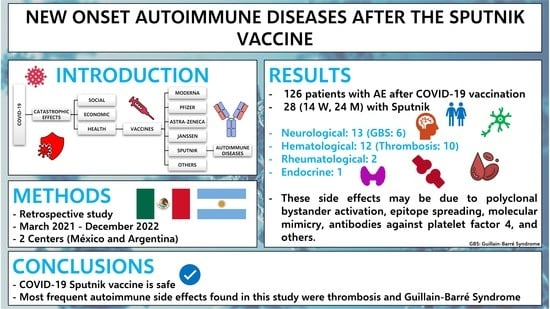
New Onset Autoimmune Diseases after the Sputnik Vaccine
Abstract
1. Introduction
2. Patients and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615 . [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.; Shuai, Z.; Ye, D.; Pan, H. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Adu, P.; Popoola, T.; Medvedev, O.N.; Collings, S.; Mbinta, J.; Aspin, C.; Simpson, C.R. Implications for COVID-19 vaccine uptake: A systematic review. J. Infect. Public Health 2023, 16, 441–466. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of a rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomized controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Erra, L.; Uriarte, I.; Colado, A.; Paolini, M.V.; Seminario, G.; Fernández, J.B.; Tau, L.; Bernatowiez, J.; Moreira, I.; Vishnopolska, S.; et al. COVID-19 Vaccination Responses with Different Vaccine Platforms in Patients with Inborn Errors of Immunity. J. Clin. Immunol. 2022, 43, 271–285. [Google Scholar] [CrossRef]
- Babamahmoodi, F.; Saeedi, M.; Alizadeh-Navaei, R.; Hedayatizadeh-Omran, A.; Mousavi, S.A.; Ovaise, G.; Kordi, S.; Akbari, Z.; Azordeh, M.; Ahangarkani, F.; et al. Side effects and Immunogenicity following administration of the Sputnik V COVID-19 vaccine in health care workers in Iran. Sci Rep. 2021, 11, 21464. [Google Scholar] [CrossRef]
- Oghazian, S.; Tavanaei Tamanaei, T.; Haghighi, R.; Faregh, M.; Oghazian, M.B. Side effects of Sputnik V, Oxford-AstraZeneca, Sinopharm, and Covaxin and their associations with other variables among healthcare workers of a tertiary hospital in Iran. Int. Immunopharmacol. 2023, 117, 109784. [Google Scholar] [CrossRef]
- Shokraee, K.; Rezaei, S.S.; Shahriarian, S.; Masoumi, M.; Parsaei, A.; Amini, B. Reactive Arthritis after Gam-COVID-Vac Vaccination: A Case Report. Mod. Rheumatol. Case Rep. 2023, 7, 368–372. [Google Scholar] [CrossRef]
- Ameri, M.; Abolmaali, M.; Alwedaie, S.M.J.; Nabavi, M.; Rahimian, N.; Hamidabad, N.M. Severe Persistent Eczema in a Recipient of the Gam-COVID-Vac vaccine. Eur. J. Case Rep. Intern. Med. 2022, 9, 003042. [Google Scholar] [CrossRef]
