Evaluating the Adipose Tissue Depth as a Predictor Factor for Gestational Diabetes in Later Pregnancy—A Systematic Review
Abstract
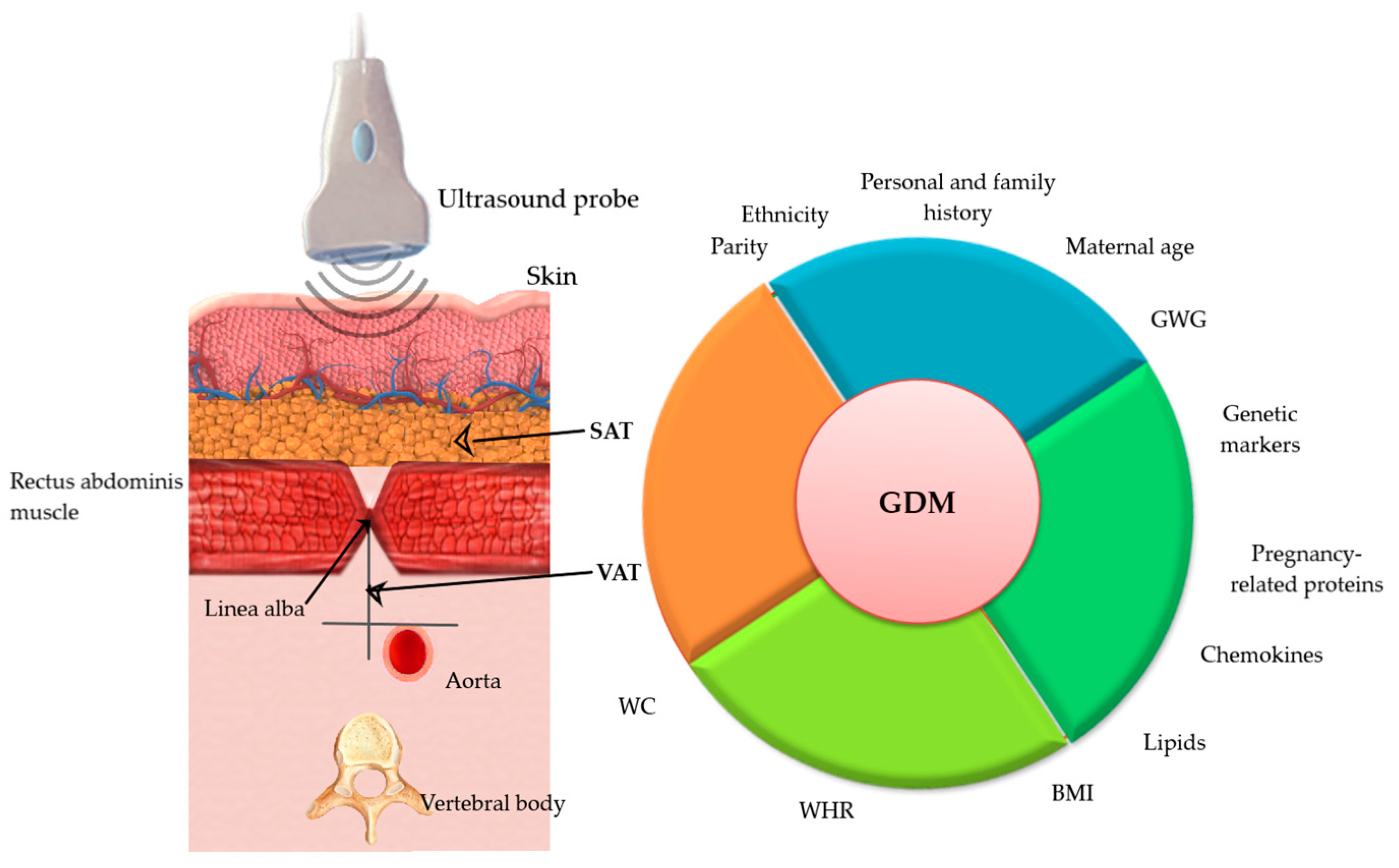
1. Introduction
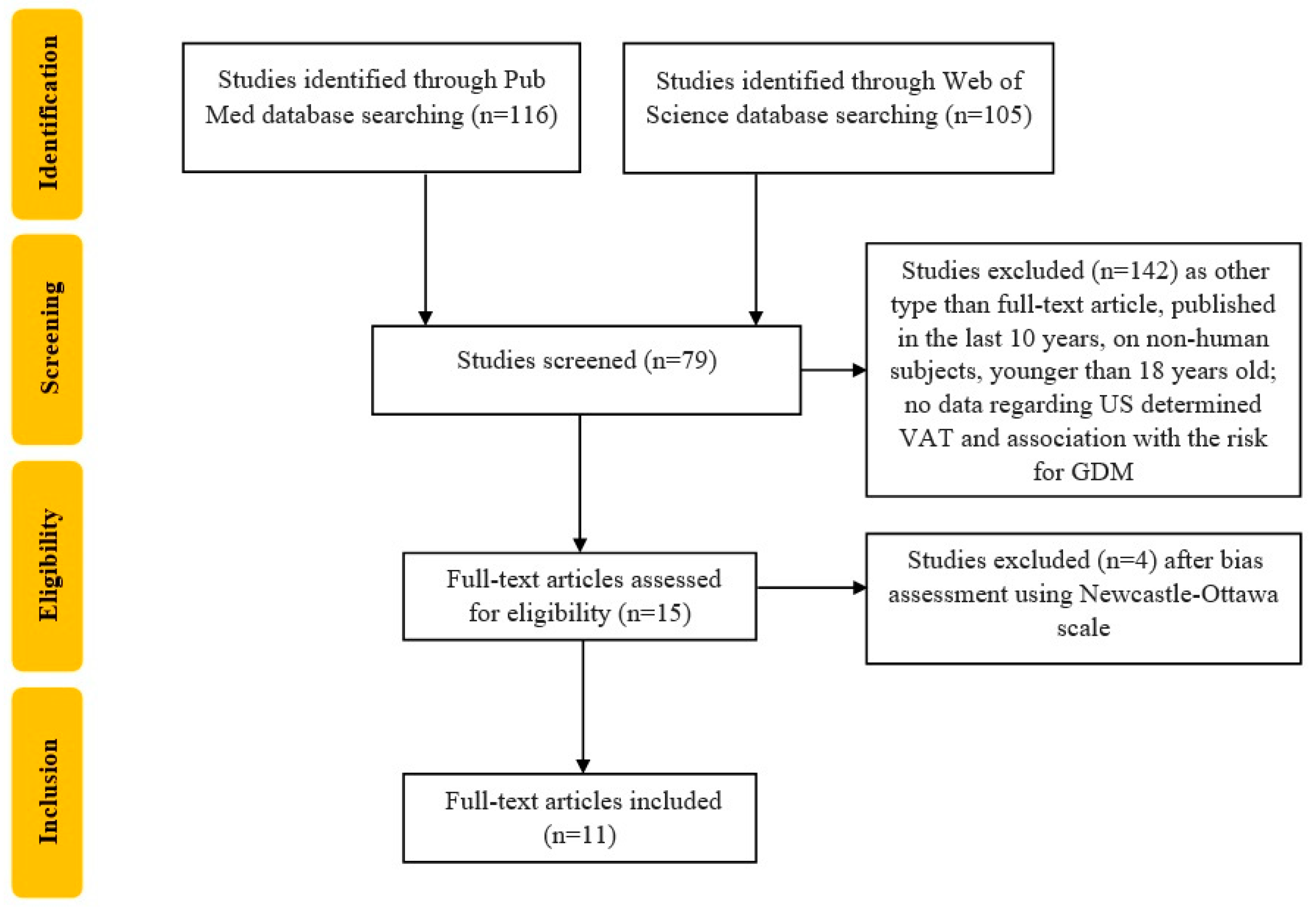
2. Materials and Methods
3. Results
4. Discussion
4.1. Used Techniques
4.2. Different Thresholds for Predictive Value
4.3. Is Pre-Pregnancy BMI Better Than VAT in the Detection of GDM?
4.4. Adipose Tissue and Metabolic Syndrome (MetS) Features
4.5. An Exhaustive Formula That Estimates the GDM Risks
4.6. Possible Confounders
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. Gestational Diabetes. Available online: https://www.idf.org/our-activities/care-prevention/gdm#:~:text=There%20were%20an%20estimated%20223,were%20due%20to%20gestational%20diabetes (accessed on 10 November 2022).
