The Potential of Glucose Treatment to Reduce Reactive Oxygen Species Production and Apoptosis of Inflamed Neural Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Study Design
2.2. Assay for Active Cell Metabolism
2.3. Measurement of Reactive Oxygen Species (ROS)
2.4. Apoptosis Assay and Cell Cycle Analysis
2.5. Luminex Assay
2.6. Statistical Analysis
3. Results
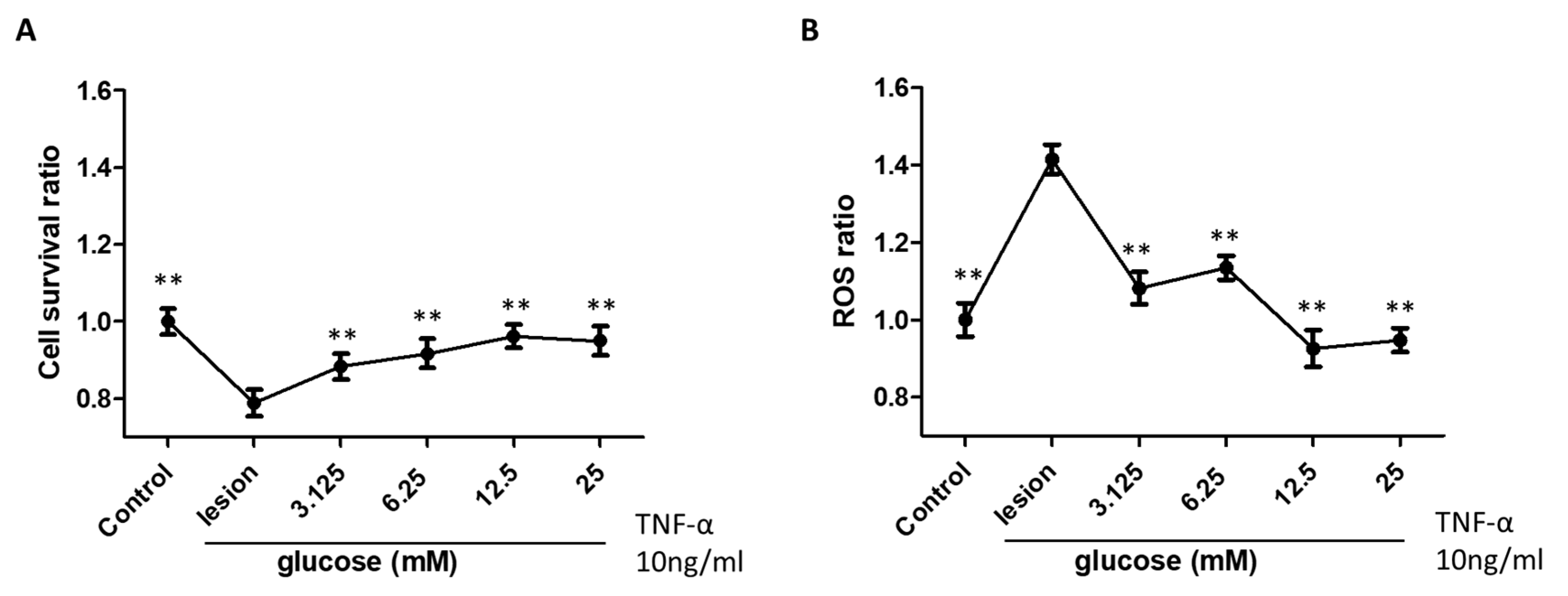
3.1. Glucose Treatment Enhances Neural Cell Survival and Reduces ROS Activity
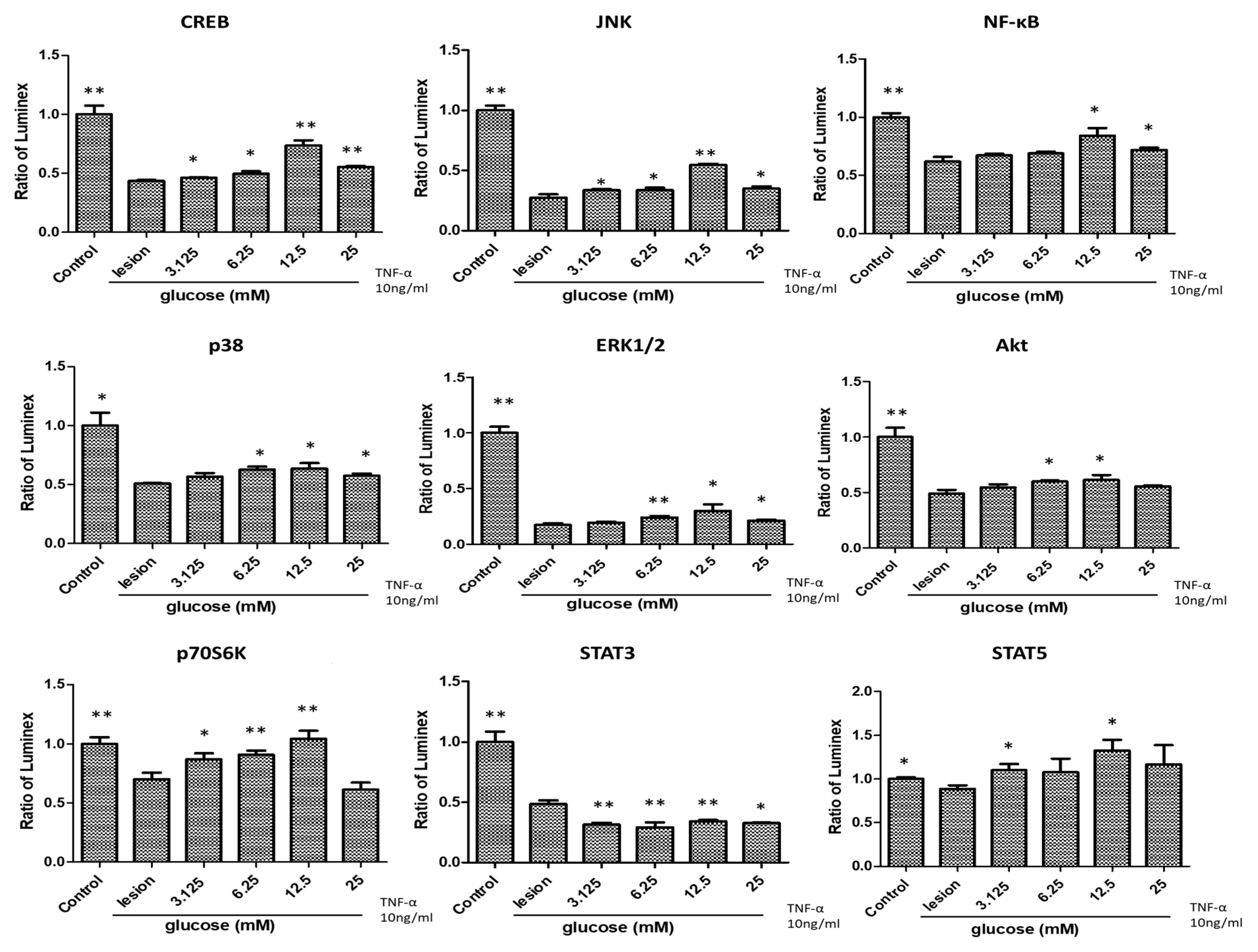
3.2. Glucose Treatment Positively Regulates Cytokines with Anti-Inflammatory/Anti-Oxidative Effects
3.3. Effects of Glucose Treatment on the Cell Cycle Regulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmid, A.B.; Fundaun, J.; Tampin, B. Entrapment neuropathies: A contemporary approach to pathophysiology, clinical assessment, and management. Pain Rep. 2020, 5, e829. [Google Scholar] [CrossRef] [PubMed]
- Doughty, C.T.; Bowley, M.P. Entrapment Neuropathies of the Upper Extremity. Med. Clin. N. Am. 2019, 103, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.B.; Coppieters, M.W.; Ruitenberg, M.J.; McLachlan, E.M. Local and remote immune-mediated inflammation after mild peripheral nerve compression in rats. J. Neuropathol. Exp. Neurol. 2013, 72, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, L.; Hausheer, A.C.; Baskozos, G.; Bennett, D.L.H.; Schmid, A.B. Somatosensory and psychological phenotypes associated with neuropathic pain in entrapment neuropathy. Pain 2021, 162, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef]
- Shamji, M.F.; Allen, K.D.; So, S.; Jing, L.; Adams, S.B., Jr.; Schuh, R.; Huebner, J.; Kraus, V.B.; Friedman, A.H.; Setton, L.A.; et al. Gait abnormalities and inflammatory cytokines in an autologous nucleus pulposus model of radiculopathy. Spine 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Gupta, R.; Channual, J.C. Spatiotemporal pattern of macrophage recruitment after chronic nerve compression injury. J. Neurotrauma 2006, 23, 216–226. [Google Scholar] [CrossRef]
- Lam, K.H.S.; Wu, Y.T.; Reeves, K.D.; Galluccio, F.; Allam, A.E.; Peng, P.W.H. Ultrasound-Guided Interventions for Carpal Tunnel Syndrome: A Systematic Review and Meta-Analyses. Diagnostics 2023, 13, 1138. [Google Scholar] [CrossRef]
- Maniquis-Smigel, L.; Reeves, K.D.; Rosen, H.J.; Lyftogt, J.; Graham-Coleman, C.; Cheng, A.L.; Rabago, D. Analgesic Effect and Potential Cumulative Benefit from Caudal Epidural D5W in Consecutive Participants with Chronic Low-Back and Buttock/Leg Pain. J. Altern. Complement. Med. 2018, 24, 1189–1196. [Google Scholar] [CrossRef]
