Outcome of BCG Vaccination in ADA-SCID Patients: A 12-Patient Series
Abstract
1. Introduction
2. Aim of this Study
3. Materials and Methods
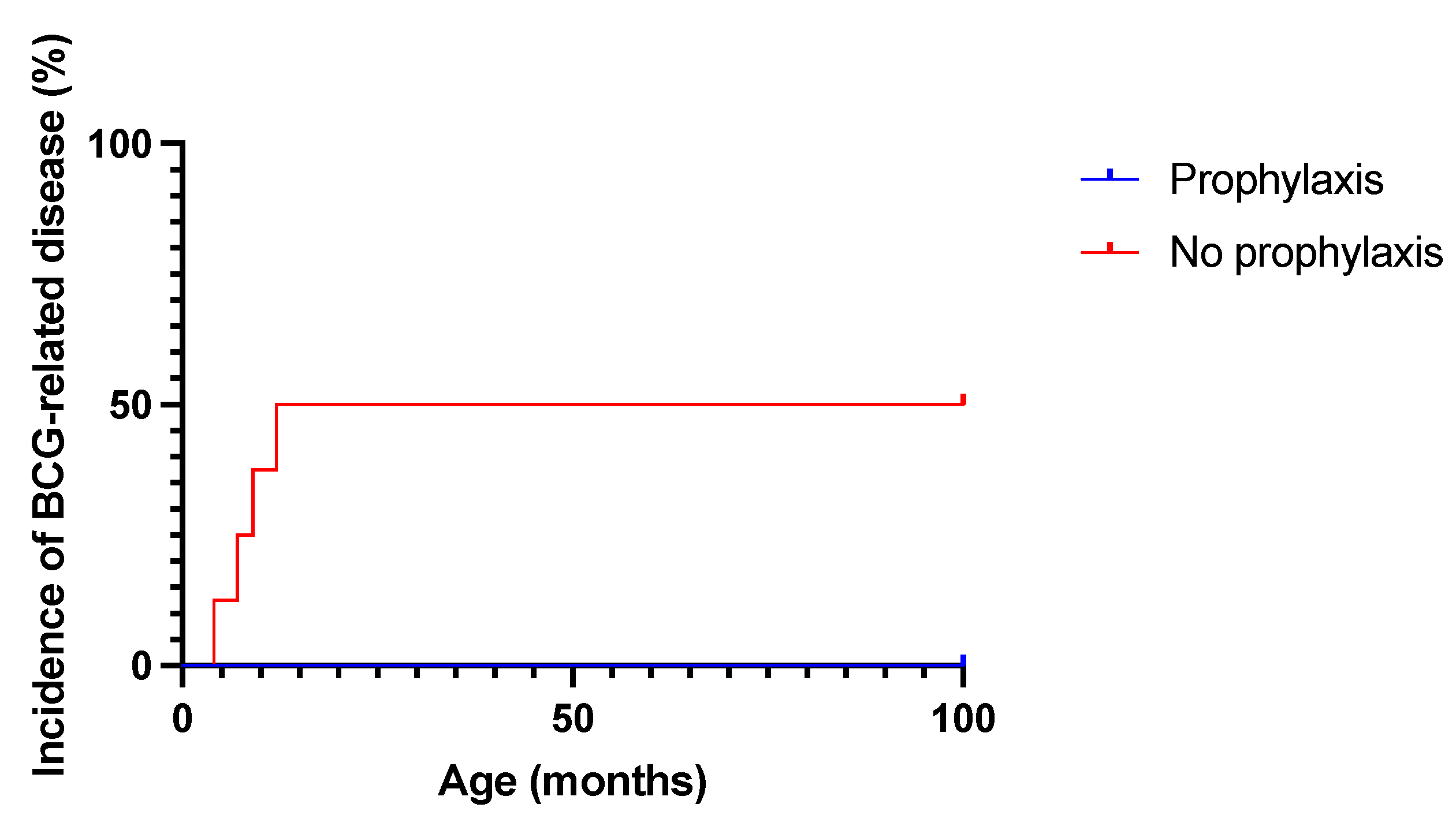
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yerramsetti, S.; Cohen, T.; Atun, R.; A Menzies, N. Global estimates of paediatric tuberculosis incidence in 2013–19: A mathematical modelling analysis. Lancet Glob. Heal. 2021, 10, e207–e215. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.E.; Yuen, C.M.; Rodriguez, C.A.; Nathavitharana, R.R.; McLaughlin, M.M.; Donald, P.; Marais, B.J.; Becerra, M.C. Mortality in children diagnosed with tuberculosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2016, 17, 285–295. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. BCG vaccines: WHO position paper-Recommendations. Wkly. Epidemiol. Rec. 2018, 93, 73–96. [Google Scholar]
- Zwerling, A.; Behr, M.A.; Verma, A.; Brewer, T.F.; Menzies, D.; Pai, M. The BCG World Atlas: A Database of Global BCG Vaccination Policies and Practices. PLOS Med. 2011, 8, e1001012. [Google Scholar] [CrossRef] [PubMed]
- Lancione, S.; Alvarez, J.V.; Alsdurf, H.; Pai, M.; Zwerling, A.A. Tracking changes in national BCG vaccination policies and practices using the BCG World Atlas. BMJ Glob. Heal. 2022, 7, e007462. [Google Scholar] [CrossRef] [PubMed]
- Marciano, B.E.; Huang, C.-Y.; Joshi, G.; Rezaei, N.; Carvalho, B.C.; Allwood, Z.; Ikinciogullari, A.; Reda, S.M.; Gennery, A.; Thon, V.; et al. BCG vaccination in patients with severe combined immunodeficiency: Complications, risks, and vaccination policies. J. Allergy Clin. Immunol. 2014, 133, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, N.; Jacobsen, E.-M.; Hoenig, M.; Schulz, A.; Schuetz, C. BCG Disease in SCID: Three Decades of Experience in a Pediatric Transplant Center. J. Clin. Immunol. 2021, 42, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Tucci, F.; Galimberti, S.; Naldini, L.; Valsecchi, M.G.; Aiuti, A. A systematic review and meta-analysis of gene therapy with hematopoietic stem and progenitor cells for monogenic disorders. Nat. Commun. 2022, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Laberko, A.; Yukhacheva, D.; Rodina, Y.; Abramov, D.; Konovalov, D.; Radygina, S.; Shelikhova, L.; Pershin, D.; Kadnikova, O.; Maschan, M.; et al. BCG-Related Inflammatory Syndromes in Severe Combined Immunodeficiency After TCRαβ+/CD19+ Depleted HSCT. J. Clin. Immunol. 