Intraamygdaloid Oxytocin Increases Time Spent on Social Interaction in Valproate-Induced Autism Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Stereotaxic Surgery
2.3. Drugs and Injection Procedure
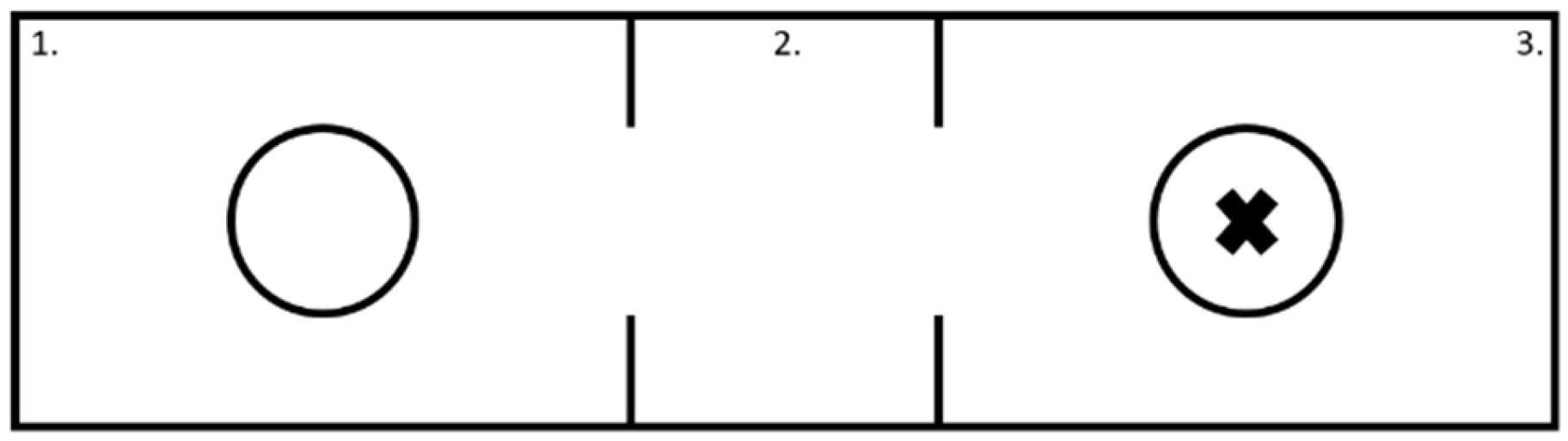
2.4. Social Interaction Test
2.5. Histology
2.6. Statistical Analysis
3. Results
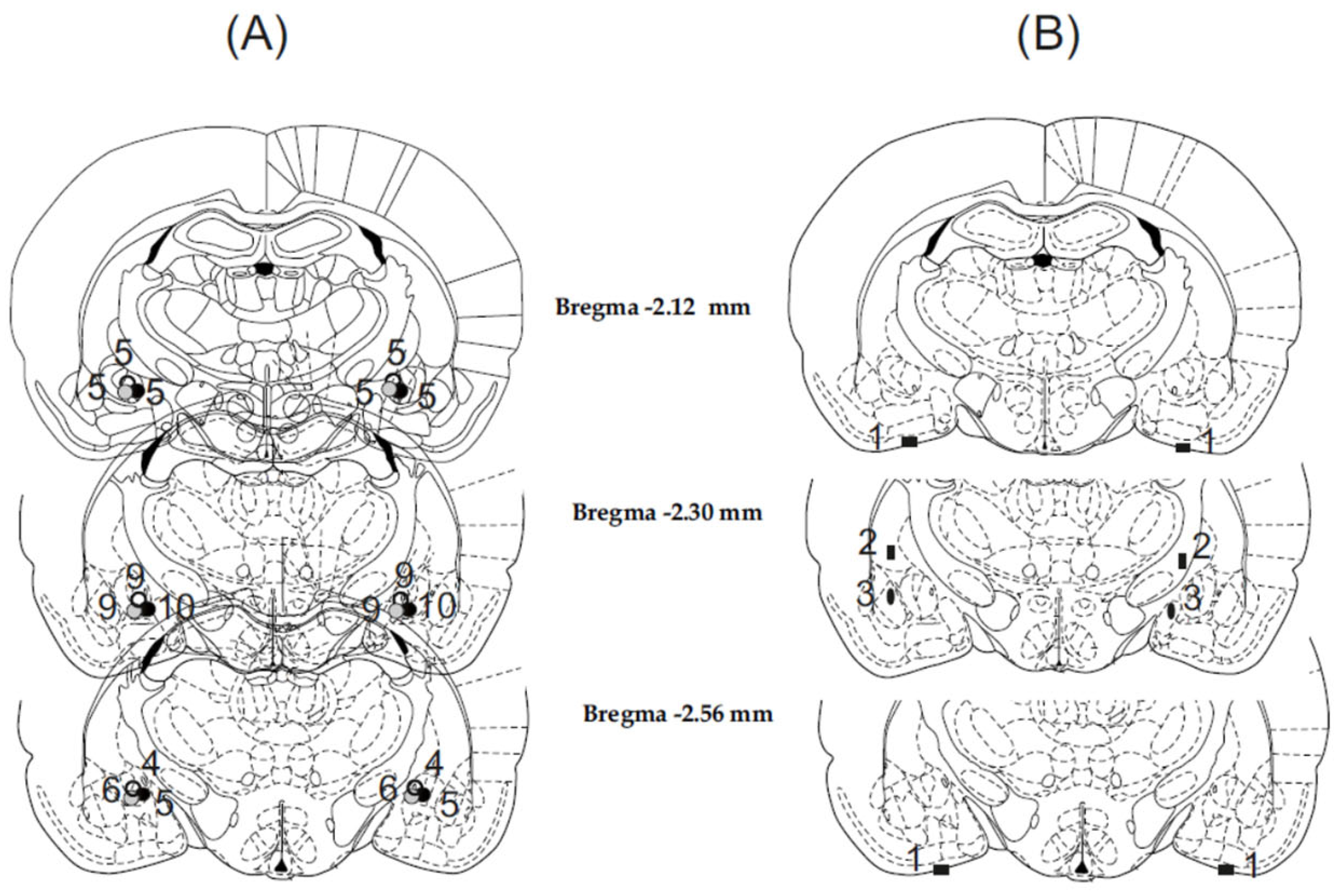
3.1. Histology
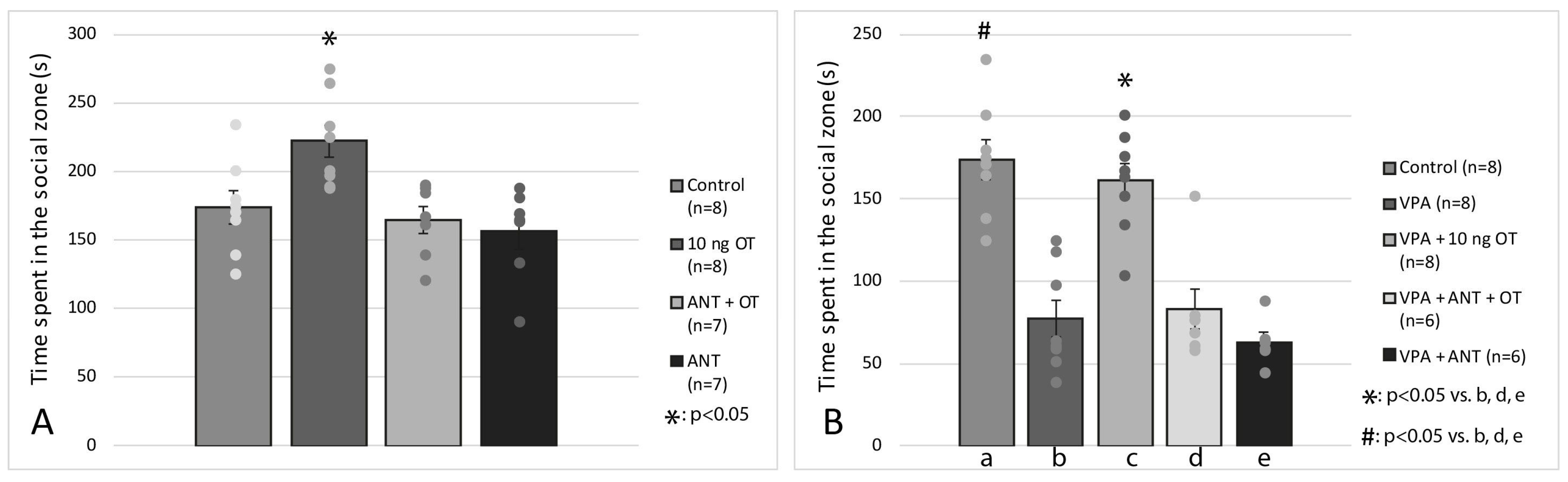
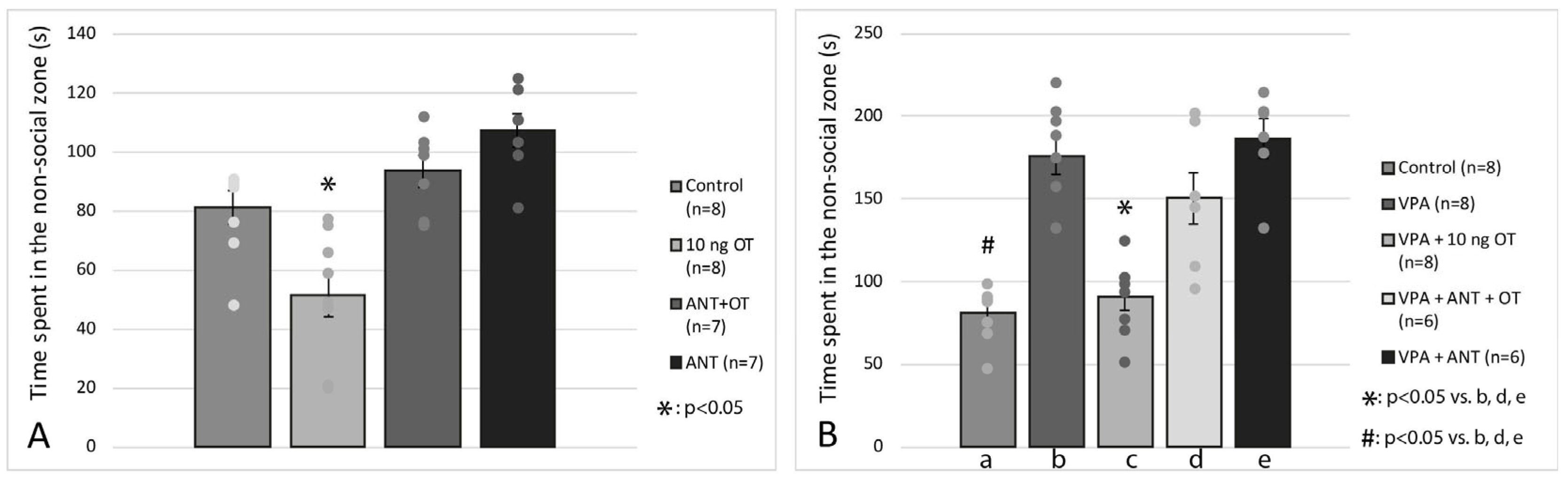
3.2. Social Interaction Test
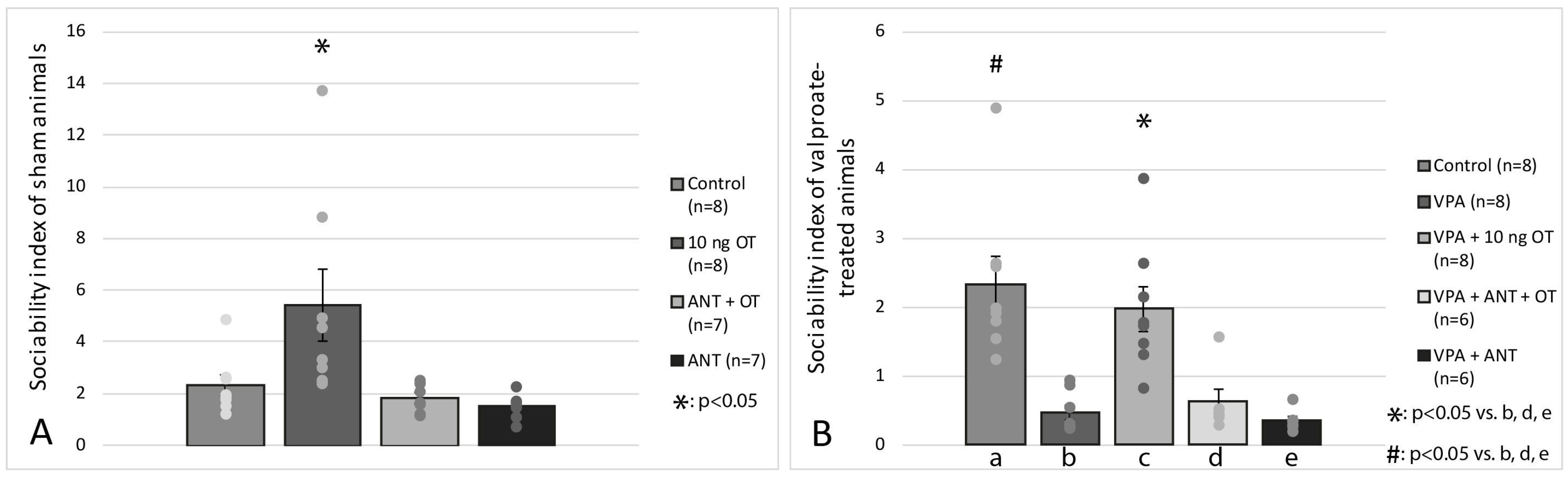
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Karande, S. Autism: A Review for Family Physicians. Indian J. Med. Sci. 2006, 60, 205. [Google Scholar] [CrossRef] [PubMed]
- Bellani, M.; Calderoni, S.; Muratori, F.; Brambilla, P. Brain Anatomy of Autism Spectrum Disorders II. Focus on Amygdala. Epidemiol. Psychiatr. Sci. 2013, 22, 309–312. [Google Scholar] [CrossRef]
- Lai, M.-C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Rogge, N.; Janssen, J. The Economic Costs of Autism Spectrum Disorder: A Literature Review. J. Autism Dev. Disord. 2019, 49, 2873–2900. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.R.; Gonda, X.; Tarazi, F.I. Autism Spectrum Disorder: Classification, Diagnosis and Therapy. Pharmacol. Ther. 2018, 190, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J. What Causes Autism? Exploring the Environmental Contribution. Curr. Opin. Pediatr. 2010, 22, 219–225. [Google Scholar] [CrossRef]
- Herrero, M.J.; Velmeshev, D.; Hernandez-Pineda, D.; Sethi, S.; Sorrells, S.; Banerjee, P.; Sullivan, C.; Gupta, A.R.; Kriegstein, A.R.; Corbin, J.G. Identification of Amygdala-Expressed Genes Associated with Autism Spectrum Disorder. Mol. Autism 2020, 11, 39. [Google Scholar] [CrossRef]