- Topol, G.A.; Pestalardo, I.G.; Reeves, K.D.; Elias, F.; Steinmetz, N.J.; Cheng, A.L.; Rabago, D. Dextrose Prolotherapy for Symptomatic Grade IV Knee Osteoarthritis: A Pilot Study of Early and Longer-Term Analgesia and Pain-Specific Cytokine Concentrations. Clin. Pract. 2022, 12, 926–938. [Google Scholar] [CrossRef]
- Topol, G.A.; Podesta, L.A.; Reeves, K.D.; Giraldo, M.M.; Johnson, L.L.; Grasso, R.; Jamín, A.; Clark, T.; Rabago, D. Chondrogenic Effect of Intra-articular Hypertonic-Dextrose (Prolotherapy) in Severe Knee Osteoarthritis. PM R 2016, 8, 1072–1082. [Google Scholar] [CrossRef]
- Yoshii, Y.; Zhao, C.; Schmelzer, J.D.; Low, P.A.; An, K.N.; Amadio, P.C. Effects of multiple injections of hypertonic dextrose in the rabbit carpal tunnel: A potential model of carpal tunnel syndrome development. Hand 2014, 9, 52–57. [Google Scholar] [CrossRef]
- Ryan, M.; Wong, A.; Rabago, D.; Lee, K.; Taunton, J. Ultrasound-guided injections of hyperosmolar dextrose for overuse patellar tendinopathy: A pilot study. Br. J. Sport. Med. 2011, 45, 972–977. [Google Scholar] [CrossRef]
- Wu, Y.T.; Ho, T.Y.; Chou, Y.C.; Ke, M.J.; Li, T.Y.; Tsai, C.K.; Chen, L.C. Six-month Efficacy of Perineural Dextrose for Carpal Tunnel Syndrome: A Prospective, Randomized, Double-Blind, Controlled Trial. Mayo Clin. Proc. 2017, 92, 1179–1189. [Google Scholar] [CrossRef]
- Wu, Y.T.; Ke, M.J.; Ho, T.Y.; Li, T.Y.; Shen, Y.P.; Chen, L.C. Randomized double-blinded clinical trial of 5% dextrose versus triamcinolone injection for carpal tunnel syndrome patients. Ann. Neurol. 2018, 84, 601–610. [Google Scholar] [CrossRef]
- Chen, L.C.; Ho, T.Y.; Shen, Y.P.; Su, Y.C.; Li, T.Y.; Tsai, C.K.; Wu, Y.T. Perineural Dextrose and Corticosteroid Injections for Ulnar Neuropathy at the Elbow: A Randomized Double-blind Trial. Arch. Phys. Med. Rehabil. 2020, 101, 1296–1303. [Google Scholar] [CrossRef]
- Lin, M.T.; Liao, C.L.; Hsiao, M.Y.; Hsueh, H.W.; Chao, C.C.; Wu, C.H. Volume Matters in Ultrasound-Guided Perineural Dextrose Injection for Carpal Tunnel Syndrome: A Randomized, Double-Blinded, Three-Arm Trial. Front. Pharmacol. 2020, 11, 625830. [Google Scholar] [CrossRef]
- Mansiz-Kaplan, B.; Nacir, B.; Pervane-Vural, S.; Tosun-Meric, O.; Duyur-Cakit, B.; Genc, H. Effect of Perineural Dextrose Injection on Ulnar Neuropathy at the Elbow: A Randomized, Controlled, Double-Blind Study. Arch. Phys. Med. Rehabil. 2022, 103, 2085–2091. [Google Scholar] [CrossRef]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.; Cahill, C.M. TNF-alpha and neuropathic pain—A review. J. Neuroinflamm. 