SGLT2 Inhibitors as Potential Anticancer Agents
Abstract
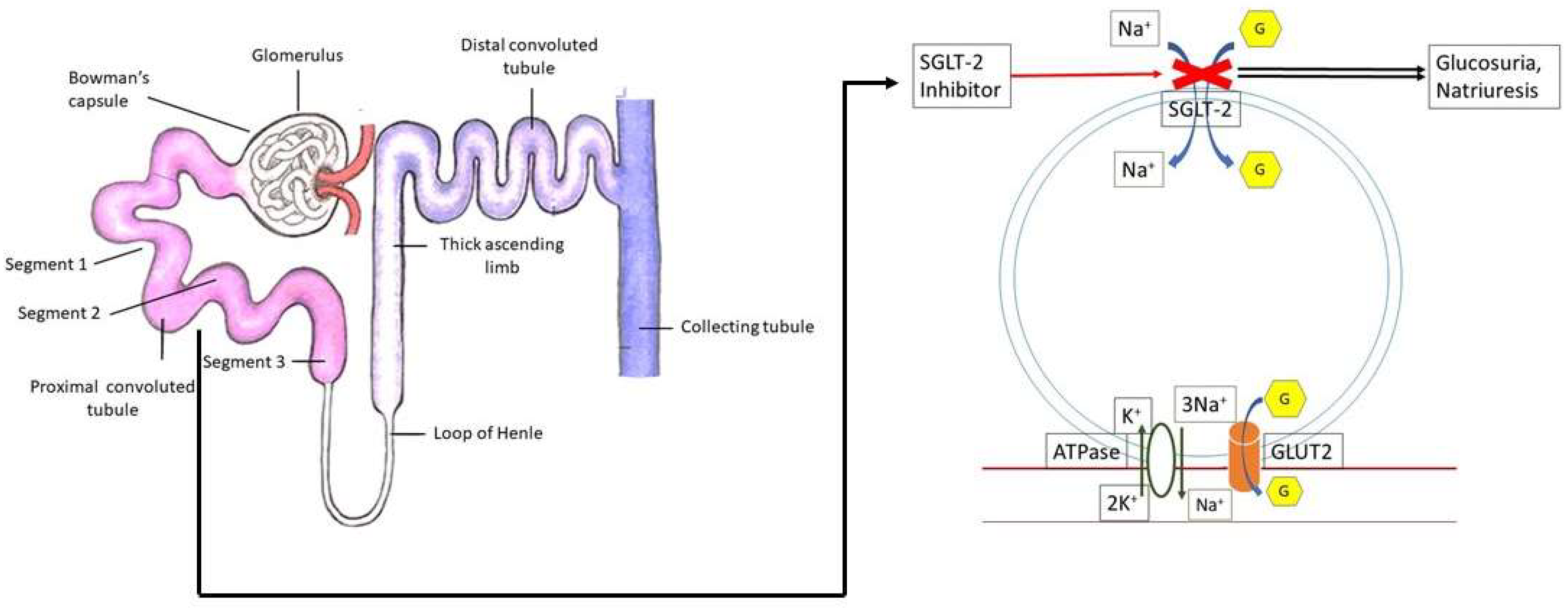
1. Introduction
2. Role of Glucose and Its Transporters in Cancer
3. Expression of SGLT2 in Cancerous Cells/Tissues
4. Properties of SGLT2 Inhibitors
| Drug Name | SGLT2/1 Affinity | Absorption | Distribution | Metabolism | Elimination | Adverse Effects |
|---|---|---|---|---|---|---|
| Bexagliflozin | TBD | F: 78%, PPT: 2–4 h | 93% bound, Vd: 262 L. | Glucuronidation (minor CYP role) | T1/2: 12 h Clearance: 19.1 L/h Feces: 51.1% Urine: 40.5% | Female genital mycotic infections, UTI, and increased urination |
| Canagliflozin | 155:1 | F: 65%, PPT: 1–2 h | 99% bound, Vd: 119 L | Glucuronidation, (minor CYP role) | T1/2: 12 h Clearance: 192 mL/min Feces: 51.7% Urine: 33% | Genital mycotic infections (>10% [women]) UTI, thirst, constipation, volume depletion |
| Dapagliflozin | 1242:1 | F: 78%, PPT: 2 h | 91% bound | Glucuronidation, (minor CYP role) | T1/2: 8–12 h Feces: 21% Urine: 75% | Renal adjustments for >65YO & eGFR 30–60 mL/min, nasopharyngitis, UTI, back pain |
| Empagliflozin | 2680:1 | F: 90% PPT: 1.5 h AUCSS: 1870 nmol*h/L | 86.2% bound, Vd: 73.8 L | Glucuronidation | T1/2: 14–18 h Clearance: 10.6 L/h Feces: 41.2% Urine: 54.4% | UTI, URTI, diuresis, arthralgia, (postmarket) angioedema & AKI |
| Ertugliflozin | 2235:1 | F: 100% PPT: 1 h AUCSS: 398 ng*h/mL | 93.6% bound, Vd: 85.5 L | Glucuronidation, | T1/2: 16.6 h Clearance: 11.2 L/h Feces: 40.9% Urine: 50.2% | Female: Genital mycotic infections (>10%), vaginal pruritus, Volume depletion, UTI, HA (postmarket) Fournier’s Gangrene |
| Ipragliflozin * | 254:1 | PPT: 2.6 ± 1.3 h AUC 27,299 ± 4622 | TBD | Glucu- ronidation | T1/2: 10.3 ± 1.6 Low urinary excretion ~1% | GI disorders |
| Luseogliflozin * | 1770:1 | Tmax 0.5 (0.5–1.0) h AUCinf(ng·h/mL) 2010 ± 508 | TBD | CYP-mediated metabolism | T1/2: 10.4 ± 0.832 h | Minimal AE, No UTI (mostly male patients) |
| Tofogliflozin * | 2912:1 | AUC0–24 h (h × ng/mL): 6 740 ± 1 680 Tmax (h) 0.750 (0.50–4.00) | TBD | CYP-mediated metabolism (CYP2C18, 4A11, and 4F3B) | T1/2: 3.98 ± 0.520 h | Increase in blood ketone body |
5. Current Status of SGLT2 Inhibitors in Cancers
5.1. Anticancer Potential of SGLT2 Inhibitors in Cancers
| Tumor Site | Cancer Model | SGLT2i for Intervention | Study Type | Results |
|---|---|---|---|---|
| Breast | MCF-7 (human) | Canagliflozin | in vitro | Inhibition of cell proliferation, clonogenic survival, and colony formation |
| MCF-7 (human) | Canagliflozin and Dapagliflozin | in vitro/in vivo | Significant reduction in tumor volume; Potently inhibited cell proliferation; Induced cell cycle arrest and apoptosis | |