- Perucca, E. Pharmacological and Therapeutic Properties of Valproate. CNS Drugs 2002, 16, 695–714. [Google Scholar] [CrossRef]
- Andrade, C. Valproate in Pregnancy. J. Clin. Psychiatry 2018, 79, 18f12351. [Google Scholar] [CrossRef]
- Christensen, J.; Grønborg, T.K.; Sørensen, M.J.; Schendel, D.; Parner, E.T.; Pedersen, L.H.; Vestergaard, M. Prenatal Valproate Exposure and Risk of Autism Spectrum Disorders and Childhood Autism. JAMA 2013, 309, 1696. [Google Scholar] [CrossRef]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Yang, S.-I.; Cheong, J.H.; Shin, C.Y.; Ko, K.H. The Critical Period of Valproate Exposure to Induce Autistic Symptoms in Sprague–Dawley Rats. Toxicol. Lett. 2011, 201, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, A.M.; Schiavi, S.; Calamandrei, G.; Trezza, V. Prenatal Valproate in Rodents as a Tool to Understand the Neural Underpinnings of Social Dysfunctions in Autism Spectrum Disorder. Neuropharmacology 2019, 159, 107477. [Google Scholar] [CrossRef]
- Chomiak, T.; Karnik, V.; Block, E.; Hu, B. Altering the Trajectory of Early Postnatal Cortical Development Can Lead to Structural and Behavioural Features of Autism. BMC Neurosci. 2010, 11, 102. [Google Scholar] [CrossRef]
- Raza, S.; Himmler, B.T.; Himmler, S.M.; Harker, A.; Kolb, B.; Pellis, S.M.; Gibb, R. Effects of Prenatal Exposure to Valproic Acid on the Development of Juvenile-Typical Social Play in Rats. Behav. Pharmacol. 2015, 26, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.M.; Deckmann, I.; Fontes-Dutra, M.; Bauer-Negrini, G.; Nunes, G.D.-F.; Nunes, W.; Rabelo, B.; Riesgo, R.; Margis, R.; Bambini-Junior, V.; et al. Data on Social Transmission of Food Preference in a Model of Autism Induced by Valproic Acid and Translational Analysis of Circulating MicroRNA. Data Brief 2018, 18, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M.J.; Edgar, J.C.; Ehrlichman, R.S.; Mehta, M.; Roberts, T.P.L.; Siegel, S.J. Validating