- Herrera-Comoglio, R.; Lane, S. Vaccine-Induced Immune Thrombocytopenia and Thrombosis after the Sputnik V Vaccine. N. Engl. J. Med. 2022, 387, 1431–1432. [Google Scholar] [CrossRef]
- Ismail, I.I.; Salama, S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J. Neuroimmunol. 2022, 362, 577765. [Google Scholar] [CrossRef]
- Albakri, K.; Khaity, A.; Atwan, H.; Saleh, O.; Al-Hajali, M.; Cadri, S.; Diab, R.A.; Albazee, E.; Negida, A. Bell’s Palsy and COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2023, 11, 236. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Palankar, R.; Wesche, J.; Handtke, S.; Wolff, M.; Aurich, K.; Lalk, M.; Methling, K.; Völker, U.; et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood 2021, 138, 2256–2268. [Google Scholar] [CrossRef]
- Klok, F.A.; Pai, M.; Huisman, M.V.; Makris, M. Vaccine-induced immune thrombotic thrombocitopenia. Lancet Haematol. 2022, 9, e73–e80. [Google Scholar] [CrossRef]
- Vallone, M.G.; Falcón, A.L.; Castro, H.M.; Ferraris, A.; Cantarella, R.F.; Staneloni, M.I.; Aliperti, V.I.; Ferloni, A.; Mezzarobba, D.; Vázquez, F.J.; et al. Thrombotic events following COVID-19 vaccines. Eur. J. Intern. Med. 2022, 99, 82–88. [Google Scholar] [CrossRef]
- Mani, A.; Ojha, V. Thromboembolism after COVID-19 Vaccination: A Systematic Review of Such Events in 286 Patients. Ann. Vasc. Surg. 2022, 84, 12–20.e1. [Google Scholar] [CrossRef]
- McGonagle, D.; Bridgewood, C.; Ramanan, A.V.; Meaney, J.F.M.; Watad, A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021, 3, e224–e233. [Google Scholar] [CrossRef]
- Harrison, S.R.; Klassen, J.R.L.; Bridgewood, C.; Scarsbrook, A.; Marzo-Ortega, H.; McGonagle, D. Chest pain mimicking pulmonary embolism may be a common presentation of COVID-19 in ambulant patients without other typical features of infection. J. Intern. Med. 2021, 290, 349–358. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Medum. Gum-COVID-Wak, Sputnik V. Instructions for Use. Available online: https://medum.ru/sputnik-v (accessed on 2 May 2023).
- Jenne, C.N.; Kubes, P. Virus-induced NETs–critical component of host defense or pathogenic mediator? PLoS Pathog. 2015, 11, e1004546. [Google Scholar] [CrossRef]