2010, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Chen, Y.P.; Lam, K.H.S.; Reeves, K.D.; Lin, J.A.; Kuo, C.Y. Mechanism of Glucose Water as a Neural Injection: A Perspective on Neuroinflammation. Life 2022, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Bhandi, S.; Alkahtani, A.; Mashyakhy, M.; Abumelha, A.S.; Albar, N.H.M.; Renugalakshmi, A.; Alkahtany, M.F.; Robaian, A.; Almeslet, A.S.; Patil, V.R.; et al. Effect of Ascorbic Acid on Differentiation, Secretome and Stemness of Stem Cells from Human Exfoliated Deciduous Tooth (SHEDs). J. Pers. Med. 2021, 11, 589. [Google Scholar] [CrossRef]
- Mahendra, J.; Mahendra, L.; Mugri, M.H.; Sayed, M.E.; Bhandi, S.; Alshahrani, R.T.; Balaji, T.M.; Varadarajan, S.; Tanneeru, S.; Patil, S.; et al. Role of Periodontal Bacteria, Viruses, and Placental mir155 in Chronic Periodontitis and Preeclampsia-A Genetic Microbiological Study. Curr. Issues Mol. Biol. 2021, 43, 831–844. [Google Scholar] [CrossRef]
- Peng, Y.; Chu, S.; Yang, Y.; Zhang, Z.; Pang, Z.; Chen, N. Neuroinflammatory In Vitro Cell Culture Models and the Potential Applications for Neurological Disorders. Front. Pharmacol. 2021, 12, 671734. [Google Scholar] [CrossRef]
- Zhi, S.M.; Fang, G.X.; Xie, X.M.; Liu, L.H.; Yan, J.; Liu, D.B.; Yu, H.Y. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1524–1536. [Google Scholar] [CrossRef]
- Wang, S.; Xia, B.; Qiao, Z.; Duan, L.; Wang, G.; Meng, W.; Liu, Z.; Wang, Y.; Zhang, M. Tetramethylpyrazine attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells through regulating apoptosis, autophagy and oxidative damage. Drug Des. Dev. Ther. 2019, 13, 1187–1196. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Plas, D.R.; Rathmell, J.C.; Fox, C.J.; Harris, M.H.; Thompson, C.B. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol. Cell. Biol. 2001, 21, 5899–5912. [Google Scholar] [CrossRef]
- Mason, E.F.; Rathmell, J.C. Cell metabolism: An essential link between cell growth and apoptosis. Biochim. Biophys. Acta 2011, 1813, 645–654. [Google Scholar] [CrossRef]
- Allen, D.A.; Yaqoob, M.M.; Harwood, S.M. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J. Nutr. Biochem. 2005, 16, 705–713. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, H.; Wei, T.; Wang, D.; Xia, H.; Zhao, L.; Ji, J.; Gao, H. High Glucose-Induced PC12 Cell Death by Increasing Glutamate Production and Decreasing Methyl Group Metabolism. BioMed Res. Int. 2016, 2016, 4125731. [Google Scholar] [CrossRef]
- Najafi, R.; Sharifi, A.M.; Hosseini, A. Protective effects of alpha lipoic acid on high glucose-induced neurotoxicity in PC12 cells. Metab. Brain Dis. 2015, 30, 731–738. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Liu, S.Y.; Sun, Q.Y.; Ren, J.; Liu, H.X.; Li, H. Proanthocyanidin B2 attenuates high-glucose-induced neurotoxicity of dorsal root ganglion neurons through the PI3K/Akt signaling pathway. Neural Regen. Res. 2018, 13, 1628–1636. [Google Scholar] [CrossRef]
