HLA-DR Expression on Monocytes and Sepsis Index Are Useful in Predicting Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definitions
2.3. Samples
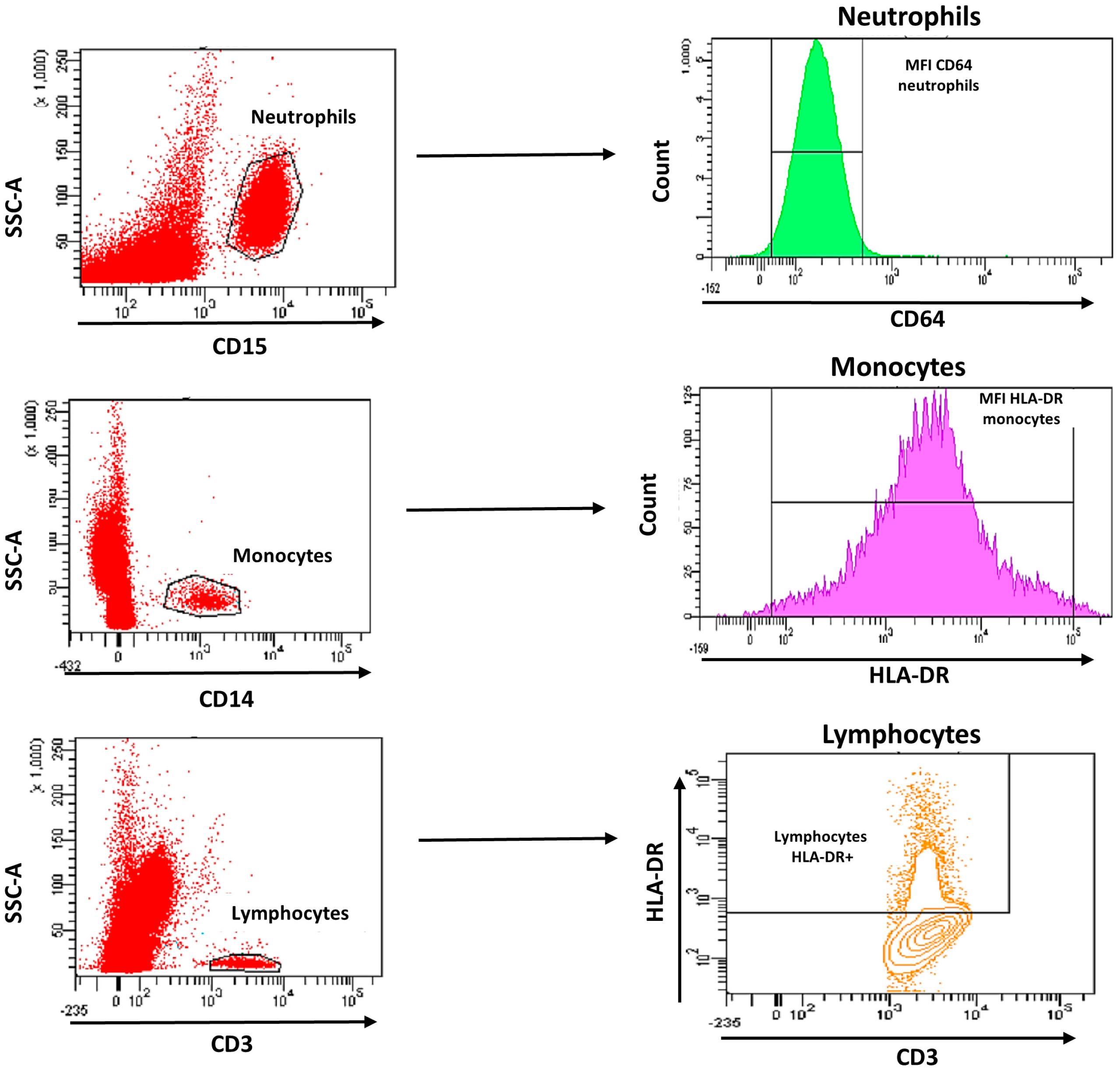
2.4. Flow Cytometry Staining
2.5. Flow Cytometry Calibration
2.6. Flow Cytometry Analysis
2.7. Plasma Analysis
2.8. Statistical Analysis
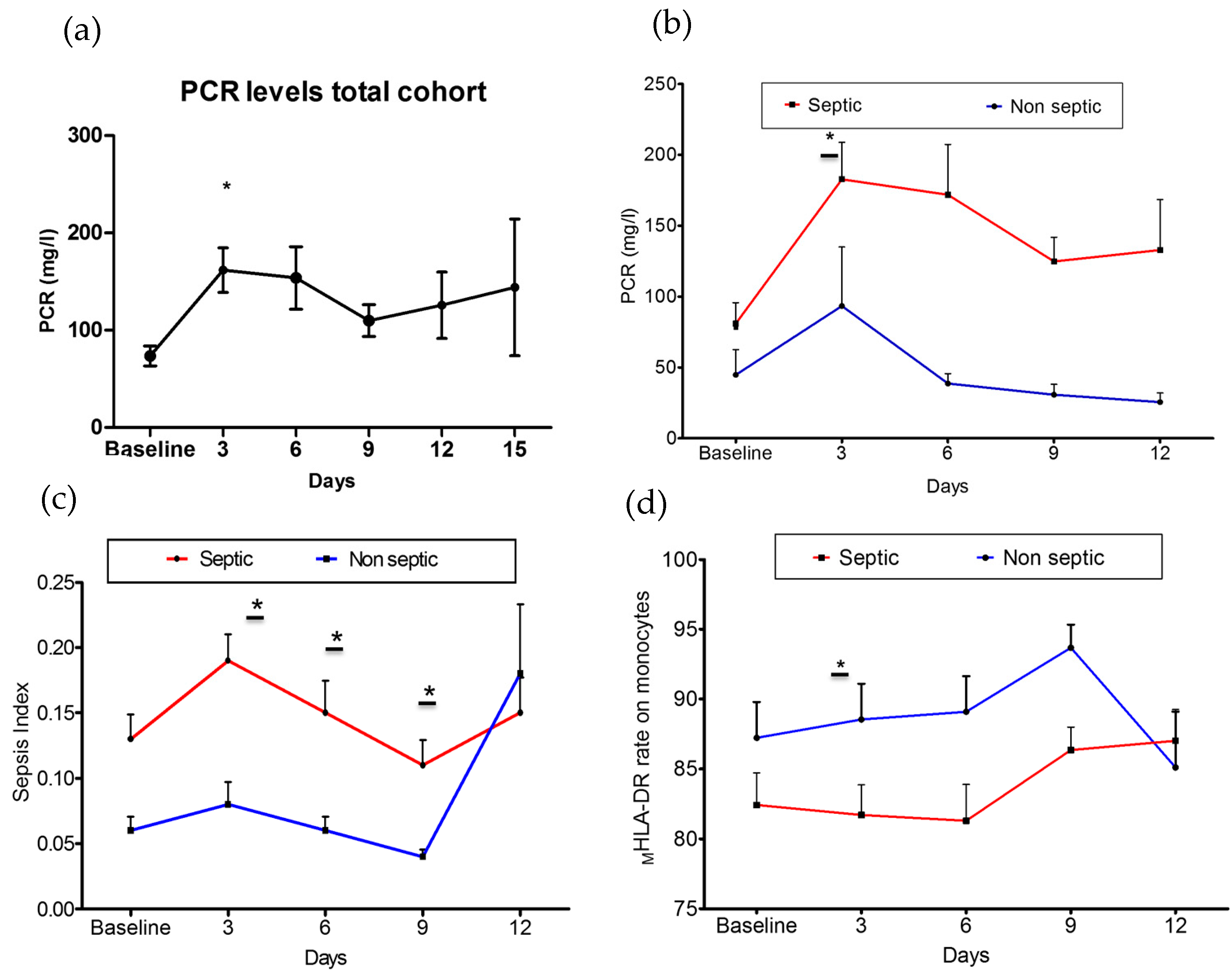
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vincent, J.-L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef]
- Focht, A.; Jones, A.E.; Lowe, T.J. Early goal-directed therapy: Improving mortality and morbidity of sepsis in the emergency department. Jt. Comm. J. Qual. Patient Saf. 2009, 35, 186–191. [Google Scholar] [CrossRef]
- Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.; LoVecchio, F.; et al. A Randomized Trial of Protocol-Based Care for Early Septic Shock. New Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [PubMed]
- Jones, S.L.; Ashton, C.M.; Kiehne, L.; Gigliotti, E.; Bell-Gordon, C.; Disbot, M.; Masud, F.; Shirkey, B.A.; Wray, N.P. Reductions in Sepsis Mortality and Costs After Design and Implementation of a Nurse-Based Early Recognition and Response Program. Jt. Comm. J. Qual. Patient Saf. 2015, 41, 483–491. [Google Scholar] [CrossRef]
- Damiani, E.; Donati, A.; Serafini, G.; Rinaldi, L.; Adrario, E.; Pelaia, P.; Busani, S.; Girardis, M. Effect of performance improvement programs on compliance with sepsis bundles and mortality: A systematic review and meta-analysis of observational studies. PLoS ONE 2015, 10, e0125827. [Google Scholar] [CrossRef]
- Lakbar, I.; Munoz, M.; Pauly, V.; Orleans, V.; Fabre, C.; Fond, G.; Vincent, J.L.; Boyer, L.; Leone, M. Septic shock: Incidence, mortality and hospital readmission rates in French intensive care units from 2014 to 2018. Anaesth. Crit. Care Pain Med. 2022, 41, 101082. [Google Scholar] [CrossRef]
- Shalova, I.N.; Lim, J.Y.; Chittezhath, M.; Zinkernagel, A.S.; Beasley, F.; Hernández-Jiménez, E.; Toledano, V.; Cubillos-Zapata, C.; Rapisarda, A.; Chen, J.; et al. Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1α. Immunity 2015, 42, 484–498. [Google Scholar] [CrossRef]