γ Oscillations and Delayed Auditory Responses as Translational Biomarkers of Autism. Biol. Psychiatry 2010, 68, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Moldrich, R.X.; Leanage, G.; She, D.; Dolan-Evans, E.; Nelson, M.; Reza, N.; Reutens, D.C. Inhibition of Histone Deacetylase in Utero Causes Sociability Deficits in Postnatal Mice. Behav. Brain Res. 2013, 257, 253–264. [Google Scholar] [CrossRef]
- Melancia, F.; Schiavi, S.; Servadio, M.; Cartocci, V.; Campolongo, P.; Palmery, M.; Pallottini, V.; Trezza, V. Sex-Specific Autistic Endophenotypes Induced by Prenatal Exposure to Valproic Acid Involve Anandamide Signalling. Br. J. Pharmacol. 2018, 175, 3699–3712. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Ring, H.A.; Bullmore, E.T.; Wheelwright, S.; Ashwin, C.; Williams, S.C.R. The Amygdala Theory of Autism. Neurosci. Biobehav. Rev. 2000, 24, 355–364. [Google Scholar] [CrossRef]
- Pitkänen, A.; Savander, V.; LeDoux, J.E. Organization of Intra-Amygdaloid Circuitries in the Rat: An Emerging Framework for Understanding Functions of the Amygdala. Trends Neurosci. 1997, 20, 517–523. [Google Scholar] [CrossRef]
- Munson, J.; Dawson, G.; Abbott, R.; Faja, S.; Webb, S.J.; Friedman, S.D.; Shaw, D.; Artru, A.; Dager, S.R. Amygdalar Volume and Behavioral Development in Autism. Arch. Gen. Psychiatry 2006, 63, 686. [Google Scholar] [CrossRef] [PubMed]
- Sweeten, T.L.; Posey, D.J.; Shekhar, A.; McDougle, C.J. The Amygdala and Related Structures in the Pathophysiology of Autism. Pharmacol. Biochem. Behav. 2002, 71, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.G.; Schumann, C.M.; Nordahl, C.W. Neuroanatomy of Autism. Trends Neurosci. 2008, 31, 137–145. [Google Scholar] [CrossRef]