- Peiró, C.; Romacho, T.; Azcutia, V.; Villalobos, L.; Fernández, E.; Bolaños, J.P.; Moncada, S.; Sánchez-Ferrer, C.F. Inflammation, glucose, and vascular cell damage: The role of the pentose phosphate pathway. Cardiovasc. Diabetol. 2016, 15, 82. [Google Scholar] [CrossRef]
- Le Goffe, C.; Vallette, G.; Charrier, L.; Candelon, T.; Bou-Hanna, C.; Bouhours, J.F.; Laboisse, C.L. Metabolic control of resistance of human epithelial cells to H2O2 and NO stresses. Biochem. J. 2002, 364 Pt 2, 349–359. [Google Scholar] [CrossRef]
- Wu, S.B.; Wei, Y.H. AMPK-mediated increase of glycolysis as an adaptive response to oxidative stress in human cells: Implication of the cell survival in mitochondrial diseases. Biochim. Biophys. Acta 2012, 1822, 233–247. [Google Scholar] [CrossRef]
- Rumsey, S.C.; Kwon, O.; Xu, G.W.; Burant, C.F.; Simpson, I.; Levine, M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 1997, 272, 18982–18989. [Google Scholar] [CrossRef]
- Liemburg-Apers, D.C.; Willems, P.H.; Koopman, W.J.; Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015, 89, 1209–1226. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef]
- Landeira, B.S.; Santana, T.; Araújo, J.A.M.; Tabet, E.I.; Tannous, B.A.; Schroeder, T.; Costa, M.R. Activity-Independent Effects of CREB on Neuronal Survival and Differentiation during Mouse Cerebral Cortex Development. Cerebral Cortex 2018, 28, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Alberini, C.M. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009, 89, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Sugo, N.; Morimatsu, M.; Arai, Y.; Yanagida, T.; Yamamoto, N. Activity-Dependent Dynamics of the Transcription Factor of cAMP-Response Element Binding Protein in Cortical Neurons Revealed by Single-Molecule Imaging. J. Neurosci. 2017, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jansson, D.; Ng, A.C.; Fu, A.; Depatie, C.; Al Azzabi, M.; Screaton, R.A. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc. Natl. Acad. Sci. USA 2008, 105, 10161–10166. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Qian, W.; Yin, X.; Zhang, L.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.X.; Liu, F. CREB regulates the expression of neuronal glucose transporter 3: A possible mechanism related to impaired brain glucose uptake in Alzheimer’s disease. Nucleic Acids Res. 2013, 41, 3240–3256. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Karelina, K.; Obrietan, K. CREB: A multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011, 116, 1–9. [Google Scholar] [CrossRef]