| MCF-7 (human) | Ipragliflozin | in vitro | Inhibition of glucose and sodium influx; Alteration in mitochondrial membrane potential | |
| SKBR3, BT-474, MCF-7 (human) | Canagliflozin | in vitro | Inhibition of oxygen consumption and glutamine metabolism | |
| Liver | Huh7, HepG2 (human) | Canagliflozin | in vitro | Inhibition of glucose uptake |
| Huh7, Hep3B | Canagliflozin | in vitro/in vivo | Inhibition of β-catenin pathway | |
| Humans | Canagliflozin | Patient sample | suppression of angiogenesis | |
| Huh7, Hep3B | Canagliflozin | in vitro | Downregulation of ATP synthase F1 subunit alpha (mitochondrial electron transport system protein) | |
| Pancreas | Capan-1, PANC-1 | Canagliflozin | in vitro/in vivo | Suppression of glucose transporter-1 and lactate dehydrogenase A |
| Thyroid | TPC-1, BCPAP, Nthy-ori-3–1 | Canagliflozin | in vitro/in vivo | Inhibition of glucose uptake, glycolysis, and AKT/mTOR signaling activation; increased AMPK activation |
| Bone | MNNG/HOS, MG-63, 143B, U2OS, K7M2 | Canagliflozin | in vitro/in vivo | Activation of STING/IRF3/IFN-β pathway and suppression of AKT phosphorylation |
| Lung | A549 (human) | Canagliflozin | in vitro | Inhibition of cell cycle progression |
| HCC827, H1975 | Canagliflozin | in vitro | Inhibition of EGFR kinase | |
| Prostate | PC3 | Canagliflozin | in vitro | Inhibition of complex I supported mitochondrial respiration |
5.2. Molecular Mechanisms of Anticancer Activities
5.3. Clinical Evidence of SGLT2 Inhibitors in Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Zhang, C.; Le, A. Glucose Metabolism in Cancer: The Warburg Effect and Beyond. Adv. Exp. Med. Biol. 2021, 1311, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ryu, T.Y.; Park, J.; Scherer, P.E. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab. J. 2014, 38, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Abudawood, M.; Tabassum, H.; Almaarik, B.; Aljohi, A. Interrelationship between oxidative stress, DNA damage and cancer risk in diabetes (Type 2) in Riyadh, KSA. Saudi J. Biol. Sci. 2020, 27, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Tasevska, N.; Jiao, L.; Cross, A.J.; Kipnis, V.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A.; Potischman, N. Sugars in diet and risk of cancer in the NIH-AARP Diet and Health Study. Int. J. Cancer 2012, 130, 159–169. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Yin, J.; Zhang, J.; Jiang, Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: A challenge for cancer therapy. J. Hematol. Oncol. 2020, 13, 64. [Google Scholar] [CrossRef]
- Zhu, B.; Qu, S. The Relationship between Diabetes Mellitus and Cancers and Its Underlying Mechanisms. Front. Endocrinol. 2022, 13, 800995. [Google Scholar] [CrossRef]