- Gregory, E.C.; Ely, D.M. Trends and Characteristics in Gestational Diabetes: United States, 2016–2020. Natl. Vital Stat. Rep. 2022, 71, 1–15. [Google Scholar] [PubMed]
- Bilous, R.W.; Jacklin, P.B.; Maresh, M.J.; Sacks, D.A. Resolving the Gestational Diabetes Diagnosis Conundrum: The Need for a Randomized Controlled Trial of Treatment. Diabetes Care 2021, 44, 858–864. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Ambrosius Hoegfeldt, C.; Powe, C.E.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef]
- Waugh, N.; Pearson, D.; Royle, P. Screening for hyperglycaemia in pregnancy: Consensus and controversy. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 553–571. [Google Scholar] [CrossRef]
- Alves, J.G.; Souza, A.; Figueiroa, J.N.; de Araújo, C.; Guimarães, A.; Ray, J.G. Visceral Adipose Tissue Depth in Early Pregnancy and Gestational Diabetes Mellitus—A Cohort Study. Sci. Rep. 2020, 10, 2032. [Google Scholar] [CrossRef]
- Lovati, E.; Beneventi, F.; Simonetta, M.; Laneri, M.; Quarleri, L.; Scudeller, L.; Albonico, G.; Locatelli, E.; Cavagnoli, C.; Tinelli, C.; et al. Gestational diabetes mellitus: Including serum pregnancy-associated plasma protein-A testing in the clinical management of primiparous women? A case-control study. Diabetes Res. Clin. Pract. 2013, 100, 340–347. [Google Scholar] [CrossRef]
- Sandu, C.; Bica, C.; Salmen, T.; Stoica, R.; Bohiltea, R.; Gherghiceanu, F.; Pacu, I.; Stefan, S.; Serafinceanu, C.; Stoian, A.P. Gestational diabetes—Modern management and therapeutic approach (Review). Exp. Ther. Med. 2021, 21, 81. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Nagendra, L.; Krishnamurthy, A.; Lakhani, O.J.; Kapoor, N.; Kalra, B.; Kalra, S. Early Gestational Diabetes Mellitus: Diagnostic Strategies and Clinical Implications. Med. Sci. 2021, 9, 59. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Williams, M.A.; Holt, V.L.; Weiss, N.S.; Ferrara, A. Body mass index and weight gain prior to pregnancy and risk of gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2008, 198, 409.e1–409.e4097. [Google Scholar] [CrossRef]
- Kim, C.; Liu, T.; Valdez, R.; Beckles, G.L. Does frank diabetes in first-degree relatives of a pregnant woman affect the likelihood of her developing gestational diabetes mellitus or nongestational diabetes? Am. J. Obstet. Gynecol. 2009, 201, 576.e1–576.e6. [Google Scholar] [CrossRef]
- Zhang, C.; Ning, Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: Review of epidemiologic evidence. Am. J. Clin. Nutr. 2011, 94, 1975S–1979S. [Google Scholar] [CrossRef]
- Gibson, K.S.; Waters, T.P.; Catalano, P.M. Maternal weight gain in women who develop gestational diabetes mellitus. Obstet. Gynecol. 2012, 119, 560–565. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Gunderson, E.P.; Ferrara, A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet. Gynecol. 2010, 115, 597–604. [Google Scholar] [CrossRef]
- Getahun, D.; Fassett, M.J.; Jacobsen, S.J. Gestational diabetes: Risk of recurrence in subsequent pregnancies. Am. J. Obstet. Gynecol. 2010, 203, 467.e1–467.e6. [Google Scholar] [CrossRef]
- Zilberlicht, A.; Feferkorn, I.; Younes, G.; Damti, A.; Auslender, R.; Riskin-Mashiah, S. The mutual effect of pregestational body mass index, maternal hyperglycemia and gestational weight gain on adverse pregnancy outcomes. Gynecol. Endocrinol. 2016, 32, 416–420. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar]
- Bobirca, A.; Bobirca, F.; Ancuta, I.C.; Mihai, C.; Tataru, C.; Comsa, M.; Bojinca, M.; Micu, A.; Musetescu, C.; Ancuta, V.; et al. Pregnancy in rheumatoid arthritis—A Romanian cohort. Ann. Rheum. Dis. 2017, 76, 1151–1152. [Google Scholar]