2020, 40, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, G.D.E.; Logan, B.R.; Prockop, S.E.; Buckley, R.H.; Kuo, C.Y.; Griffith, L.M.; Liu, X.; Yip, A.; Hershfield, M.S.; Ayoub, P.G.; et al. Outcomes following treatment for ADA-deficient severe combined immunodeficiency: A report from the PIDTC. Blood 2022, 140, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Aiuti, A.; Cattaneo, F.; Galimberti, S.; Benninghoff, U.; Cassani, B.; Callegaro, L.; Scaramuzza, S.; Andolfi, G.; Mirolo, M.; Brigida, I.; et al. Gene Therapy for Immunodeficiency Due to Adenosine Deaminase Deficiency. N. Engl. J. Med. 2009, 360, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Cicalese, M.P.; Ferrua, F.; Castagnaro, L.; Pajno, R.; Barzaghi, F.; Giannelli, S.; Dionisio, F.; Brigida, I.; Bonopane, M.; Casiraghi, M.; et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood 2016, 128, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hesseling, A.C.; Rabie, H.; Marais, B.J.; Manders, M.; Lips, M.; Schaaf, H.S.; Gie, R.P.; Cotton, M.F.; van Helden, P.D.; Warren, R.M.; et al. Bacille Calmette-Guerin Vaccine--Induced Disease in HIV-Infected and HIV-Uninfected Children. Clin. Infect. Dis. 2006, 42, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Tucci, F.; Gallo, V.; Barzaghi, F.; Ferrua, F.; Migliavacca, M.; Calbi, V.; Doglio, M.; Fratini, E.S.; Karakas, Z.; Guner, S.; et al. Emapalumab treatment in an ADA-SCID patient with refractory hemophagocytic lymphohistiocytosis- related graft failure and disseminated bacillus Calmette-Guérin infection. Haematologica 2020, 106, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Swindells, S.; Ramchandani, R.; Gupta, A.; Benson, C.A.; Leon-Cruz, J.; Mwelase, N.; Juste, M.A.J.; Lama, J.R.; Valencia, J.; Omoz-Oarhe, A.; et al. One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis. N. Engl. J. Med. 2019, 380, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Tuberculosis Programme. WHO Operational Handbook on Tuberculosis. Module 5: Management of Tuberculosis in Children and Adolescents; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Bode, S.F.; Ammann, S.; Al-Herz, W.; Bataneant, M.; Dvorak, C.C.; Gehring, S.; Gennery, A.; Gilmour, K.C.; Gonzalez-Granado, L.I.; Groß-Wieltsch, U.; et al. The syndrome of hemophagocytic lymphohistiocytosis in primary immunodeficiencies: Implications for differential diagnosis and pathogenesis. Haematologica 2015, 100, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Cords, O.; Liu, Q.; Acuna-Villaorduna, C.; Bonnet, M.; Fox, G.J.; Carvalho, A.C.C.; Chan, P.-C.; Croda, J.; Hill, P.C.; et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: A systematic review and individual participant data meta-analysis. Lancet Glob. Heal. 2022, 10, e1307–e1316. [Google Scholar] [CrossRef] [PubMed]