- Assiri, S.A.; Althaqafi, R.M.M.; Alswat, K.; Alghamdi, A.A.; Alomairi, N.E.; Nemenqani, D.M.; Ibrahim, Z.S.; Elkady, A. Post COVID-19 Vaccination-Associated Neurological Complications. Neuropsychiatr. Dis. Treat. 2022, 18, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.T.; Lin, Y.Y.; Chiang, W.F.; Lin, C.Y.; Chen, M.H.; Wu, K.A.; Chan, J.S.; Kao, Y.H.; Shyu, H.Y.; Hsiao, P.J. COVID-19 vaccine-induced encephalitis and status epilepticus. Qjm Int. J. Med. 2022, 115, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Askari, N. A review of neurological side effects of COVID-19 vaccination. Eur. J. Med. Res. 2023, 28, 102. [Google Scholar] [CrossRef] [PubMed]
- Altman, A.; Szyper-Kravitz, M.; Shoenfeld, Y. HBV vaccine and dermatomyositis: Is there an association? Rheumatol. Int. 2008, 28, 609–612. [Google Scholar] [CrossRef]
- Winkelmann, R.K. Influenza vaccine and dermatomyositis. Lancet 1982, 2, 495. [Google Scholar] [CrossRef]
- Orbach, H.; Tanay, A. Vaccines as a trigger for myopathies. Lupus 2009, 18, 1213–1216. [Google Scholar] [CrossRef]
- Liu, J.; Wu, H.; Xia, S.L. New-Onset Arthritis Following COVID-19 Vaccination: A Systematic Review of Case Reports. Vaccines 2023, 11, 665. [Google Scholar] [CrossRef]
- Rai, A.; Aashish Priya Karmani, S.; Abbas, W.; Khatri, G. Prevalence of rheumatoid arthritis following COVID-19 vaccine: An autoimmune disorder. Ann. Med. Surg. 2022, 82, 104628. [Google Scholar] [CrossRef]
- Mercado, C.; Perez-Rueda, M. An Atypical Case of Miller Fisher Syndrome with Multiple Autoimmunity. Neuroophthalmology 2021, 46, 122–125. [Google Scholar] [CrossRef]
- Fnu, Z.; Uddin, A.; Navetta-Modrov, B.; Patnaik, A.; Kaell, A. Inpatient Rheumatology Consultation Prompted by Positive Autoantibodies in Patients Receiving Intravenous Immunoglobulin Therapy: A Case Series and Literature Review. Cureus 2023, 15, e37008. [Google Scholar] [CrossRef]
- Fekih-Romdhane, F.; Ghrissi, F.; Hallit, S.; Cheour, M. New-onset acute psychosis as a manifestation of lupus cerebritis following concomitant COVID-19 infection and vaccination: A rare case report. BMC Psychiatry 2023, 23, 419. [Google Scholar] [CrossRef]
- Garrido, I.; Lopes, S.; Simões, M.S.; Liberal, R.; Lopes, J.; Carneiro, F.; Macedo, G. J Autoimmune hepatitis after COVID-19 vaccine—More than a coincidence. J. Autoimmun. 2021, 125, 102741. [Google Scholar] [CrossRef]