- Wu, X.; Liang, Y.; Jing, X.; Lin, D.; Chen, Y.; Zhou, T.; Peng, S.; Zheng, D.; Zeng, Z.; Lei, M.; et al. Rifampicin Prevents SH-SY5Y Cells from Rotenone-Induced Apoptosis via the PI3K/Akt/GSK-3β/CREB Signaling Pathway. Neurochem. Res. 2018, 43, 886–893. [Google Scholar] [CrossRef]
- Thornton, T.M.; Rincon, M. Non-classical p38 map kinase functions: Cell cycle checkpoints and survival. Int. J. Biol. Sci. 2009, 5, 44–51. [Google Scholar] [CrossRef]
- Lucas, R.M.; Luo, L.; Stow, J.L. ERK1/2 in immune signalling. Biochem. Soc. Trans. 2022, 50, 1341–1352. [Google Scholar] [CrossRef]
- Ku, J.M.; Hong, S.H.; Kim, H.I.; Kim, M.J.; Kim, S.K.; Kim, M.; Choi, S.Y.; Park, J.; Kim, H.K.; Kim, J.H.; et al. Synergistic anticancer effect of combined use of Trichosanthes kirilowii with cisplatin and pemetrexed enhances apoptosis of H1299 non-small-cell lung cancer cells via modulation of ErbB3. Phytomedicine 2020, 66, 153109. [Google Scholar] [CrossRef]
- Walker, S.R.; Nelson, E.A.; Yeh, J.E.; Pinello, L.; Yuan, G.C.; Frank, D.A. STAT5 outcompetes STAT3 to regulate the expression of the oncogenic transcriptional modulator BCL6. Mol. Cell. Biol. 2013, 33, 2879–2890. [Google Scholar] [CrossRef]
- Linher-Melville, K.; Singh, G. The complex roles of STAT3 and STAT5 in maintaining redox balance: Lessons from STAT-mediated xCT expression in cancer cells. Mol. Cell. Endocrinol. 2017, 451, 40–52. [Google Scholar] [CrossRef]
- Walker, S.R.; Xiang, M.; Frank, D.A. Distinct roles of STAT3 and STAT5 in the pathogenesis and targeted therapy of breast cancer. Mol. Cell. Endocrinol. 2014, 382, 616–621. [Google Scholar] [CrossRef]
- Mora, L.B.; Buettner, R.; Seigne, J.; Diaz, J.; Ahmad, N.; Garcia, R.; Bowman, T.; Falcone, R.; Fairclough, R.; Cantor, A.; et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: Direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002, 62, 6659–6666. [Google Scholar]
- Cui, Y.; Riedlinger, G.; Miyoshi, K.; Tang, W.; Li, C.; Deng, C.X.; Robinson, G.W.; Hennighausen, L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell. Biol. 2004, 24, 8037–8047. [Google Scholar] [CrossRef]
- Hand, T.W.; Cui, W.; Jung, Y.W.; Sefik, E.; Joshi, N.S.; Chandele, A.; Liu, Y.; Kaech, S.M. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc. Natl. Acad. Sci. USA 2010, 107, 16601–16606. [Google Scholar] [CrossRef]
- Harada, H.; Andersen, J.S.; Mann, M.; Terada, N.; Korsmeyer, S.J. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc. Natl. Acad. Sci. USA 2001, 98, 9666–9670. [Google Scholar] [CrossRef]