- Bobirca, A.; Ancuta, I.; Bojincă, M.; Stoica, V.; Ceaușu, I.; Toader, O.; Micu, M.; Ancuta, C.; Mușetescu, A.; Bobirca, F. Risk factors of adverse pregnancy outcome in Romanian rheumatoid arthritis patients. In Proceedings of the 4th Congress of the Romanian Society for Minimal Invasive Surgery in Ginecology/Annual Days of the National Institute for Mother and Child Health Alessandrescu-Rusescu, Bucharest, Romania, 1–3 November 2019; pp. 148–151. [Google Scholar]
- Torloni, M.R.; Betrán, A.P.; Horta, B.L.; Nakamura, M.U.; Atallah, A.N.; Moron, A.F.; Valente, O. Pre-pregnancy BMI and the risk of gestational diabetes: A systematic review of the literature with meta-analysis. Obes. Rev. 2009, 10, 194–203. [Google Scholar] [CrossRef]
- Madhavan, A.; Beena Kumari, R.; Sanal, M.G. A pilot study on the usefulness of body mass index and waist hip ratio as a predictive tool for gestational diabetes in Asian Indians. Gynecol. Endocrinol. 2008, 24, 701–707. [Google Scholar] [CrossRef]
- Basraon, S.K.; Mele, L.; Myatt, L.; Roberts, J.M.; Hauth, J.C.; Leveno, K.J.; Varner, M.W.; Wapner, R.J.; Thorp, J.M., Jr.; Peaceman, A.M.; et al. Relationship of Early Pregnancy Waist-to-Hip Ratio versus Body Mass Index with Gestational Diabetes Mellitus and Insulin Resistance. Am. J. Perinatol. 2016, 33, 114–121. [Google Scholar]
- Sina, M.; Hoy, W.E.; Callaway, L.; Wang, Z. The associations of anthropometric measurements with subsequent gestational diabetes in Aboriginal women. Obes. Res. Clin. Pract. 2015, 9, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Bernardi, J.R.; Matos, S.; Kretzer, D.C.; Schöffel, A.C.; Goldani, M.Z.; de Azevedo Magalhães, J.A. Maternal visceral adipose tissue during the first half of pregnancy predicts gestational diabetes at the time of delivery—A cohort study. PLoS ONE. 2020, 15, e0232155. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.; Liu, A.; Poulton, A.; Quinton, A.; Amer, Z.; Mongelli, M.; Martin, A.; Benzie, R.; Peek, M.; Nanan, R. Comparison of maternal abdominal subcutaneous fat thickness and body mass index as markers for pregnancy outcomes: A stratified cohort study. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Pontual, A.C.; Figueiroa, J.N.; De Souza, L.R.; Ray, J.G.; Alves, J.G. Visceral Adiposity in the First Half of Pregnancy in Association with Glucose, Lipid and Insulin Profiles in Later Pregnancy: A Cohort Study. Matern. Child Health J. 2016, 20, 1720–1725. [Google Scholar] [CrossRef]
- Thaware, P.K.; Patterson, C.C.; Young, I.S.; Casey, C.; McCance, D.R. Clinical utility of ultrasonography-measured visceral adipose tissue depth as a tool in early pregnancy screening for gestational diabetes: A proof-of-concept study. Diabet. Med. 2019, 36, 898–901. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosi, F.; Rossi, G.; Soldavini, C.M.; Di Maso, M.; Carbone, I.F.; Cetera, G.E.; Colosi, E.; Ferrazzi, E. Ultrasound assessment of maternal adipose tissue during 1st trimester screening for aneuploidies and risk of developing gestational diabetes. Acta Obstet. Gynecol. Scand. 2020, 99, 644–650. [Google Scholar] [CrossRef]
- Bourdages, M.; Demers, M.É.; Dubé, S.; Gasse, C.; Girard, M.; Boutin, A.; Ray, J.G.; Bujold, E.; Demers, S. First-Trimester Abdominal Adipose Tissue Thickness to Predict Gestational Diabetes. J. Obstet. Gynaecol. Can. 2018, 40, 883–887. [Google Scholar] [CrossRef]
- Aydın, G.A.; Özsoy, H.G.T.; Akdur, P.Ö.; Özgen, G. The predictive value of first-trimester anthropo-metric and ultrasonographic adipose tissue measurements in gestational diabetes mellitus. J. Obstet. Gynaecol. Res. 2021, 47, 3071–3077. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (N.O.S.) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; The Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011; Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 30 December 2022).