- Kim, S.; Jung, J.; Cho, H.; Lee, J.; Go, H.; Lee, J.H. A child with crescentic glomerulonephritis following SARS-CoV-2 mRNA (Pfizer-BioNTech) vaccination. Pediatr. Nephrol. 2023, 38, 299–302. [Google Scholar] [CrossRef]
- Markewitz, R.; Pauli, D.; Dargvainiene, J.; Steinhagen, K.; Engel, S.; Herbst, V.; Zapf, D.; Krüger, C.; Sharifzadeh, S.; Schomburg, B.; et al. The temporal course of T- and B-cell responses to vaccination with BNT162b2 and mRNA-1273. Clin. Microbiol. Infect. 2021, 28, 701–709. [Google Scholar] [CrossRef]
- Noureldine, H.A.; Maamari, J.; El Helou, M.O.; Chedid, G.; Farra, A.; Husni, R.; Mokhbat, J.E. The effect of the BNT162b2 vaccine on antinuclear antibody and antiphospholipid antibody levels. Immunol. Res. 2022, 70, 800–810. [Google Scholar] [CrossRef]
- Bello-Chavolla, O.Y.; Antonio-Villa, N.E.; Valdés-Ferrer, S.I.; Fermín-Martínez, C.A.; Fernández-Chirino, L.; Vargas-Vázquez, A.; Ramírez-García, D.; Mancilla-Galindo, J.; Kammar-García, A.; Ávila-Funes, J.A.; et al. Effectiveness of a nationwide COVID-19 vaccination program in Mexico against symptomatic COVID-19, hospitalizations, and death: A retrospective analysis of national surveillance data. Int. J. Infect. Dis. 2023, 129, 188–196. [Google Scholar] [CrossRef]
- Gushchin, V.A.; Dolzhikova, I.V.; Shchetinin, A.M.; Odintsova, A.S.; Siniavin, A.E.; Nikiforova, M.A.; Pochtovyi, A.A.; Shidlovskaya, E.V.; Kuznetsova, N.A.; Burgasova, O.A.; et al. Neutralizing Activity of Sera from Sputnik V-Vaccinated People against Variants of Concern (VOC: B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.617.3) and Moscow Endemic SARS-CoV-2 Variants. Vaccines 2021, 9, 779. [Google Scholar] [CrossRef]
- Abdel-Qader, D.H.; Abdel-Qader, H.; Silverthorne, J.; Kongkaew, C.; Al Meslamani, A.Z.; Hayajneh, W.; Abu Ata, O.M.; Shnaigat, W.; AbuRuz, S.; Al Nsour, M.; et al. Active Safety Surveillance of Four Types of COVID-19 Vaccines: A National Study from Jordan. Clin. Drug Investig. 2022, 42, 813–827. [Google Scholar] [CrossRef]