- Cejková, P.; Chromá, V.; Cerná, M.; Marková, M.; Marek, J.; Lacinová, Z.; Haluzík, M. Monitoring of the course of sepsis in hematooncological patients by extrapituitary prolactin expression in peripheral blood monocytes. Physiol Res. 2012, 61, 481–488. [Google Scholar] [CrossRef]
- Cheng, S.C.; Scicluna, B.P.; Arts, R.J.; Gresnigt, M.S.; Lachmandas, E.; Giamarellos-Bourboulis, E.J.; Kox, M.; Manjeri, G.R.; Wagenaars, J.A.; Cremer, O.L.; et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat. Immunol. 2016, 17, 406–413. [Google Scholar] [CrossRef]
- Grondman, I.; Arts, R.J.W.; Koch, R.M.; Leijte, G.P.; Gerretsen, J.; Bruse, N.; Kempkes, R.W.M.; Ter Horst, R.; Kox, M.; Pickkers, P.; et al. Frontline Science: Endotoxin-induced immunotolerance is associated with loss of monocyte metabolic plasticity and reduction of oxidative burst. J. Leukoc. Biol. 2019, 106, 11–25. [Google Scholar] [CrossRef]
- Juskewitch, J.E.; Abraham, R.S.; League, S.C.; Jenkins, S.M.; Smith, C.Y.; Enders, F.T.; Grebe, S.K.; Carey, W.A.; Huskins, W.C. Monocyte HLA-DR expression and neutrophil CD64 expression as biomarkers of infection in critically ill neonates and infants. Pediatr. Res. 2015, 78, 683–690. [Google Scholar] [CrossRef]
- Cazalis, M.A.; Friggeri, A.; Cavé, L.; Demaret, J.; Barbalat, V.; Cerrato, E.; Lepape, A.; Pachot, A.; Monneret, G.; Venet, F. Decreased HLA-DR antigen-associated invariant chain (CD74) mRNA expression predicts mortality after septic shock. Crit. Care 2013, 17, R287. [Google Scholar] [CrossRef] [PubMed]
- Vester, H.; Dargatz, P.; Huber-Wagner, S.; Biberthaler, P.; van Griensven, M. HLA-DR expression on monocytes is decreased in polytraumatized patients. Eur. J. Med. Res. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Harrison, P.T.; Davis, W.; Norman, J.C.; Hockaday, A.R.; Allen, J.M. Binding of monomeric immunoglobulin G triggers Fc RI-mediated endocytosis. J. Biol. Chem. 1994, 269, 24396–24402. [Google Scholar] [CrossRef]
- van der Poel, C.E.; Spaapen, R.M.; van de Winkel, J.G.; Leusen, J.H. Functional characteristics of the high affinity IgG receptor, FcRI. J. Immunol. 2011, 186, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on Neutrophil Function in Severe Inflammation. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef] [PubMed]