- De Souza, L.R.; Berger, H.; Retnakaran, R.; Maguire, J.L.; Nathens, A.B.; Connelly, P.W.; Ray, J.G. First-Trimester Maternal Abdominal Adiposity Predicts Dysglycemia and Gestational Diabetes Mellitus in Midpregnancy. Diabetes Care 2016, 39, 61–64. [Google Scholar] [CrossRef]
- Gur, E.B.; Ince, O.; Turan, G.A.; Karadeniz, M.; Tatar, S.; Celik, E.; Yalcin, M.; Guclu, S. Ultrasonographic visceral fat thickness in the first trimester can predict metabolic syndrome and gestational diabetes mellitus. Endocrine 2014, 47, 478–484. [Google Scholar] [CrossRef]
- De Souza, L.R.; Berger, H.; Retnakaran, R.; Vlachou, P.A.; Maguire, J.L.; Nathens, A.B.; Connelly, P.W.; Ray, J.G. Hepatic fat and abdominal adiposity in early pregnancy together predict impaired glucose homeostasis in mid-pregnancy. Nutr. Diabetes 2016, 6, e229. [Google Scholar] [CrossRef]
- SaifElnasr, I.; Ammar, H. Ultrasound markers for prediction of gestational diabetes mellitus in early pregnancy in Egyptian women: Observational study. J. Matern.-Fetal Neonatal Med. 2021, 34, 3120–3126. [Google Scholar] [CrossRef]
- Tunc, S.; Oglak, S.C.; Olmez, F.; Ozkose, Z.G. The Value of First-trimester Maternal Abdominal Visceral Adipose Tissue Thickness in Predicting the Subsequent Development of Gestational Diabetes Mellitus. J. Coll. Physicians Surg. Pak. 2022, 32, 722–727. [Google Scholar]
- Gupta, S.; Gupta, A.; Swarnakar, C.P.; Rathore, M.; Beniwal, R.; Meena, K.; Simlot, A.; Gupta, N. The Early Sonographic Prediction of Gestational Diabetes in Women from India. J. Diagn. Med. Sonogr. 2022, 38, 18–24. [Google Scholar] [CrossRef]
- D’Ambrosi, F.; Crovetto, F.; Colosi, E.; Fabietti, I.; Carbone, F.; Tassis, B.; Motta, S.; Bulfoni, A.; Fedele, L.; Rossi, G.; et al. Maternal Subcutaneous and Visceral Adipose Ultrasound Thickness in Women with Gestational Diabetes Mellitus at 24–28 Weeks’ Gestation. Fetal Diagn. Ther. 2018, 43, 143–147. [Google Scholar] [CrossRef]
- Martin, A.M.; Berger, H.; Nisenbaum, R.; Lausman, A.Y.; MacGarvie, S.; Crerar, C.; Ray, J.G. Abdominal visceral adiposity in the first trimester predicts glucose intolerance in later pregnancy. Diabetes Care 2009, 32, 1308–1310. [Google Scholar] [CrossRef]
- Rahnemaei, F.A.; Abdi, F.; Pakzad, R.; Sharami, S.H.; Mokhtari, F.; Kazemian, E. Association of body composition in early pregnancy with gestational diabetes mellitus: A meta-analysis. PLoS ONE 2022, 17, e0271068. [Google Scholar] [CrossRef]
- Alwash, S.M.; McIntyre, H.D.; Mamun, A. The association of general obesity, central obesity and visceral body fat with the risk of gestational diabetes mellitus: Evidence from a systematic review and meta-analysis. Obes. Res. Clin. Pract. 2021, 15, 425–430. [Google Scholar] [CrossRef]
- Armellini, F.; Zamboni, M.; Rigo, L.; Todesco, T.; Bergamo-Andreis, I.A.; Procacci, C.; Bosello, O. The contribution of sonography to the measurement of intra-abdominal fat. J. Clin. Ultrasound 1990, 18, 563–567. [Google Scholar] [CrossRef]
- Suzuki, R.; Watanabe, S.; Hirai, Y.; Akiyama, K.; Nishide, T.; Matsushima, Y.; Murayama, H.; Ohshima, H.; Shinomiya, M.; Shirai, K. Abdominal wall fat index, estimated by ultrasonography, for assessment of the ratio of visceral fat to subcutaneous fat in the abdomen. Am. J. Med. 1993, 95, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Cremona, A.; Hayes, K.; O’Gorman, C.S.; Laighin, C.N.; Ismail, K.I.; Donnelly, A.E.; Hamilton, J.; Cotter, A. Inter and intra-reliability of ultrasonography for the measurement of abdominal subcutaneous & visceral adipose tissue thickness at 12 weeks gestation. BMC Med. Imaging 2019, 19, 95. [Google Scholar]