- Isnardi, C.A.; Schneeberger, E.E.; Kreimer, J.L.; Luna, P.C.; Echeverría, C.; Roberts, K.; de la Vega, M.C.; Virasoro, B.M.; Landi, M.; Quintana, R.; et al. An Argentinean cohort of patients with rheumatic and immune-mediated diseases vaccinated for SARS-CoV-2: The SAR-CoVAC Registry-protocol and preliminary data. Clin. Rheumatol. 2022, 41, 3199–3209. [Google Scholar] [CrossRef]
- Mirmosayyeb, O.; Barzegar, M.; Rezaei, M.; Baharlouie, N.; Shaygannejad, V. Bell’s palsy after Sputnik V COVID-19 (Gam-COVID-Vac) vaccination. Clin. Case Rep. 2022, 10, e05468. [Google Scholar] [CrossRef]
- Etemadifar, M.; Sigari, A.A.; Sedaghat, N.; Salari, M.; Nouri, H. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum. Vaccin. Immunother. 2021, 17, 3481–3483. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi Hamidabad, N.; Mafi, A.R.; Abolmaali, M. Mild Facial Paresis in a Recipient of Gam-COVID-Vac Vaccine: A Case Report. Clin. Med. Insights Case Rep. 2022, 15, 11795476221129120. [Google Scholar] [CrossRef] [PubMed]
- Naghashzadeh, F.; Shafaghi, S.; Dorudinia, A.; Naji, S.A.; Marjani, M.; Amin, A.; Mohamadifar, A.; Noorali, S.; Kashani, B.S. Myocarditis following rAd26 and rAd5 vector-based COVID-19 vaccine: Case report. ESC Heart Fail. 2022, 9, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.M.; Bertoli, A.M.; Rigo, D.; Sanchez Freytes, M. Relapse of lupus nephritis in temporal association with anti SARS-CoV-2 vaccination. Medicina 2022, 82, 971–973. [Google Scholar] [PubMed]
- Teigler, J.E.; Iampietro, M.J.; Barouch, D.H. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J. Virol. 2012, 86, 9590–9598. [Google Scholar] [CrossRef]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef]
- Herve, C.; Laupeze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccin. 2019, 4, 39. [Google Scholar] [CrossRef]
- Jara, L.J.; Vera-Lastra, O.; Mahroum, N.; Pineda, C.; Shoenfeld, Y. Autoimmune post-COVID vaccine syndromes: Does the spectrum of autoimmune/inflammatory syndrome expand? Clin. Rheumatol. 2022, 41, 1603–1609. [Google Scholar] [CrossRef]
- Dotan, A.; Kanduc, D.; Muller, S.; Makatsariya, A.; Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 and the female reproductive system. Am. J. Reprod. Immunol. 2021, 86, e13494. [Google Scholar] [CrossRef]