- Graeff, F.G.; Silveira, M.C.L.; Nogueira, R.L.; Audi, E.A.; Oliveira, R.M.W. Role of the Amygdala and Periaqueductal Gray in Anxiety and Panic. Behav. Brain Res. 1993, 58, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Gimpl, G.; Fahrenholz, F. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [PubMed]
- Dumais, K.M.; Alonso, A.G.; Bredewold, R.; Veenema, A.H. Role of the Oxytocin System in Amygdala Subregions in the Regulation of Social Interest in Male and Female Rats. Neuroscience 2016, 330, 138–149. [Google Scholar] [CrossRef]
- Bale, T.L.; Davis, A.M.; Auger, A.P.; Dorsa, D.M.; McCarthy, M.M. CNS Region-Specific Oxytocin Receptor Expression: Importance in Regulation of Anxiety and Sex Behavior. J. Neurosci. 2001, 21, 2546–2552. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Stevens, F.L.; Wiesman, O.; Feldman, R.; Hurley, R.A.; Taber, K.H. Oxytocin and Behavior: Evidence for Effects in the Brain. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 96–102. [Google Scholar] [CrossRef]
- Krettek, J.E.; Price, J.L. A Description of the Amygdaloid Complex in the Rat and Cat with Observations on Intra-Amygdaloid Axonal Connections. J. Comp. Neurol. 1978, 178, 255–279. [Google Scholar] [CrossRef]
- Mehler, W.R. Subcortical Afferent Connections of the Amygdala in the Monkey. J. Comp. Neurol. 1980, 190, 733–762. [Google Scholar] [CrossRef] [PubMed]
- Price, J.; Amaral, D. An Autoradiographic Study of the Projections of the Central Nucleus of the Monkey Amygdala. J. Neurosci. 1981, 1, 1242–1259. [Google Scholar] [CrossRef] [PubMed]
- Schulkin, J. Autism and the Amygdala: An Endocrine Hypothesis. Brain Cogn. 2007, 65, 87–99. [Google Scholar] [CrossRef]
- Gottschalk, M.G.; Domschke, K. Oxytocin and Anxiety Disorders. Curr. Top. Behav. Neurosci. 2018, 35, 467–498. [Google Scholar] [PubMed]