- Habibi, N.; Mousa, A.; Tay, C.T.; Khomami, M.B.; Patten, R.K.; Andraweera, P.H.; Wassie, M.; Vandersluys, J.; Aflatounian, A.; Bianco-Miotto, T.; et al. Maternal metabolic factors and the association with gestational diabetes: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2022, 38, e3532. [Google Scholar] [CrossRef] [PubMed]
- Bartha, J.L.; Marin-Segura, P.; Gonzalez- Gonzalez, N.L.; Wagner, F.; Aguilar-Diosdado, M.; Hervi-as-Vivancos, B. Ultrasound evaluation of visceral fat and metabolic risk factors during early pregnancy. Obesity (Silver Spring) 2007, 15, 2233–2239. [Google Scholar] [CrossRef]
- De Souza, L.R.; Kogan, E.; Berger, H.; Alves, J.G.; Lebovic, G.; Retnakaran, R.; Maguire, J.L.; Ray, J.G. Abdominal adiposity and insulin resistance in early pregnancy. J. Obstet. Gynaecol. Can. 2014, 36, 969–975. [Google Scholar] [CrossRef]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef]
- Lewandowski, K.; Głuchowska, M.; Garnysz, K.; Horzelski, W.; Grzesiak, M.; Lewiński, A. High prevalence of early (1st trimester) gestational diabetes mellitus in Polish women is accompanied by marked insulin resistance—Comparison to PCOS model. Endokrynol. Pol. 2022, 73, 1–7. [Google Scholar]
- Swainson, M.G.; Batterham, A.M.; Tsakirides, C.; Rutherford, Z.H.; Hind, K. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS ONE 2017, 12, e0177175. [Google Scholar] [CrossRef]
- Peña-Cano, M.I.; Valencia-Ortega, J.; Morales-Ávila, E.; Díaz-Velázquez, M.F.; Gómez-Díaz, R.; Saucedo, R. Omentin-1 and its relationship with inflammatory factors in maternal plasma and visceral adipose tissue of women with gestational diabetes mellitus. J. Endocrinol. Investig. 2022, 45, 453–462. [Google Scholar] [CrossRef]
- Rancourt, R.C.; Ott, R.; Ziska, T.; Schellong, K.; Melchior, K.; Henrich, W.; Plagemann, A. Visceral Adipose Tissue Inflammatory Factors (TNF-Alpha, SOCS3) in Gestational Diabetes (GDM): Epigenetics as a Clue in GDM Pathophysiology. Int. J. Mol. Sci. 2020, 21, 479. [Google Scholar] [CrossRef]
- Kelley, D.E.; Thaete, F.L.; Troost, F.; Huwe, T.; Goodpaster, B.H. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E941–E948. [Google Scholar] [CrossRef]
- Yang, S.H.; Kim, C.; An, H.S.; An, H.; Lee, J.S. Prediction of gestational diabetes mellitus in pregnant Korean women based on abdominal subcutaneous fat thickness as measured by ultrasonography. Diabetes Metab. J. 2017, 41, 486–491. [Google Scholar] [CrossRef]
- Kennedy, N.J.; Peek, M.J.; Quinton, A.E.; Lanzarone, V.; Martin, A.; Benzie, R.; Nanan, R. Maternal abdominal subcutaneous fat thickness as a predictor for adverse pregnancy outcome: A longitudinal cohort study. BJOG 2016, 123, 225–232. [Google Scholar] [CrossRef]
- Cooray, S.D.; Wijeyaratne, L.A.; Soldatos, G.; Allotey, J.; Boyle, J.A.; Teede, H.J. The Unrealized Potential for Predicting Pregnancy Complications in Women with Gestational Diabetes: A Systematic Review and Critical Appraisal. Int. J. Environ. Res. Public Health 2020, 17, 3048. [Google Scholar] [CrossRef]
- Kretzer, D.C.; Matos, S.; Von Diemen, L.; de Azevedo Magalhães, J.A.; Schöffel, A.C.; Goldani, M.Z.; da Silva Rocha, A.; Bernardi, J.R. Anthropometrical measurements and maternal visceral fat during first half of pregnancy: A cross-sectional survey. BMC Pregnancy Childbirth 2020, 20, 576. [Google Scholar] [CrossRef]
- Liu, J.; Song, G.; Meng, T.; Zhao, G. Epicardial adipose tissue thickness as a potential predictor of gestational diabetes mellitus: A prospective cohort study. BMC Cardiovasc. Disord. 2020, 20, 184. [Google Scholar] [CrossRef]
- Zhao, B.; Han, X.; Meng, Q.; Luo, Q. Early second trimester maternal serum markers in the prediction of gestational diabetes mellitus. J. Diabetes Investig. 2018, 9, 967–974. [Google Scholar] [CrossRef]