- Thysen, S.M.; Jensen, A.M.; Vedel, J.O.; da Silva Borges, I.; Aaby, P.; Jensen, A.K.G.; Benn, C.S.; Fisker, A.B. Can BCG vaccination at first health-facility contact reduce early infant mortality? Study protocol for a cluster-randomised trial (CS-BCG). BMJ Open 2022, 12, e063872. [Google Scholar] [CrossRef] [PubMed]
- Currier, R.; Puck, J.M. SCID newborn screening: What we’ve learned. J. Allergy Clin. Immunol. 2021, 147, 417–426. [Google Scholar] [CrossRef] [PubMed]
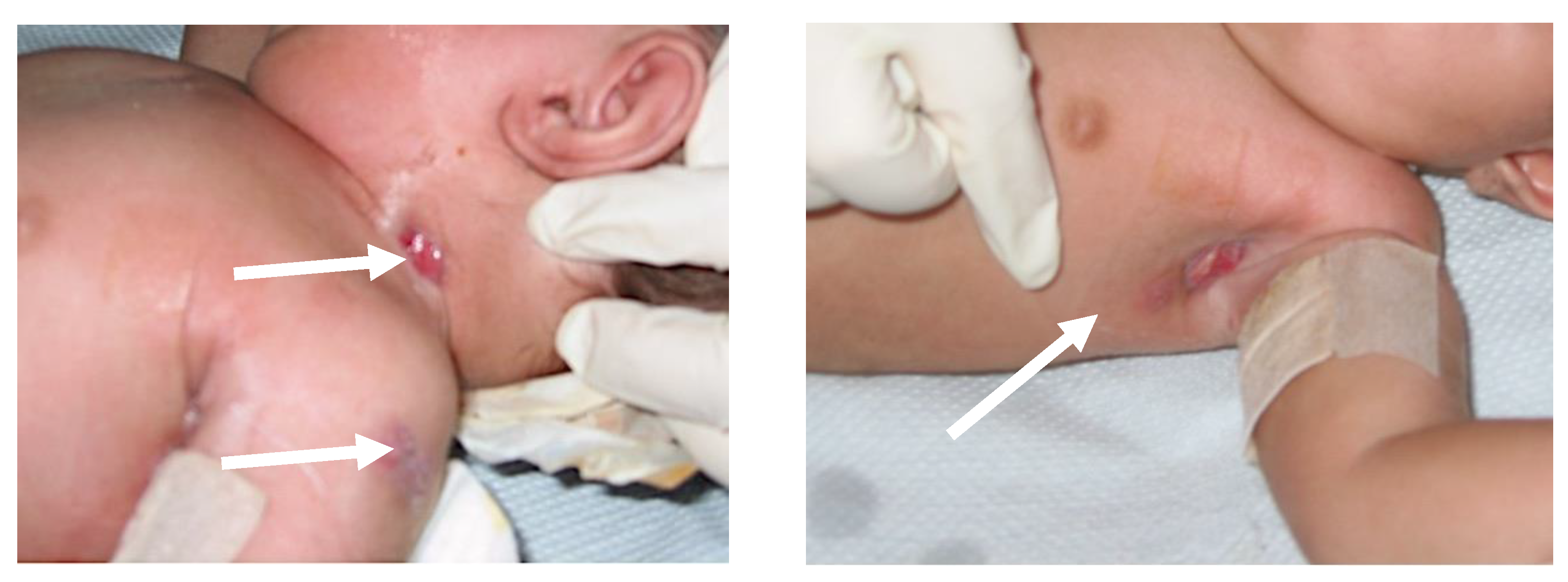
| Cohort | n (%) | Anti-Mycobacterial Therapy |
|---|---|---|
| Vaccinated patients | 349 | 238/349 (68%) |
| BCG-related disease | 177/349 (51%) | 160/177 (90%) |
| Disseminated | 118/177 (34%) | 107/118 (90%) |
| Localized | 59/177 (17%) | 53/59 (89%) |
| No BCG-related disease | 172/349 (49%) | 78/172 (45%) |
| SCID treatment and IRIS | ||
| HSCT/GT/Thymus transplantation/ERT (% of total patients) | 184/3/1/2 (54%) | |
| IRIS | 55/190 (29%) | |
| Previously symptomatic for BCG-related disease | 47/55 (85%) |
| Patient # | Sex | Country of Birth | Lymphocyte Count before ERT (×109/L) | Age at ADA-SCID Diagnosis/Age at Start of ERT (months) | Previous HSCT | Weight Centile at Referral | Comorbidities | Age at BCG Vaccination | Age at BCG Disease Onset (months) | Disease Classification | Treatment or (Secondary Prophylaxis)/Duration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Saudi Arabia | 0.1 | 6/20 | Yes | <5° | Lung interstitial disease, Arnold–Chiari malformation | Birth | 9 | Local (inoculum site) | Rifampicin, ethambutol, ciprofloxacin > ofloxacin/6 months |
| 2 | M | Saudi Arabia | 0.14 | 2/8 | Yes | <3° | Disorder of sexual development, bilateral peripheral hearing loss, psycho-motor retardation, hypothyroidism, pes pronatus | Unknown | 4 | Distant (skin, lymph nodes, lung) | Isoniazid, rifampicin, ethambutol, amikacin/14 months |
| 3 | M | Brazil | <0.25 | 4/10 | No | 3° | Pulmonary stenosis, hypothyroidism, arterial hypertension | 20 days | 7 | Regional (lymph nodes) | Isoniazid, rifampicin, ethambutol/18 months |
| 4 | F | Turkey | 0.039 T-cells | 12/13 | No | <3° | Anemia | Birth | <12 | Disseminated (skin abscesses, central nervous system) | Isoniazid, rifampicin, ethambutol, moxifloxacin/12 month + isoniazid, rifampicin/6 months [14] |