- Yatawara, C.J.; Einfeld, S.L.; Hickie, I.B.; Davenport, T.A.; Guastella, A.J. The Effect of Oxytocin Nasal Spray on Social Interaction Deficits Observed in Young Children with Autism: A Randomized Clinical Crossover Trial. Mol. Psychiatry 2016, 21, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Yamasue, H.; Okada, T.; Munesue, T.; Kuroda, M.; Fujioka, T.; Uno, Y.; Matsumoto, K.; Kuwabara, H.; Mori, D.; Okamoto, Y.; et al. Effect of Intranasal Oxytocin on the Core Social Symptoms of Autism Spectrum Disorder: A Randomized Clinical Trial. Mol. Psychiatry 2020, 25, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Zhang, L.; Zhao, W.; Zhu, S.; Lan, C.; Kou, J.; Zhang, Q.; Zhang, Y.; Li, Q.; Chen, Z.; et al. Infrequent Intranasal Oxytocin Followed by Positive Social Interaction Improves Symptoms in Autistic Children: A Pilot Randomized Clinical Trial. Psychother. Psychosom. 2022, 91, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. Rat Brain Stereotaxic Coordinates; Academic Press: New York, NY, USA, 1986; Volume 2. [Google Scholar]
- Jiménez, J.A.; Zylka, M.J. Controlling Litter Effects to Enhance Rigor and Reproducibility with Rodent Models of Neurodevelopmental Disorders. J. Neurodev. Disord. 2021, 13, 2. [Google Scholar] [CrossRef]
- László, K.; Kiss, O.; Vörös, D.; Mintál, K.; Ollmann, T.; Péczely, L.; Kovács, A.; Zagoracz, O.; Kertes, E.; Kállai, V.; et al. Intraamygdaloid Oxytocin Reduces Anxiety in the Valproate-Induced Autism Rat Model. Biomedicines 2022, 10, 405. [Google Scholar] [CrossRef]
- Crawley, J.N. Designing Mouse Behavioral Tasks Relevant to Autistic-like Behaviors. Ment. Retard. Dev. Disabil. Res. Rev. 2004, 10, 248–258. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, B. Memantine Ameliorates Autistic Behavior, Biochemistry & Blood Brain Barrier Impairments in Rats. Brain Res. Bull. 2016, 124, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Persico, A.M.; Ricciardello, A.; Lamberti, M.; Turriziani, L.; Cucinotta, F.; Brogna, C.; Vitiello, B.; Arango, C. The Pediatric Psychopharmacology of Autism Spectrum Disorder: A Systematic Review—Part I: The Past and the Present. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110326. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Przewłocki, R. Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Animal Model of Autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef]
- Mintál, K.; Tóth, A.; Hormay, E.; Kovács, A.; László, K.; Bufa, A.; Marosvölgyi, T.; Kocsis, B.; Varga, A.; Vizvári, Z.; et al. Novel Probiotic Treatment of Autism Spectrum Disorder Associated Social Behavioral Symptoms in Two Rodent Models. Sci. Rep. 2022, 12, 5399. [Google Scholar] [CrossRef] [PubMed]
- Zalla, T. Amygdala, Oxytocin, and Social Cognition in Autism Spectrum Disorders. Biol. Psychiatry 2014, 76, 356–357. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, F.; Folloni, D.; Møller, A.; Landau, A.M.; Scheel-Krüger, J.; Winterdahl, M. Suppressed Play Behaviour and Decreased Oxytocin Receptor Binding in the Amygdala after Prenatal Exposure to Low-Dose Valproic Acid. Behav. Pharmacol. 2017, 28, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Ago, Y.; Higuchi, M.; Hasebe, S.; Nakazawa, T.; Hashimoto, H.; Matsuda, T.; Takuma, K. Oxytocin Attenuates Deficits in Social Interaction but Not Recognition Memory in a Prenatal Valproic Acid-Induced Mouse Model of Autism. Horm. Behav. 2017, 96, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, K.; Bernaerts, S.; Prinsen, J.; Dillen, C.; Steyaert, J.; Wenderoth, N. Oxytocin Induces Long-Lasting Adaptations within Amygdala Circuitry in Autism: A Treatment-Mechanism Study with Randomized Placebo-Controlled Design. Neuropsychopharmacology 2020, 45, 1141–1149. [Google Scholar] [CrossRef]