- Kobayashi, M.; Uematsu, T.; Tokura, Y.; Takei, K.; Sakamoto, K.; Narimatsu, T.; Nukui, A.; Kamai, T. Immunohistochemical expression of Sodium-Dependent Glucose Transporter-2 (SGLT-2) in clear cell renal carcinoma: Possible prognostic implications. Int. Braz. J. Urol. 2019, 45, 169–178. [Google Scholar] [CrossRef]
- Scafoglio, C.; Hirayama, B.A.; Kepe, V.; Liu, J.; Ghezzi, C.; Satyamurthy, N.; Moatamed, N.A.; Huang, J.; Koepsell, H.; Barrio, J.R.; et al. Functional expression of sodium-glucose transporters in cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E4111–E4119. [Google Scholar] [CrossRef]
- Lau, K.T.K.; Ng, L.; Wong, J.W.H.; Loong, H.H.F.; Chan, W.W.L.; Lee, C.H.; Wong, C.K.H. Repurposing Sodium-Glucose Co-Transporter 2 inhibitors (SGLT2i) for cancer treatment—A Review. Rev. Endocr. Metab. Disord. 2021, 22, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097. [Google Scholar] [CrossRef]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef]
- Vaupel, P. The role of hypoxia-induced factors in tumor progression. Oncologist 2004, 9 (Suppl. S5), 10–17. [Google Scholar] [CrossRef]
- Hay, N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat. Rev. Cancer 2016, 16, 635–649. [Google Scholar] [CrossRef]
- Burns, J.S.; Manda, G. Metabolic Pathways of the Warburg Effect in Health and Disease: Perspectives of Choice, Chain or Chance. Int. J. Mol. Sci. 2017, 18, 2755. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, P.; Deb, A.; Shepal, V.; Bhat, M.K. Hyperglycemia Associated Metabolic and Molecular Alterations in Cancer Risk, Progression, Treatment, and Mortality. Cancers 2019, 11, 1402. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Hohn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Chang, C.H.; Pauklin, S. ROS and TGFβ: From pancreatic tumour growth to metastasis. J. Exp. Clin. Cancer Res. 2021, 40, 152. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Bialkowska, A.M.; Koziolkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, T.S.; Park, K.H.; Kwon, W.S.; Kim, J.J. Serum concentration of sex hormone-binding globulin in healthy volunteers and patients with breast cancer stratified by sex and age. Oncol. Lett. 2020, 20, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Oguri, T.; Isobe, T.; Fujitaka, K.; Kohno, N. SGLT gene expression in primary lung cancers and their metastatic lesions. Jpn. J. Cancer Res. 2001, 92, 874–879. [Google Scholar] [CrossRef]
- Wright, E.M. SGLT2 and cancer. Pflugers Arch. 2020, 472, 1407–1414. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. BRENZAVVY (Bexagliflozin) Tablets, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/214373s000lbl.pdf (accessed on 25 May 2023).