- Lavi, Y.; Vojdani, A.; Halpert, G.; Sharif, K.; Ostrinski, Y.; Zyskind, I.; Lattin, M.T.; Zimmerman, J.; I Silverberg, J.; Rosenberg, A.Z.; et al. Dysregulated Levels of Circulating Autoantibodies against Neuronal and Nervous System Autoantigens in COVID-19 Patients. Diagnostics 2023, 13, 687. [Google Scholar] [CrossRef]
- Shoenfeld, Y. Corona (COVID-19) time musings: Our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun. Rev. 2020, 19, 102538. [Google Scholar] [CrossRef]
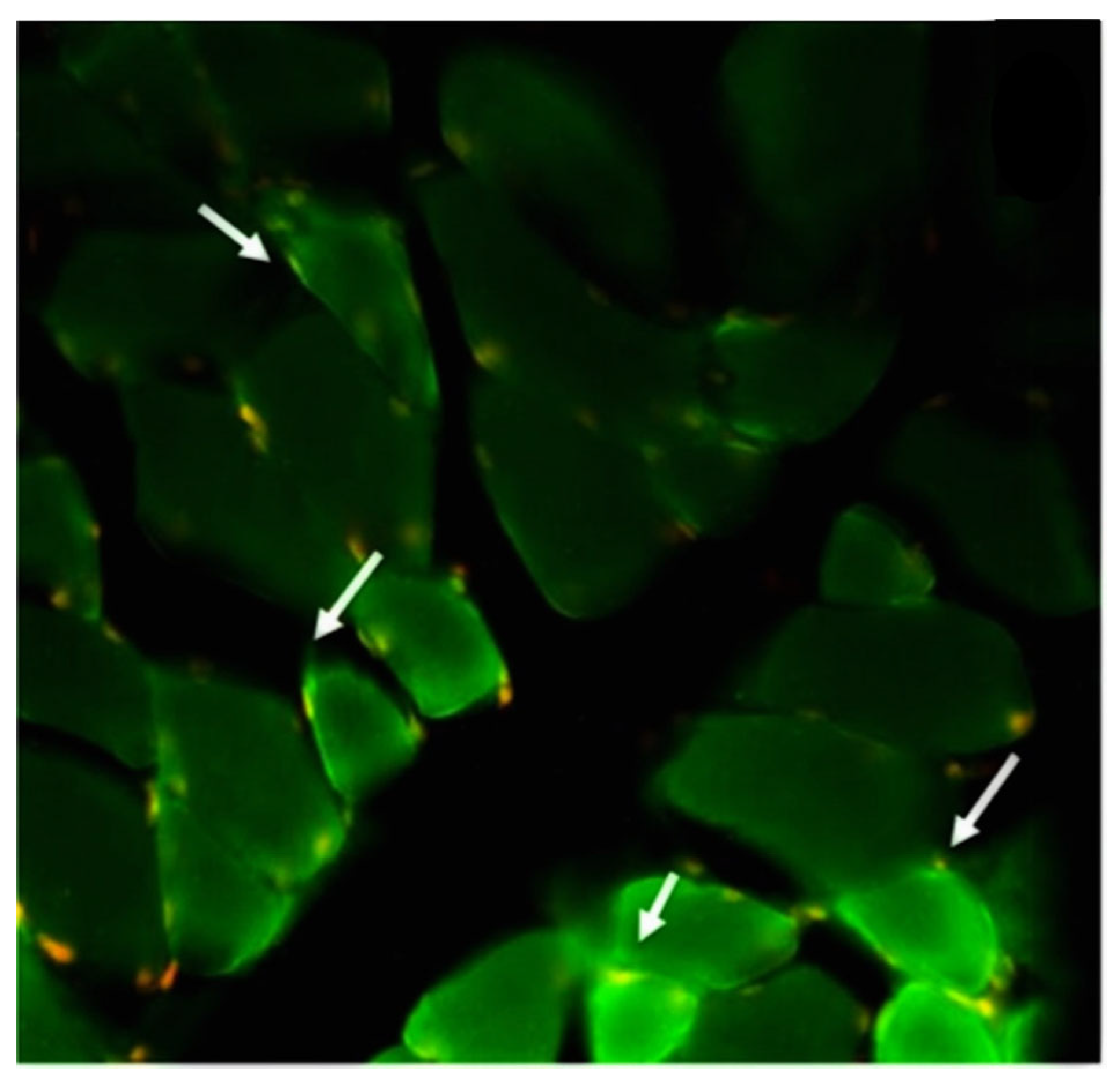
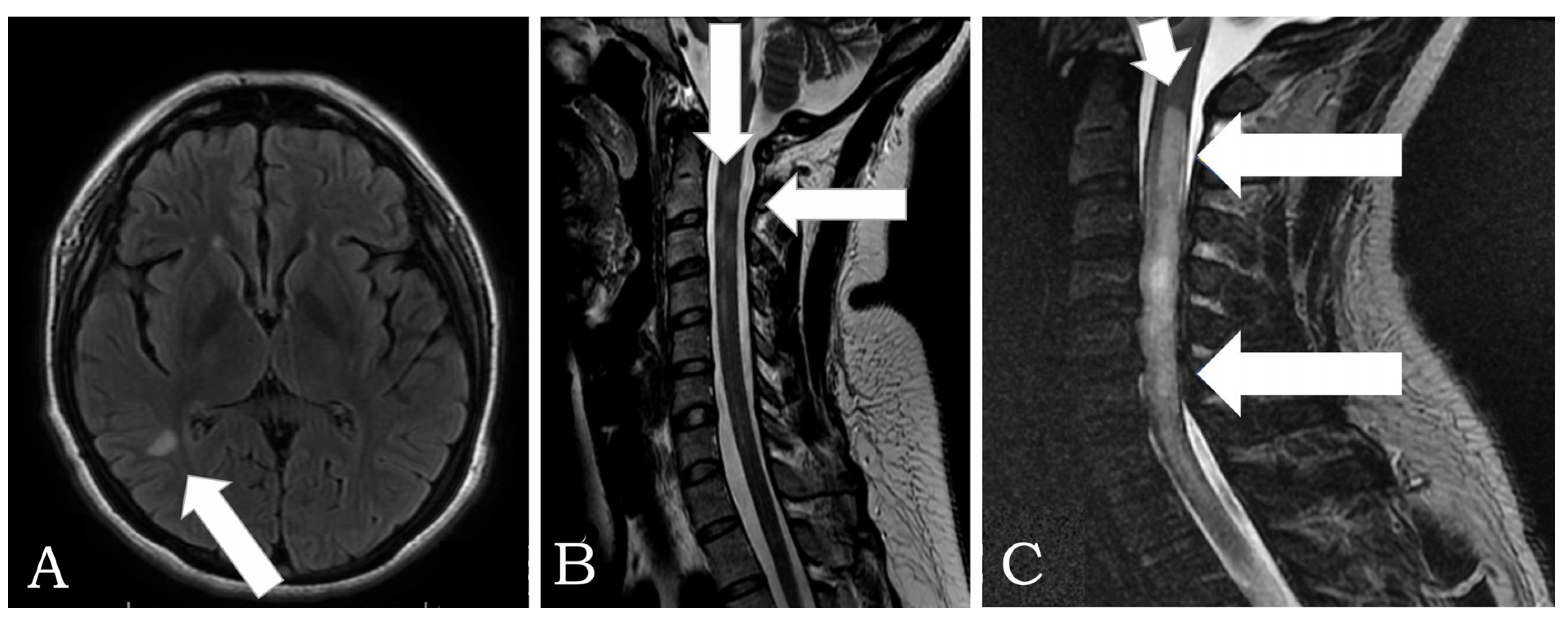
| Syndrome or Disease | Number of Cases | Sex | Age (Years) | Comorbidities | Autoantibodies | 1st/2nd Dose | Time to Clinical Manifestations (Days) |
|---|---|---|---|---|---|---|---|
| Neurological Manifestation | |||||||
| Guillain Barré Syndrome | 6 | F: 2 M: 4 | 32 ±10.5 | None: 4 AH:2 | ANA: 2, 1:80 (Positive) | 1st: 2 2nd: 4 | 11 (1–30) |
| Neuromyelitis Optica Spectrum Disorder | 2 | F: 2 | 37± 15.6 | None | AQP-4: 2 Positive Anti-NMDAr: Negative * Anti- GABA: Negative * Anti-GADAb: Negative * | 2nd: 2 | 7 (5–10) |
| Myasthenia Gravis | 2 | M: 1 F: 1 | 56 ± 20 | None | AChR: 2: Positive * | 2nd: 2 | 30 |
| Transverse Myelitis | 1 | F:1 | 55 | None | ANA: 1:80 (Positive) | 2nd | 7 |
| Opsoclonus-myoclonus-ataxia syndrome | 1 | F:1 | 45 | None | ANA: Negative | 2nd | 24 |
| Chronic Immune Demyelinating Polyneuropathy | 1 | M: 1 | 45 | AH | ANA: Negative | 2nd | 45 |
| Hematological Manifestations | |||||||
| Deep venous thrombosis | 6 | F:4 M: 2 | 73 ± 18 | AH: 4 Breast cancer: 1 | APL: Not done | 1st: 3 2nd: 3 | 32 (10–90) |
| Multiple venous thrombosis | 2 | M: 2 | 54 ± 11 | AH | APL: Not done | 1st: 2 | 45 (5–90) |
| Pulmonary thromboembolism | 2 | M: 2 | 85 ± 6 | Prostate cancer | APL: Not done | 1st: 2 | 33 |
| Autoimmune hemolytic anemia | 2 | M: 2 | 54 ± 11 | None | APL: Not done | 1st: 2 | 7 |
| Rheumatological Manifestations | |||||||
| Dermatomyositis | 1 | F: 1 | 40 | None | ANA: 1:80 (Positive) Anti-JO1: 10 AU/mL (Negative) Anti-SSA: 12 AU/mL (Negative) Anti-SSB: 15 AU/mL (Negative) | 2nd | 60 |
| Adult-onset Still’s disease | 1 | F: 1 | 20 | None | Ferritin: 1000 ng/mL (Positive) RF: 9 IU/mL (Negative) Anti-CCP: 9 AU/mL (Negative) Anti-JO1: 8 AU/mL (Negative) Anti-SSA: 10 AU/mL (Negative) Anti-SSB: 10 AU/mL (Negative) | 2nd | 60 |