- Bernaerts, S.; Boets, B.; Steyaert, J.; Wenderoth, N.; Alaerts, K. Oxytocin Treatment Attenuates Amygdala Activity in Autism: A Treatment-Mechanism Study with Long-Term Follow-Up. Transl. Psychiatry 2020, 10, 383. [Google Scholar] [CrossRef]
- Roullet, F.I.; Wollaston, L.; De Catanzaro, D.; Foster, J.A. Behavioral and Molecular Changes in the Mouse in Response to Prenatal Exposure to the Anti-Epileptic Drug Valproic Acid. Neuroscience 2010, 170, 514–522. [Google Scholar] [CrossRef]
- Lebowitz, E.R.; Leckman, J.F.; Feldman, R.; Zagoory-Sharon, O.; McDonald, N.; Silverman, W.K. Salivary Oxytocin in Clinically Anxious Youth: Associations with Separation Anxiety and Family Accommodation. Psychoneuroendocrinology 2016, 65, 35–43. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.A.; Hernandez, R.M.; Reichel, C.M.; Farley, C.M. The Partial D2-like Dopamine Receptor Agonist Terguride Acts as a Functional Antagonist in States of High and Low Dopaminergic Tone: Evidence from Preweanling Rats. Psychopharmacology 2005, 178, 431–439. [Google Scholar] [CrossRef]
- Carter, C. Sex Differences in Oxytocin and Vasopressin: Implications for Autism Spectrum Disorders? Behav. Brain Res. 2007, 176, 170–186. [Google Scholar] [CrossRef]
- Lee, H.-J.; Macbeth, A.H.; Pagani, J.; Young, W.S. Oxytocin: The Great Facilitator of Life. Prog. Neurobiol. 2009, 88, 127–151. [Google Scholar] [CrossRef]
- Baskerville, T.A.; Allard, J.; Wayman, C.; Douglas, A.J. Dopamine–Oxytocin Interactions in Penile Erection. Eur. J. Neurosci. 2009, 30, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- László, K.; Kovács, A.; Zagoracz, O.; Ollmann, T.; Péczely, L.; Kertes, E.; Lacy, D.G.; Lénárd, L. Positive Reinforcing Effect of Oxytocin Microinjection in the Rat Central Nucleus of Amygdala. Behav. Brain Res. 2016, 296, 279–285. [Google Scholar] [CrossRef] [PubMed]