- Saisho, Y. SGLT2 Inhibitors: The Star in the Treatment of Type 2 Diabetes? Diseases 2020, 8, 14. [Google Scholar] [CrossRef]
- Bersoff-Matcha, S.J.; Chamberlain, C.; Cao, C.; Kortepeter, C.; Chong, W.H. Fournier Gangrene Associated with Sodium-Glucose Cotransporter-2 Inhibitors: A Review of Spontaneous Postmarketing Cases. Ann. Intern. Med. 2019, 170, 764–769. [Google Scholar] [CrossRef]
- Danne, T.; Pettus, J.; Giaccari, A.; Cariou, B.; Rodbard, H.; Weinzimer, S.A.; Bonnemaire, M.; Sawhney, S.; Stewart, J.; Wang, S.; et al. Sotagliflozin Added to Optimized Insulin Therapy Leads to Lower Rates of Clinically Relevant Hypoglycemic Events at Any HbA1c at 52 Weeks in Adults with Type 1 Diabetes. Diabetes Technol. Ther. 2019, 21, 471–477. [Google Scholar] [CrossRef]
- Tang, H.; Dai, Q.; Shi, W.; Zhai, S.; Song, Y.; Han, J. SGLT2 inhibitors and risk of cancer in type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Diabetologia 2017, 60, 1862–1872. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A.; Norton, L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 2013, 62, 3324–3328. [Google Scholar] [CrossRef]
- Dhillon, S. Dapagliflozin: A Review in Type 2 Diabetes. Drugs 2019, 79, 1135–1146. [Google Scholar] [CrossRef]
- Hailat, M.; Zakaraya, Z.; Al-Ani, I.; Meanazel, O.A.; Al-Shdefat, R.; Anwer, M.K.; Saadh, M.J.; Abu Dayyih, W. Pharmacokinetics and Bioequivalence of Two Empagliflozin, with Evaluation in Healthy Jordanian Subjects under Fasting and Fed Conditions. Pharmaceuticals 2022, 15, 193. [Google Scholar] [CrossRef]
- Li, Y.; Nucci, G.; Yamamoto, Y.; Fediuk, D.J.; Sahasrabudhe, V. Pharmacokinetics and Pharmacodynamics of Ertugliflozin in Healthy Japanese and Western Subjects. Clin. Pharmacol. Drug Dev. 2021, 10, 765–776. [Google Scholar] [CrossRef]
- Song, L.; Yao, X.; Liu, Y.; Zhong, W.; Jiang, J.; Liu, H.; Zhou, H.; Shi, C.; Zong, K.; Wang, C.; et al. Translational prediction of first-in-human pharmacokinetics and pharmacodynamics of janagliflozin, a selective SGLT2 inhibitor, using allometric scaling, dedrick and PK/PD modeling methods. Eur. J. Pharm. Sci. 2020, 147, 105281. [Google Scholar] [CrossRef] [PubMed]
- Kasahara-Ito, N.; Fukase, H.; Ogama, Y.; Saito, T.; Ohba, Y.; Shimada, S.; Takano, Y.; Ichihara, T.; Terao, K.; Nakamichi, N.; et al. Pharmacokinetics and Pharmacodynamics of Tofogliflozin (a Selective SGLT2 Inhibitor) in Healthy Male Subjects. Drug Res. 2017, 67, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kadokura, T.; Zhang, W.; Krauwinkel, W.; Leeflang, S.; Keirns, J.; Taniuchi, Y.; Nakajo, I.; Smulders, R. Clinical pharmacokinetics and pharmacodynamics of the novel SGLT2 inhibitor ipragliflozin. Clin. Pharmacokinet. 2014, 53, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Hendryx, M.; Dong, Y.; Ndeke, J.M.; Luo, J. Sodium-Glucose Cotransporter 2 (SGLT2) inhibitor initiation and hepatocellular carcinoma prognosis. PLoS ONE 2022, 17, e0274519. [Google Scholar] [CrossRef]
- Villani, L.A.; Smith, B.K.; Marcinko, K.; Ford, R.J.; Broadfield, L.A.; Green, A.E.; Houde, V.P.; Muti, P.; Tsakiridis, T.; Steinberg, G.R. The diabetes medication Canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-I supported respiration. Mol. Metab. 2016, 5, 1048–1056. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, J.; Yu, S.J.; Ma, H.L.; Chen, J.; Ding, X.F.; Chen, G.; Liang, Y.; Zhang, Q. Sodium-Glucose Co-Transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed. Pharmacother. 2020, 132, 110821. [Google Scholar] [CrossRef]
- Komatsu, S.; Nomiyama, T.; Numata, T.; Kawanami, T.; Hamaguchi, Y.; Iwaya, C.; Horikawa, T.; Fujimura-Tanaka, Y.; Hamanoue, N.; Motonaga, R.; et al. SGLT2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocr. J. 2020, 67, 99–106. [Google Scholar] [CrossRef]