| 5 | M | Venezuela | NA | 4/NA | Yes | 3–5° | Phimosis, feeding disorder, hemangioma of the liver | 5 days | NA | Asymptomatic | |
| 6 | M | Algeria | NA | 21/22 | No | 48° | Hypothyroidism, increased IgE | 28 days | NA | Asymptomatic | |
| 7 | M | Turkey | 0.1 | 18/18 | No | 48° | 2 months | NA | Asymptomatic | ||
| 8 | F | Turkey | 0.14 | 2/2 | No | <−3 SDS | Ileostomy, lymphoma | Unknown | NA | Asymptomatic | |
| 9 | F | Argentina | 0.11–0.47 | 7/8 | No | <−3 SDS | Psychomotor development delay, congenital complex heart malformation | 15 days | NA | Asymptomatic | (Isoniazid, rifampin)/5 months |
| 10 | F | Argentina | 0.13 | 7/8 | No | <−3 SDS | Psychomotor development delay | 15 days | NA | Asymptomatic | (Isoniazid, rifampin, ciprofloxacin)/5 months |
| 11 | M | Poland | 0.36 | 7/10 | No | 62° | Birth | NA | Asymptomatic | (Isoniazid, rifampin)/6 months | |
| 12 | M | Turkey | 0.10 | 2/3 | No | 17° | Unknown | NA | Asymptomatic | (Isoniazid, rifampin)/10 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canarutto, D.; Oltolini, C.; Barzaghi, F.; Calbi, V.; Migliavacca, M.; Tucci, F.; Gallo, V.; Consiglieri, G.; Ferrua, F.; Recupero, S.; et al. Outcome of BCG Vaccination in ADA-SCID Patients: A 12-Patient Series. Biomedicines 2023, 11, 1809. https://doi.org/10.3390/biomedicines11071809
Canarutto D, Oltolini C, Barzaghi F, Calbi V, Migliavacca M, Tucci F, Gallo V, Consiglieri G, Ferrua F, Recupero S, et al. Outcome of BCG Vaccination in ADA-SCID Patients: A 12-Patient Series. Biomedicines. 2023; 11(7):1809. https://doi.org/10.3390/biomedicines11071809
Chicago/Turabian StyleCanarutto, Daniele, Chiara Oltolini, Federica Barzaghi, Valeria Calbi, Maddalena Migliavacca, Francesca Tucci, Vera Gallo, Giulia Consiglieri, Francesca Ferrua, Salvatore Recupero, and et al. 2023. "Outcome of BCG Vaccination in ADA-SCID Patients: A 12-Patient Series" Biomedicines 11, no. 7: 1809. https://doi.org/10.3390/biomedicines11071809
APA StyleCanarutto, D., Oltolini, C., Barzaghi, F., Calbi, V., Migliavacca, M., Tucci, F., Gallo, V., Consiglieri, G., Ferrua, F., Recupero, S., Cervi, M. C., Al-Mousa, H., Pituch-Noworolska, A., Tassan Din, C., Scarpellini, P., Silvani, P., Fossati, C., Casiraghi, M., Cirillo, D. M., ... Cicalese, M. P. (2023). Outcome of BCG Vaccination in ADA-SCID Patients: A 12-Patient Series. Biomedicines, 11(7), 1809. https://doi.org/10.3390/biomedicines11071809