| Covered Distance (cm) | |
|---|---|
| Control | 1717.33 ± 55.33 |
| 10 ng OT | 1821.25 ± 61.86 |
| ANT + OT | 1788.50 ± 48.45 |
| ANT | 1675.52 ± 90.12 |
| VPA | 1699.52 ± 52.66 |
| VPA + 10 ng OT | 1709.33 ± 67.71 |
| VPA + ANT + OT | 1785.15 ± 68.89 |
| VPA + ANT | 1689.58 ± 98.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vörös, D.; Kiss, O.; Ollmann, T.; Mintál, K.; Péczely, L.; Zagoracz, O.; Kertes, E.; Kállai, V.; László, B.R.; Berta, B.; et al. Intraamygdaloid Oxytocin Increases Time Spent on Social Interaction in Valproate-Induced Autism Animal Model. Biomedicines 2023, 11, 1802. https://doi.org/10.3390/biomedicines11071802
Vörös D, Kiss O, Ollmann T, Mintál K, Péczely L, Zagoracz O, Kertes E, Kállai V, László BR, Berta B, et al. Intraamygdaloid Oxytocin Increases Time Spent on Social Interaction in Valproate-Induced Autism Animal Model. Biomedicines. 2023; 11(7):1802. https://doi.org/10.3390/biomedicines11071802
Chicago/Turabian StyleVörös, Dávid, Orsolya Kiss, Tamás Ollmann, Kitti Mintál, László Péczely, Olga Zagoracz, Erika Kertes, Veronika Kállai, Bettina Réka László, Beáta Berta, and et al. 2023. "Intraamygdaloid Oxytocin Increases Time Spent on Social Interaction in Valproate-Induced Autism Animal Model" Biomedicines 11, no. 7: 1802. https://doi.org/10.3390/biomedicines11071802
APA StyleVörös, D., Kiss, O., Ollmann, T., Mintál, K., Péczely, L., Zagoracz, O., Kertes, E., Kállai, V., László, B. R., Berta, B., Toth, A., Lénárd, L., & László, K. (2023). Intraamygdaloid Oxytocin Increases Time Spent on Social Interaction in Valproate-Induced Autism Animal Model. Biomedicines, 11(7), 1802. https://doi.org/10.3390/biomedicines11071802