- Lorenzo-Almorós, A.; Hang, T.; Peiró, C.; Soriano-Guillén, L.; Egido, J.; Tuñón, J.; Lorenzo, Ó. Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases. Cardiovasc. Diabetol. 2019, 18, 140. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Q.; Chen, Y.; Sui, L.; Jiang, L.; Shen, Q.; Li, M.; Li, G.; Wang, Q. The possible role of visceral fat in early pregnancy as a predictor of gestational diabetes mellitus by regulating adipose-derived exosomes miRNA-148 family: Protocol for a nested case-control study in a cohort study. BMC Pregnancy Childbirth 2021, 21, 262. [Google Scholar]
- Nanda, S.; Savvidou, M.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat. Diagn. 2011, 31, 135–141. [Google Scholar] [CrossRef]
- Di Filippo, D.; Wanniarachchi, T.; Wei, D.; Yang, J.J.; Mc Sweeney, A.; Havard, A.; Henry, A.; Welsh, A. The diagnostic indicators of gestational diabetes mellitus from second trimester to birth: A systematic review. Clin. Diabetes Endocrinol. 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Study Design | Country | No of Patients | Diagnostic Criteria | GW of Assessment | US Techinique | GDM Incidence | Results | Adjusting Factors |
|---|---|---|---|---|---|---|---|---|---|
| Alves et al., 2020 [7] | prospective cohort study | Brazil | 518 | IADPSG | <20 GW Mean 14.4 GW | Armellini et al., slightly modified by Martin et al. | 87 (16.8%) | increased risk of GDM in relation to VAT in early pregnancy, similar after adjusting for BMI. OR 2.00, 95% CI 1.61 to 2.50 | Age and pregestational BMI |
| D’Ambrosi et al., 2020 [29] | single-center study | Italy | 295 | IADPSG | 112/7–136/7 GW | Suzuki et al. | 57 (19.32%) 1st trimester (n = 29) 2nd trimester (n = 28) | Signifficant association for VAT and for 1st and 2nd trimester GDM | maternal age, BMI at 12 GW, GWG at 12 GW, parity and family history of DM |
| Bourdages et al., 2018 [30] | planned sub-cohort study of a large prospective cohort study | Canada | 1048 | ACOG | 110/7 to 140/7 weeks | Armellini et al. Martin et al. | 61 (5.8%) developed GDM, of which 36 (3.4%) insulin-requiring GDM. | VAT associated with subsequent GDM (AUC 0.65, 95% CI 0.58–0.73) | maternal age and BMI |
| Thaware et al., 2019 [28] | prospective observational study | United Kingdom | 80 | IADPSG/WHO 2013 criteria | 9–18 GW | Armellini et al., slightly modified by Martin et al. | 15 (19%) | VAT was associated with greater GDM risk 2.09 (95% CI 1.06–4.12; p = 0.03) for 1-SD increase | age, parity, years in education and pre-pregnancy BMI |
| Rocha et al., 2020 [25] | prospective cohort study | Brazil | 133 | IADPSG | ≤20 GW | Armel-lini et al. | 8 (13.5%) | Strong association between VAT at a 45 mm treshold andGDM. Crude and aOR for GDM were 13.4 (95% CI 2.9–61.1) and 8.9 (95% CI 1.9–42.2) | maternal age and pre-gravid BMI |
| De Souza et al., 2016 [33] | prospective cohort study | Canada | 485 | IADPSG | 11–14 GW | Martin et al. | 45 (9.27%) (9.3%, 95% CI 7.0–12.2) | The highest quartile of VAT (aOR 3.1, 95% CI 1.1–9.5) was associated with the composite outcome of GDM, IFG or IGT 3.4, 95% CI; 1.5–8.3 for IFG | maternal age, ethnicity, family history of type 2 DM, BMI at 11–14 GW and change in BMI from 11–14 to 24–28 GW |
| Gur et al., 2014 [34] | prospective cohort study | Turkey | 94 | FBG > 105 mg/dL 1 h glucose >190 mg/dL 2 h glucose > 165 mg/dL 3 h glucose > 145 mg/dL
| 4–14 GW | Martin et al. | IGT 6 (6.3%) GDM 10 (10.2%) MS 9 (9.5%) | VAT was significantly higher in the GDM group (p = 0.04) VAT in early pregnancy correlated with hyperglycemia, dyslipidemia, high diastolic BP, and IR. VAT was a more sensitive predictor of GDM than WC and BMI. | diastolic BP, TG, FBG, insulin level, HOMA-IR, HDL, BMI, WC, age, DM family history and GDM history |