- Ban, K.; Kozar, R.A. Protective role of p70S6K in intestinal ischemia/reperfusion injury in mice. PLoS ONE 2012, 7, e41584. [Google Scholar] [CrossRef]
- Lezaja, A.; Altmeyer, M. Inherited DNA lesions determine G1 duration in the next cell cycle. Cell Cycle 2018, 17, 24–32. [Google Scholar] [CrossRef]
- Stark, G.R.; Taylor, W.R. Analyzing the G2/M checkpoint. Methods Mol. Biol. 2004, 280, 51–82. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, N.Y.; Lee, K.A.; Park, T.S.; Jin, H.Y. Influence of Glucose Fluctuation on Peripheral Nerve Damage in Streptozotocin-Induced Diabetic Rats. Diabetes Metab. J. 2022, 46, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Sabanayagam, C.; Liew, G.; Tai, E.S.; Shankar, A.; Lim, S.C.; Subramaniam, T.; Wong, T.Y. Relationship between glycated haemoglobin and microvascular complications: Is there a natural cut-off point for the diagnosis of diabetes? Diabetologia 2009, 52, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Muona, P.; Peltonen, J.; Jaakkola, S.; Uitto, J. Increased matrix gene expression by glucose in rat neural connective tissue cells in culture. Diabetes 1991, 40, 605–611. [Google Scholar] [CrossRef]
- Fink, R.B.; Cairns, A.M. A bioenergetic basis for peripheral nerve fiber dissociation. Pain 1982, 12, 307–317. [Google Scholar] [CrossRef]
- Zhu, H.; Yu, W.J.; Le, Y.; Wang, W.J.; Li, F.; Gui, T.; Wang, Y.M.; Shi, W.D.; Ding, W.L.; Fan, X.Q. High glucose levels increase the expression of neurotrophic factors associated with p-p42/p44 MAPK in Schwann cells in vitro. Mol. Med. Rep. 2012, 6, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, J.; Shi, S.; Qin, X.; Zhang, K.; Xu, J. Kaempferol protects retinal ganglion ceils from high-glucose-induced injury by regulating vasohibin-1. Neurosci. Lett. 2020, 716, 134633. [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, Z.G. Choosing an appropriate glucose concentration according to different cell types and experimental purposes is very important. Cell Stress Chaperones 2015, 20, 1–2. [Google Scholar] [CrossRef]
- Sun, H.; Leng, T.; Zeng, Z.; Gao, X.; Inoue, K.; Xiong, Z.G. Role of TRPM7 channels in hyperglycemia-mediated injury of vascular endothelial cells. PLoS ONE 2013, 8, e79540. [Google Scholar] [CrossRef]
- Kaplan, M.; Tendler, Y.; Mahamid, R.; Shiner, M.; Aviram, M.; Hayek, T. High glucose upregulates C-reactive protein synthesis in macrophages. Clin. Chem. 2010, 56, 1036–1038. [Google Scholar] [CrossRef]
- Gandhi, G.K.; Ball, K.K.; Cruz, N.F.; Dienel, G.A. Hyperglycaemia and diabetes impair gap junctional communication among astrocytes. ASN Neuro 2010, 2, e00030. [Google Scholar] [CrossRef]