- Akinrinmade, O.A.; Chetty, S.; Daramola, A.K.; Islam, M.U.; Thepen, T.; Barth, S. CD64: An Attractive Immunotherapeutic Target for M1-type Macrophage Mediated Chronic Inflammatory Diseases. Biomedicines 2017, 5, 56. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS. International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Cheron, A.; Floccard, B.; Allaouchiche, B.; Guignant, C.; Poitevin, F.; Malcus, C.; Crozon, J.; Faure, A.; Guillaume, C.; Marcotte, G.; et al. Lack of recovery in monocyte human leukocyte antigen-DR expression is independently associated with the development of sepsis after major trauma. Crit. Care 2010, 14, R208. [Google Scholar] [CrossRef]
- Flohé, S.; Scholz, M. HLA-DR monitoring in the intensive care unit--more than a tool for the scientist in the laboratory? Crit. Care Med. 2009, 37, 2849–2850. [Google Scholar] [CrossRef]
- Schefold, J.C. Measurement of monocytic HLA-DR (mHLA-DR) expression in patients with severe sepsis and septic shock: Assessment of immune organ failure. Intensive Care Med. 2010, 36, 1810–1812. [Google Scholar] [CrossRef]
- Gámez-Díaz, L.Y.; Enriquez, L.E.; Matute, J.D.; Velásquez, S.; Gómez, I.D.; Toro, F.; Ospina, S.; Bedoya, V.; Arango, C.M.; Valencia, M.L.; et al. Diagnostic accuracy of HMGB-1, sTREM-1, and CD64 as markers of sepsis in patients recently admitted to the emergency department. Acad. Emerg. Med. 2011, 18, 807–815. [Google Scholar] [CrossRef]
- Chouhan, S.; Hansa, J.; Pradhan, S. Early diagnosis of sepsis through sepsis markers and sepsis index through flow Cytometry technology. Asian J. Pharm. Clin. Res. 2017, 10, 145. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.Y.; Zeng, L.; Zhang, A.Q.; Pan, W.; Gu, W.; Jiang, J.X. Neutrophil CD64 expression as a diagnostic marker for sepsis in adult patients: A meta-analysis. Crit Care 2015, 19, 245. [Google Scholar] [CrossRef]


| Characteristics | Total Cohort n = 77 | Septic n = 55 | Non-Septic n = 22 | p-Value |
|---|---|---|---|---|
| Female sex (n. of patients, (%)) | 26 (34) | 17 (31) | 9 (41) | 0.400 |
