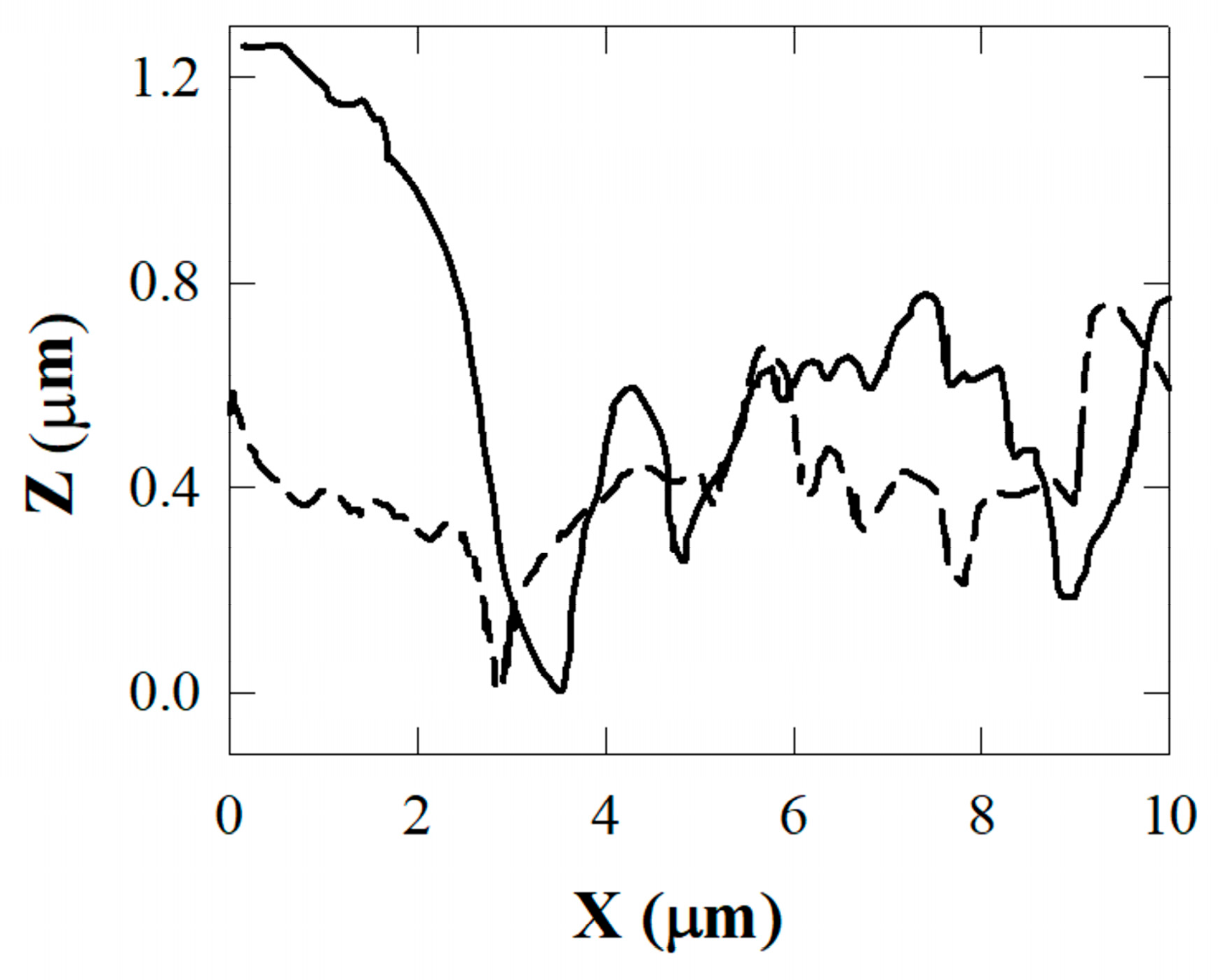
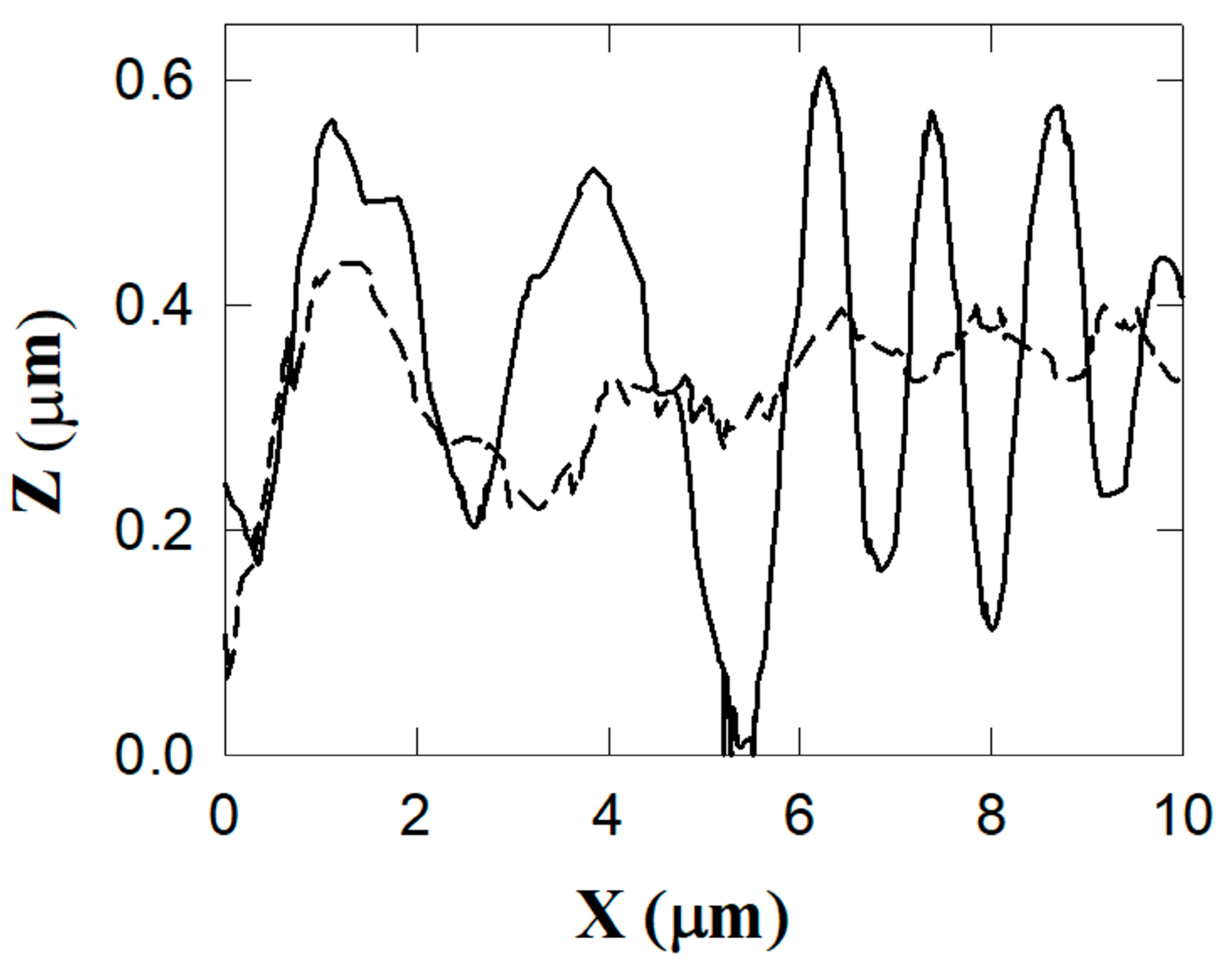
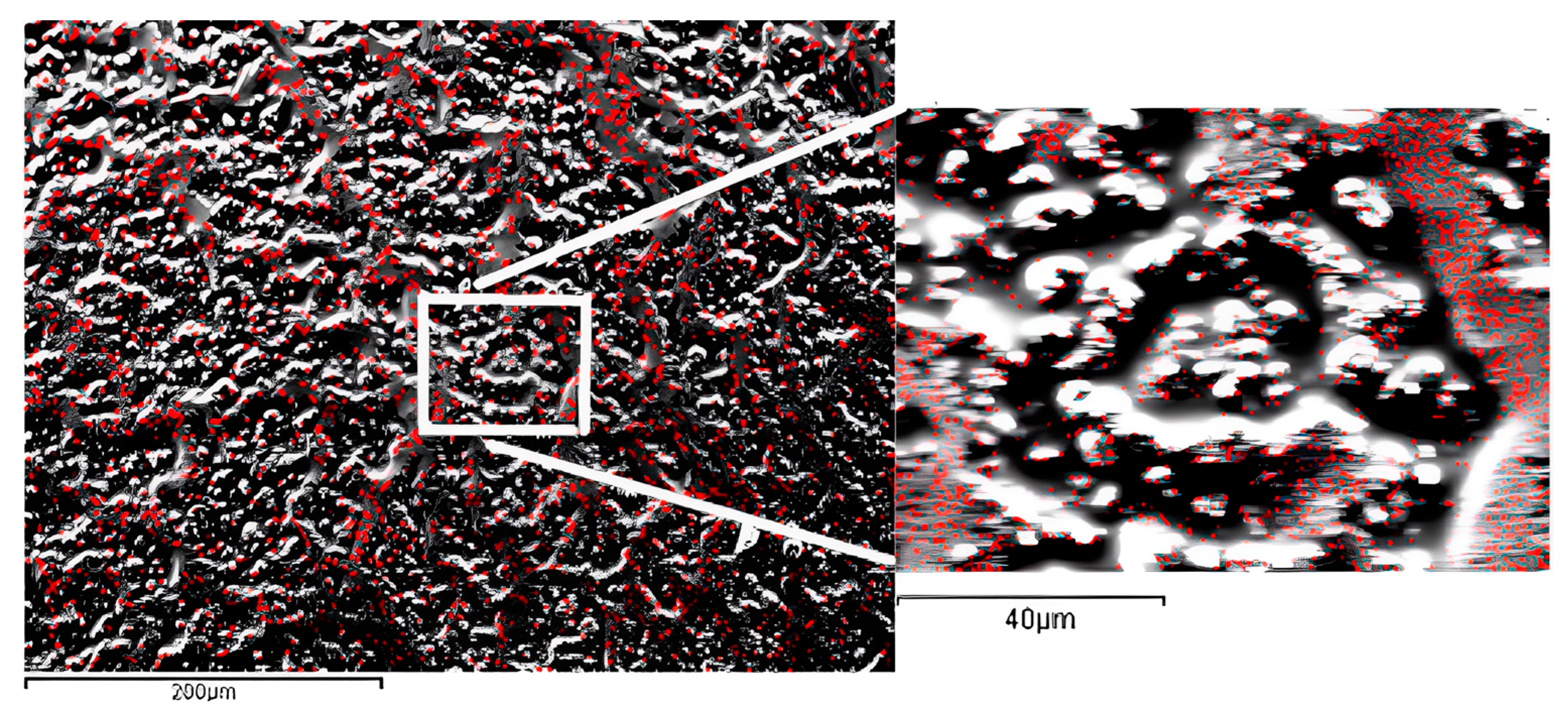
2.4. Atomic Force Microscopy (AFM), Scanning Electron Microscopy (SEM) Characterization, and X-ray Chemical Analysis
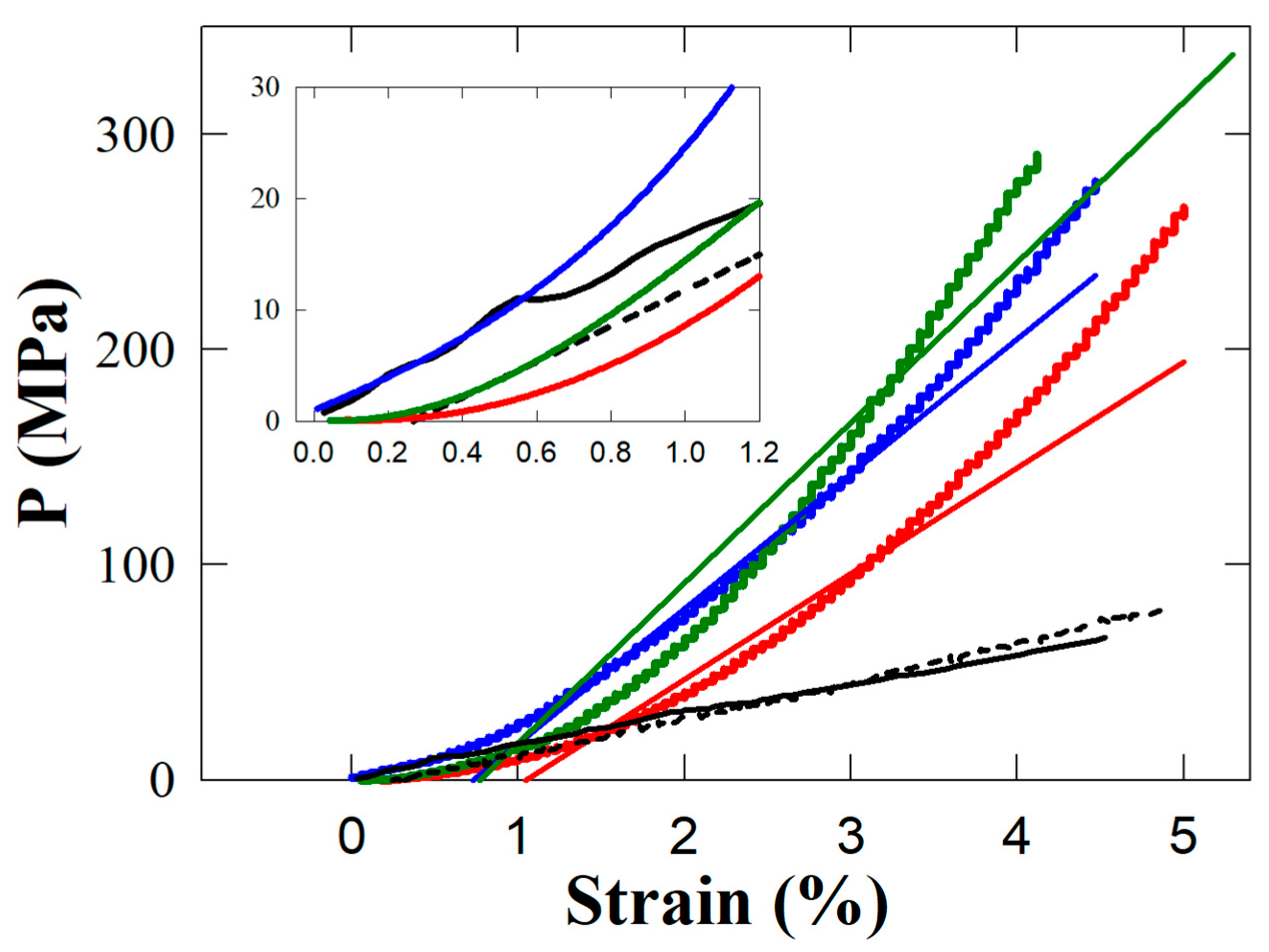
2.5. Mechanical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferracane, L. Resin composite-State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Heintze, S.D.; Rousson, V. Clinical Effectiveness of Direct Class II Restorations—A Meta-Analysis. J. Adhes. Dent. 2012, 14, 407–431. [Google Scholar] [CrossRef]
- Klapdohr, S.; Moszner, N. New Inorganic Components for Dental Filling Composites. Monatshefte Chem. 2005, 136, 21–45. [Google Scholar] [CrossRef]
- Kobayashi, M.; Abdulmajeed, A.; Moon, J.; Punkkinen, R.; Shimada, J.; Vallittu, P.; Lassila, L. Effect of UV on wettability and bacterial adhesion of TiO2-nanotubes. Dent. Mater. 2014, 30, e160–e161. [Google Scholar] [CrossRef]
- Hurst, D. Amalgam or composite fillings—Which material lasts longer? Evid. Based Dent. 2014, 15, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Rasines Alcaraz, M.G.; Veitz-Keenan, A.; Sahrmann, P.; Schmidlin, P.R.; Davis, D.; Iheozor-Ejiofor, Z. Direct composite resin fillings versus amalgam fillings for permanent or adult posterior teeth. Cochrane Database Syst. Rev. 2014, 31, CD005620. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, D.; Belli, R.; Petschelt, A.; Lohbauer, U. Are resin composites suitable replacements for amalgam? A study of two-body wear. Clin. Oral Investig. 2015, 19, 1485–1492. [Google Scholar] [CrossRef]