- Meng, X.F.; Wang, X.L.; Tian, X.J.; Yang, Z.H.; Chu, G.P.; Zhang, J.; Li, M.; Shi, J.; Zhang, C. Nod-like receptor protein 1 inflammasome mediates neuron injury under high glucose. Mol. Neurobiol. 2014, 49, 673–684. [Google Scholar] [CrossRef]
- Freeman, J.W.; Empson, Y.M.; Ekwueme, E.C.; Paynter, D.M.; Brolinson, P.G. Effect of prolotherapy on cellular proliferation and collagen deposition in MC3T3-E1 and patellar tendon fibroblast populations. Transl. Res. 2011, 158, 132–139. [Google Scholar] [CrossRef]
- Ekwueme, E.C.; Mohiuddin, M.; Yarborough, J.A.; Brolinson, P.G.; Docheva, D.; Fernandes, H.A.M.; Freeman, J.W. Prolotherapy Induces an Inflammatory Response in Human Tenocytes In Vitro. Clin. Orthop. Relat. Res. 2017, 475, 2117–2127. [Google Scholar] [CrossRef]
- Woo, M.S.; Park, J.; Ok, S.H.; Park, M.; Sohn, J.T.; Cho, M.S.; Shin, I.W.; Kim, Y.A. The proper concentrations of dextrose and lidocaine in regenerative injection therapy: In vitro study. Korean J. Pain 2021, 34, 19–26. [Google Scholar] [CrossRef]
- Oh, S.; Ettema, A.M.; Zhao, C.; Zobitz, M.E.; Wold, L.E.; An, K.N.; Amadio, P.C. Dextrose-induced subsynovial connective tissue fibrosis in the rabbit carpal tunnel: A potential model to study carpal tunnel syndrome? Hand 2008, 3, 34–40. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherng, J.-H.; Chang, S.-J.; Tsai, H.-D.; Chun, C.-F.; Fan, G.-Y.; Reeves, K.D.; Lam, K.H.S.; Wu, Y.-T. The Potential of Glucose Treatment to Reduce Reactive Oxygen Species Production and Apoptosis of Inflamed Neural Cells In Vitro. Biomedicines 2023, 11, 1837. https://doi.org/10.3390/biomedicines11071837
Cherng J-H, Chang S-J, Tsai H-D, Chun C-F, Fan G-Y, Reeves KD, Lam KHS, Wu Y-T. The Potential of Glucose Treatment to Reduce Reactive Oxygen Species Production and Apoptosis of Inflamed Neural Cells In Vitro. Biomedicines. 2023; 11(7):1837. https://doi.org/10.3390/biomedicines11071837
Chicago/Turabian StyleCherng, Juin-Hong, Shu-Jen Chang, Hsin-Da Tsai, Chung-Fang Chun, Gang-Yi Fan, Kenneth Dean Reeves, King Hei Stanley Lam, and Yung-Tsan Wu. 2023. "The Potential of Glucose Treatment to Reduce Reactive Oxygen Species Production and Apoptosis of Inflamed Neural Cells In Vitro" Biomedicines 11, no. 7: 1837. https://doi.org/10.3390/biomedicines11071837
APA StyleCherng, J.-H., Chang, S.-J., Tsai, H.-D., Chun, C.-F., Fan, G.-Y., Reeves, K. D., Lam, K. H. S., & Wu, Y.-T. (2023). The Potential of Glucose Treatment to Reduce Reactive Oxygen Species Production and Apoptosis of Inflamed Neural Cells In Vitro. Biomedicines, 11(7), 1837. https://doi.org/10.3390/biomedicines11071837