| Age (years), (IQR) | 54 ± 16 | 54 ± 16 | 56 ± 16 | 0.520 |
| Basal SOFA * score (IQR) | 7 ± 4 | 8 ± 3 | 4 ± 3 | <0.001 |
| APACHE ** II score (IQR) | 21 ± 7 | 22 ± 6 | 19 ± 9 | 0.050 |
| Median hospital days (IQR) | 21 ± 15 | 25 ± 15 | 10 ± 7 | <0.001 |
| Mechanic ventilation days (IQR) | 14 ± 13 | 17 ± 13 | 4 ± 8 | <0.001 |
| Comorbidities (n. of patients, (%)) | ||||
| COPD *** | 6 (8) | 4 (7) | 2 (10) | 0.790 |
| Smoker | 25 (32) | 16 (29) | 9 (41) | 0.320 |
| Alcoholism | 15 (19) | 9 (16) | 6 (27) | 0.270 |
| Cardiopathy | 8 (10) | 6 (11) | 2 (10) | 0.810 |
| Chronic kidney disease | 7 (9) | 5 (9) | 2 (10) | 1.000 |
| Cirrhosis | 2 (3) | 2 (4) | 0 (0) | 0.360 |
| Exitus (n.patients, (%)) | 14 (18) | 11 (20) | 3 (14) | 0.510 |
| Blood culture (n. of patients, (%)) | 36 (48) | 36 (66) | NA | |
| Adequate antibiotic treatment (n. of patients, (%)) | 41 (53) | 41 (75) | NA | |
| Clinical suspicion sepsis days (IQR) | 4 ± 1 | 4 ± 1 | ||
| Shock septic (n. of patients, (%)) | 9 (12) | 9 (16) | NA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quirant-Sánchez, B.; Plans-Galván, O.; Lucas, E.; Argudo, E.; Martinez-Cáceres, E.M.; Arméstar, F. HLA-DR Expression on Monocytes and Sepsis Index Are Useful in Predicting Sepsis. Biomedicines 2023, 11, 1836. https://doi.org/10.3390/biomedicines11071836
Quirant-Sánchez B, Plans-Galván O, Lucas E, Argudo E, Martinez-Cáceres EM, Arméstar F. HLA-DR Expression on Monocytes and Sepsis Index Are Useful in Predicting Sepsis. Biomedicines. 2023; 11(7):1836. https://doi.org/10.3390/biomedicines11071836
Chicago/Turabian StyleQuirant-Sánchez, Bibiana, Oriol Plans-Galván, Ester Lucas, Eduard Argudo, Eva María Martinez-Cáceres, and Fernando Arméstar. 2023. "HLA-DR Expression on Monocytes and Sepsis Index Are Useful in Predicting Sepsis" Biomedicines 11, no. 7: 1836. https://doi.org/10.3390/biomedicines11071836
APA StyleQuirant-Sánchez, B., Plans-Galván, O., Lucas, E., Argudo, E., Martinez-Cáceres, E. M., & Arméstar, F. (2023). HLA-DR Expression on Monocytes and Sepsis Index Are Useful in Predicting Sepsis. Biomedicines, 11(7), 1836. https://doi.org/10.3390/biomedicines11071836