- Scholtanus, J.D.; Özcan, M. Clinical longevity of extensive direct composite restorations in amalgam replacement: Up to 3.5 years follow-up. J. Dent. 2014, 42, 1404–1410. [Google Scholar] [CrossRef]
- Bastos, N.A.; Bitencourt, S.B.; Martins, E.A.; De Souza, G.M. Review of nano-technology applications in resin-based restorative materials. J. Esthet. Restor. Dent. 2021, 33, 567–582. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jo, J.-K.; Kim, D.-A.; Patel, K.D.; Kim, H.-W.; Lee, H.-H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef]
- Kim, K.-I.; Kim, D.-A.; Patel, K.D.; Shin, U.S.; Kim, H.-W.; Lee, J.-H.; Lee, H.-H. Carbon nanotube incorporation in PMMA to prevent microbial adhesion. Sci. Rep. 2019, 9, 4921. [Google Scholar] [CrossRef] [PubMed]
- Bin Jo, S.; Kim, H.K.; Lee, H.N.; Kim, Y.-J.; Patel, K.D.; Knowles, J.C.; Lee, J.-H.; Song, M. Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro. Nanomaterials 2020, 10, 1750. [Google Scholar] [CrossRef]
- Salerno, M.; Loria, P.; Matarazzo, G.; Tomè, F.; Diaspro, A.; Eggenhöffner, R. Surface Morphology and Tooth Adhesion of a Novel Nanostructured Dental Restorative Composite. Materials 2016, 9, 203. [Google Scholar] [CrossRef]