- Papadopoli, D.; Uchenunu, O.; Palia, R.; Chekkal, N.; Hulea, L.; Topisirovic, I.; Pollak, M.; St-Pierre, J. Perturbations of cancer cell metabolism by the antidiabetic drug canagliflozin. Neoplasia 2021, 23, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tong, C.W.; Leung, Y.; Wong, M.H.; To, K.K.; Leung, K.S. Identification of Clinically Approved Drugs Indacaterol and Canagliflozin for Repurposing to Treat Epidermal Growth Factor Tyrosine Kinase Inhibitor-Resistant Lung Cancer. Front. Oncol. 2017, 7, 288. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, L.; Yamashita, S.; Nomiyama, T.; Kawanami, T.; Hamaguchi, Y.; Shigeoka, T.; Horikawa, T.; Tanaka, Y.; Yanase, T.; Kawanami, D.; et al. Sodium-glucose cotransporter 2 inhibitor canagliflozin attenuates lung cancer cell proliferation in vitro. Diabetol. Int. 2021, 12, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, Z.; Jing, D.; Huang, X.; Ren, D.; Shao, Z.; Zhang, Z. SGLT2 inhibitor activates the STING/IRF3/IFN-β pathway and induces immune infiltration in osteosarcoma. Cell Death Dis. 2022, 13, 523. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhou, Y.; Xie, X.; He, L.; Ding, J.; Pang, S.; Shen, B.; Zhou, C. Inhibitory effects of canagliflozin on pancreatic cancer are mediated via the downregulation of glucose transporter-1 and lactate dehydrogenase A. Int. J. Oncol. 2020, 57, 1223–1233. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Mao, L.; Zhang, L.; Zhu, Y.; Xu, Y.; Cheng, Y.; Sun, R.; Zhang, Y.; Ke, J.; et al. SGLT2 inhibition restrains thyroid cancer growth via G1/S phase transition arrest and apoptosis mediated by DNA damage response signaling pathways. Cancer Cell Int. 2022, 22, 74. [Google Scholar] [CrossRef]
- Nakano, D.; Kawaguchi, T.; Iwamoto, H.; Hayakawa, M.; Koga, H.; Torimura, T. Effects of canagliflozin on growth and metabolic reprograming in hepatocellular carcinoma cells: Multi-omics analysis of metabolomics and absolute quantification proteomics (iMPAQT). PLoS ONE 2020, 15, e0232283. [Google Scholar] [CrossRef]
- Hung, M.H.; Chen, Y.L.; Chen, L.J.; Chu, P.Y.; Hsieh, F.S.; Tsai, M.H.; Shih, C.T.; Chao, T.I.; Huang, C.Y.; Chen, K.F. Canagliflozin inhibits growth of hepatocellular carcinoma via blocking glucose-influx-induced β-catenin activation. Cell Death Dis. 2019, 10, 420. [Google Scholar] [CrossRef]
- Kaji, K.; Nishimura, N.; Seki, K.; Sato, S.; Saikawa, S.; Nakanishi, K.; Furukawa, M.; Kawaratani, H.; Kitade, M.; Moriya, K.; et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int. J. Cancer 2018, 142, 1712–1722. [Google Scholar] [CrossRef]
- Ansary, T.M.; Nakano, D.; Nishiyama, A. Diuretic Effects of Sodium Glucose Cotransporter 2 Inhibitors and Their Influence on the Renin-Angiotensin System. Int. J. Mol. Sci. 2019, 20, 629. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Lhaf, F.; Sathyapalan, T.; Sahebkar, A. Effects of novel antidiabetes agents on apoptotic processes in diabetes and malignancy: Implications for lowering tissue damage. Life Sci. 2019, 231, 116538. [Google Scholar] [CrossRef] [PubMed]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Balcioglu, A.S.; Celik, E.; Sahin, M.; Gocer, K.; Aksu, E.; Aykan, A.C. Dapagliflozin Improves Cardiac Autonomic Function Measures in Type 2 Diabetic Patients with Cardiac Autonomic Neuropathy. Anatol. J. Cardiol. 2022, 26, 832–840. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Borges, J.I.; Cora, N.; Sizova, A. Sympatholytic Mechanisms for the Beneficial Cardiovascular Effects of SGLT2 Inhibitors: A Research Hypothesis for Dapagliflozin’s Effects in the Adrenal Gland. Int. J. Mol. Sci. 2021, 22, 7684. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Botteri, E.; Gillis, R.D.; Lofling, L.; Le, C.P.; Ziegler, A.I.; Chung, N.C.; Rowe, M.C.; Fabb, S.A.; Hartley, B.J.; et al. Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci. Transl. Med. 2023, 15, eadf1147. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Nakano, D.; Okamura, S.; Shimose, S.; Hayakawa, M.; Niizeki, T.; Koga, H.; Torimura, T. Spontaneous regression of hepatocellular carcinoma with reduction in angiogenesis-related cytokines after treatment with sodium-glucose cotransporter 2 inhibitor in a cirrhotic patient with diabetes mellitus. Hepatol. Res. 2019, 49, 479–486. [Google Scholar] [CrossRef]
- Abdel-Rafei, M.K.; Thabet, N.M.; Rashed, L.A.; Moustafa, E.M. Canagliflozin, a SGLT-2 inhibitor, relieves ER stress, modulates autophagy and induces apoptosis in irradiated HepG2 cells: Signal transduction between PI3K/AKT/GSK-3β/mTOR and Wnt/beta-catenin pathways; in vitro. J. Cancer Res. Ther. 2021, 17, 1404–1418. [Google Scholar] [CrossRef]
- Washington University School of Medicine National Institutes of Health National Cancer Institute. Targeting Pancreatic Cancer with Sodium Glucose Transporter 2 (SGLT2) Inhibition. Available online: https://ClinicalTrials.gov/show/NCT04542291 (accessed on 25 May 2023).
- Petra Pharma. A Phase 1b/2 Study of Serabelisib in Combination with Canagliflozin in Patients with Advanced Solid Tumors. Available online: https://ClinicalTrials.gov/show/NCT04073680 (accessed on 25 May 2023).
- Memorial Sloan Kettering Cancer Center. Preventing High Blood Sugar in People Being Treated for Metastatic Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT05090358 (accessed on 25 May 2023).
- Novartis Pharmaceuticals. Study of Safety and Efficacy of Dapagliflozin + Metformin XR Versus Metformin XR in Participants With HR+, HER2−, Advanced Breast Cancer While on Treatment with Alpelisib and Fulvestrant. Available online: https://ClinicalTrials.gov/show/NCT04899349 (accessed on 25 May 2023).
- Saint Luke’s Health System Novartis Pharmaceuticals. Alpelisib, Fulvestrant and Dapagliflozin for the Treatment of HR+, HER2−, PIK3CA Mutant Metastatic Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT05025735 (accessed on 25 May 2023).
- Washington University School of Medicine Children’s Discovery Institute. Targeting Pediatric Brain Tumors with Sodium Glucose Cotransporter 2 Inhibitors (SGLT2i). Available online: https://ClinicalTrials.gov/show/NCT05521984 (accessed on 25 May 2023).
- Washington University School of Medicine. Neoadjuvant SGLT2 Inhibition in High-Risk Localized Prostate Cancer. Available online: https://ClinicalTrials.gov/show/NCT04887935 (accessed on 25 May 2023).
- Park, L.K.; Lim, K.H.; Volkman, J.; Abdiannia, M.; Johnston, H.; Nigogosyan, Z.; Siegel, M.J.; McGill, J.B.; McKee, A.M.; Salam, M.; et al. Safety, tolerability, and effectiveness of the Sodium-Glucose Cotransporter 2 inhibitor (SGLT2i) dapagliflozin in combination with standard chemotherapy for patients with advanced, inoperable pancreatic adenocarcinoma: A phase 1b observational study. Cancer Metab. 2023, 11, 6. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basak, D.; Gamez, D.; Deb, S. SGLT2 Inhibitors as Potential Anticancer Agents. Biomedicines 2023, 11, 1867. https://doi.org/10.3390/biomedicines11071867
Basak D, Gamez D, Deb S. SGLT2 Inhibitors as Potential Anticancer Agents. Biomedicines. 2023; 11(7):1867. https://doi.org/10.3390/biomedicines11071867
Chicago/Turabian StyleBasak, Debasish, David Gamez, and Subrata Deb. 2023. "SGLT2 Inhibitors as Potential Anticancer Agents" Biomedicines 11, no. 7: 1867. https://doi.org/10.3390/biomedicines11071867
APA StyleBasak, D., Gamez, D., & Deb, S. (2023). SGLT2 Inhibitors as Potential Anticancer Agents. Biomedicines, 11(7), 1867. https://doi.org/10.3390/biomedicines11071867