| Endocrinological Manifestations | |||||||
| Graves’ disease | 1 | M: 1 | 28 | None | ATSH-R & TPO: Positive * ATG: Negative * Free T3 = 15 pg/mL (2.04–4.1) Free T4 = 4.01 ng/dL (0.93–1.71) Anti TSH-R = 19 UiL(0.1.75) TSI = 340 % Baseline (<140) | 2nd | 6 |
| Author | Sex | Age (Years) | 1st/2nd Dose | Time to Clinical Manifestations (Days) | Syndrome or Disease |
|---|---|---|---|---|---|
| Shokraee K. et al. [8] | Male | 41 | 1st | 20 | Reactive arthritis |
| Ameri M. et al. [9] | Female | 40 | 1st | 2 | Severe eczema |
| Herrera-Comoglio R. et al. [10] | Female | 24 | Not mentioned | 7 | VITT |
| Mirmosayyeb O, et al. [41] | (1) Female (2) Male | (1) 27 (2) 58 | 1st | (1) 3 (2) 10 | Bell’s palsy |
| Mahmoudi N. et al. [43] | Female | 34 | 1st | 1 | Facial Paresis |
| Naghashzadeh F. et al. [44] | Male | 29 | 2nd | 2 | Myocarditis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Lastra, O.; Mora, G.; Lucas-Hernández, A.; Ordinola-Navarro, A.; Rodríguez-Chávez, E.; Peralta-Amaro, A.L.; Medina, G.; Cruz-Dominguez, M.P.; Jara, L.J.; Shoenfeld, Y. New Onset Autoimmune Diseases after the Sputnik Vaccine. Biomedicines 2023, 11, 1898. https://doi.org/10.3390/biomedicines11071898
Vera-Lastra O, Mora G, Lucas-Hernández A, Ordinola-Navarro A, Rodríguez-Chávez E, Peralta-Amaro AL, Medina G, Cruz-Dominguez MP, Jara LJ, Shoenfeld Y. New Onset Autoimmune Diseases after the Sputnik Vaccine. Biomedicines. 2023; 11(7):1898. https://doi.org/10.3390/biomedicines11071898
Chicago/Turabian StyleVera-Lastra, Olga, Gabriela Mora, Abihai Lucas-Hernández, Alberto Ordinola-Navarro, Emmanuel Rodríguez-Chávez, Ana Lilia Peralta-Amaro, Gabriela Medina, María Pilar Cruz-Dominguez, Luis J. Jara, and Yehuda Shoenfeld. 2023. "New Onset Autoimmune Diseases after the Sputnik Vaccine" Biomedicines 11, no. 7: 1898. https://doi.org/10.3390/biomedicines11071898
APA StyleVera-Lastra, O., Mora, G., Lucas-Hernández, A., Ordinola-Navarro, A., Rodríguez-Chávez, E., Peralta-Amaro, A. L., Medina, G., Cruz-Dominguez, M. P., Jara, L. J., & Shoenfeld, Y. (2023). New Onset Autoimmune Diseases after the Sputnik Vaccine. Biomedicines, 11(7), 1898. https://doi.org/10.3390/biomedicines11071898