- Salerno, M.; Caneva-Soumetz, F.; Pastorino, L.; Patra, N.; Diaspro, A.; Ruggiero, C. Adhesion and Proliferation of Osteoblast-Like Cells on Anodic Porous Alumina Substrates with Different Morphology. IEEE Trans. NanoBiosci. 2013, 12, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Alfouzan, A.; Alnouwaisar, A.; Alazzam, N.; Al-Otaibi, H.; Labban, N.; Alswaidan, M.; Al-Taweel, S.; Alshehri, H. Surface roughness analysis of prepolymerized CAD/CAM dental acrylic resins following combined surface treatments. Mater. Sci. 2021, 39, 209–218. [Google Scholar] [CrossRef]
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A review of dental composites: Challenges, chemistry aspects, filler influences, and future insights. Compos. Part B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- MikroMasch. Available online: https://www.spmtips.com/afm-tip-hq-nsc18-cr-au (accessed on 12 May 2023).
- Thorat, S.B.; Diaspro, A.; Salerno, M. In vitro investigation of coupling-agent-free dental restorative composite based on nano-porous alumina fillers. J. Dent. 2014, 42, 279–286. [Google Scholar] [CrossRef]
- Garcés, F.A.; Acquaroli, L.N.; Arce, R.D. Fabricación y caracterización de nanoporos ordenados de Al2O3 obtenidos por anodización electroquímica del alumínio. Asoc. Argent. Mater. 2010, 7, 19–26. [Google Scholar]
- Thorat, S.B. Dental Materials Based on Nano-Scale Anodic Porous Alumina. Ph.D. Thesis, Università degli Studi di Genova and Istituto Italiano di Tecnologia, Genoa, Italy, 2014. [Google Scholar]
- Toccafondi, C.; Stępniowski, W.; Leoncini, M.; Salerno, M. Advanced morphological analysis of patterns of thin anodic porous alumina. Mater. Charact. 2014, 94, 26–36. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Zhu, X. Functional fillers for dental resin composites. Acta Biomater. 2020, 122, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Dafar, M.O.; Grol, M.W.; Canham, P.B.; Dixon, S.J.; Rizkalla, A.S. Reinforcement of flowable dental com-posites with titanium dioxide nanotubes. Dent. Mater. 2016, 32, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Han, Q.; Jiang, A.; Wang, Y.; Li, R.; Wang, Y.; Xiao, S.; Wei, R.; Ma, Y. BNN/TiO2 nanocomposite system–modified dental flow resins and the mechanism of the enhancement of mechanical and antibacterial properties. Biomater. Sci. 2023, 11, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanhossaini, A.; Rafiee, R.; Pligovka, A.; Salerno, M. Dental composites with strength after aging improved by using anodic nanoporous fillers: Experimental results, modeling, and simulations. Eng. Comput. 2022, 39, 387–398. [Google Scholar] [CrossRef]
- Rüttermann, S.; Trellenkamp, T.; Bergmann, N.; Raab, W.H.-M.; Ritter, H.; Janda, R. A new approach to influence contact angle and surface free energy of resin-based dental restorative materials. Acta Biomater. 2011, 7, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Flury, S.; Peutzfeldt, A.; Lussi, A. Influence of surface roughness on mechanical properties of two computer-aided de-sign/computer-aided manufacturing (CAD/CAM) ceramic materials. Oper. Dent. 2012, 37, 617–624. [Google Scholar] [CrossRef]
- Tekçe, N.; PALA, K.; Tuncer, S.; Demirci, M. The effect of surface sealant application and accelerated aging on posterior restorative surfaces: An SEM and AFM study. Dent. Mater. J. 2017, 36, 182–189. [Google Scholar] [CrossRef]
- Chun, K.; Choi, H.; Lee, J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 2014, 5, 1758736014520809. [Google Scholar] [CrossRef]
- Gu, Y.; Bai, Y.; Xie, X. Bite Force Transducers and Measurement Devices. Front. Bioeng. Biotechnol. 2021, 9, 665081. [Google Scholar] [CrossRef]
- Thorat, S.; Diaspro, A.; Salerino, M. Effect of Alumina Reinforcing Fillers In BisGMA-based Resin Composites For Dental Applications. Adv. Mater. Lett. 2013, 4, 15–21. [Google Scholar] [CrossRef]
- Fauzi, N.A.; Ireland, A.; Sherriff, M.; Bandara, H.; Su, B. Nitrogen doped titanium dioxide as an aesthetic antimicrobial filler in dental polymers. Dent. Mater. 2020, 38, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, M.; Pan, Y.; Sun, S.; Shen, T.; Wei, X.; Zhu, Y.; Liu, J.; Huang, Q. Control of surface composition and microstructure of nano super-hydrophilic TiO2-CuOy coatings through reactive sputtering to improve antibacterial ability, corrosion resistance, and biocompatibility. Appl. Surf. Sci. 2021, 578, 151893. [Google Scholar] [CrossRef]
- Ding, Z.; He, Q.; Ding, Z.; Liao, C.; Chen, D.; Ou, L. Fabrication and Performance of ZnO Doped Tantalum Oxide Multilayer Composite Coatings on Ti6Al4V for Orthopedic Application. Nanomaterials 2019, 9, 685. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Hilton, T.J.; Heintze, S.D.; Hickel, R.; Watts, D.C.; Silikas, N.; Stansbury, J.W.; Cadenaro, M.; Ferracane, J.L. Academy of Dental Materials guidance—Resin composites: Part I—Mechanical properties. Dent. Mater. 2017, 33, 880–894. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggenhöffner, R.; Ghisellini, P.; Rando, C.; Pechkova, E.; Terencio, T.; Mazzolai, B.; Giacomelli, L.; Barbaro, K.; Benedicenti, S. Innovative Nanostructured Fillers for Dental Resins: Nanoporous Alumina and Titania Nanotubes. Biomedicines 2023, 11, 1926. https://doi.org/10.3390/biomedicines11071926
Eggenhöffner R, Ghisellini P, Rando C, Pechkova E, Terencio T, Mazzolai B, Giacomelli L, Barbaro K, Benedicenti S. Innovative Nanostructured Fillers for Dental Resins: Nanoporous Alumina and Titania Nanotubes. Biomedicines. 2023; 11(7):1926. https://doi.org/10.3390/biomedicines11071926
Chicago/Turabian StyleEggenhöffner, Roberto, Paola Ghisellini, Cristina Rando, Eugenia Pechkova, Tercio Terencio, Barbara Mazzolai, Luca Giacomelli, Katia Barbaro, and Stefano Benedicenti. 2023. "Innovative Nanostructured Fillers for Dental Resins: Nanoporous Alumina and Titania Nanotubes" Biomedicines 11, no. 7: 1926. https://doi.org/10.3390/biomedicines11071926
APA StyleEggenhöffner, R., Ghisellini, P., Rando, C., Pechkova, E., Terencio, T., Mazzolai, B., Giacomelli, L., Barbaro, K., & Benedicenti, S. (2023). Innovative Nanostructured Fillers for Dental Resins: Nanoporous Alumina and Titania Nanotubes. Biomedicines, 11(7), 1926. https://doi.org/10.3390/biomedicines11071926