| De Souza et al., 2016 [35] | prospective cohort study | Canada | 476 | CDA | 11–14 GW | De Souza et al., 2014 | 50 (10.5%) developed the composite of IFG, IGT or GDM | Maternal hepatic fat and abdominal adiposity (VAT, TAT) may independently predict disglycemia and GDM in mid-pregnancy. | maternal age, ethnicity, 1st degree relative with type 2 DM, BMI at 11–14 GW and change in BMI from 11–14 to 24–28 GW |
| Saif Elnasr et al., 2021 [36] | observational study | Egypt | 83 | ADA | 11–14 GW | Muller et al. | 12 (14.45%) GDM |
| HOMA-IR, ISI and BMI |
| Tunc et al., 2022 [37] | observationalstudy | Turkey | 100 | IADPSG | 11–14 GW | Martin et al. | 12 (12%) GDM | The most significant risk factor for the prediction of GDM was VAT (OR = 33.2, 95% CI = 7.395–149.046, p < 0.001). Other signifficant predictors were SAT, TAT, a pre-gestational BMI > 30 kg/m2 | maternal age, parity, GW at recruitment FPG, plasma insulin, HOMA-IR, HDL, LDL, VLDL, TG, systolic BP diastolic BP, BMI, Pre-gestational-BMI and body weight and GWG. |
| Gupta et al., 2022 [38] | cohort study | India | 190 | IADPSG | 11–14 GW | Muller et al. | 98 (51.57%) | There was a significant association between SAT, VAT and BMI and occurrence of GDM, p < 0.001 | age, gestational age, thyroid stimulating hormone, SAT and TAT. |
| Author and Year | Maternal Mean Age (Years + SD) | VAT Depth (mm + SD) | SAT Depth (Value/NR) | Other US Parameters (Value/NR) | Anthropometric Indices (Mean + SD) | Detailed Results | Prediction Power/Special Considerations |
|---|---|---|---|---|---|---|---|
| Alves et al., 2020 [7] | GDM group 27.5 ± 5.8 Non-GDM group 26.0 ± 5.7 | 54.4 ± 12.7 GDM group 63 ± 13 Non-GDM group 52 ± 11 | NR | NR | GDM group
| VAT and FPG (r = 0.179, 95% CI 0.094–0.261; p < 0.001) VAT and OGTT 1 h glucose (r = 0.238, 95% CI 0.154–0.319; p < 0.001) VAT and OGTT 2 h glucose (r = 0.221, 95% CI 0.136 to 0.303; p < 0.001) VAT-GDM—OR 2.00, 95% CI 1.61–2.50, p = 0.001 VAT (0.70 95% CI 0.63–0.75) vs. pre-pregnancy BMI (0.57 95% CI 0.50–0.64) (p < 0.0001) | VAT was more predictive for GDM than pre-pregnancy BMI. Optimal VAT cut-off for maximized Youden’s index was 5.1 cm, and a 1 cm increase in VAT led to unadjusted OR for developing GDM of 1.99 (95% CI 1.59–2.46) |
| D’Ambrosi et al., 2020 [29] | GDM group (1st trimester) 33.4 ± 4.3 GDM group (2nd trimester) 33.3 ± 4.1 Non-GDM group 33.0 ± 4.3 | GDM group (1st trimester) 99 ± 44 GDM group (2nd trimester) 105 ± 53 Non-GDM group 72 ± 35 | GDM group (1st trimester) 128 ± 65 GDM group (2nd trimester) 111 ± 46 Non-GDM group 98 ± 49 | NR | GDM group (1st trimester)
| VAT p = 0.01 (Multivariate analysis) BMI p < 0.01 (Univariate analysis) 1st trimester GDM OR = 1.15, 95% CI 1.02–1.29 and 2nd trimester GDM OR = 1.19, 95% CI 1.05–1.34 | In the multivariate analysis, only VAT was significantly associated with the risk of GDM. No further association was observed in the multivariate analysis |
| Bourdages et al., 2018 [30] | Insulin-requiring GDM 30.4 ± 4.6 GDM—no insulin 29.3 ± 3.2 No GDM 28.9 ± 4.1 | NR | NR | NR | Insulin-requiring GDM
| GDM
| In logistic regression, at a false-positive rate of 10%, the detection rates for insulin-requiring GDM were 19% (95% CI 8–36) for maternal age ≥35 years, 31% (95% CI 16–48) for a BMI ≥31.6 kg/m2 and 31% (95% CI 16–48) for TAT ≥61 mm, up to 42% (95% CI 26–59) in a model including all 3 measures. |
| Thaware et al., 2019 [28] | N | 43.6 ± 13.1 | 22.4 ± 10.1 | NR | NR | VAT (OR 2.09, 95% CI 1.06–4.12; p = 0.03) SAT (OR 0.62; 95% CI 0.27–1.44; p = 0.27) | Increasing VAT, but not SAT, was associated with greater GDM risk after adjusting for confounding factors. VAT ≥ 42.7 mm had greater Sen and similar Spe compared with current NICE criteria for GDM. |
| Rocha et al., 2020 [25] | 26 ± 6.2 | Pre-pregnancy BMI < 25.0 kg/m2 37.0 ± 12.5 Pre-pregnancy BMI 25.0–30.0 kg/m2 44.0 ± 11.2 Pre-pregnancy BMI > 30 kg/m2 53.1 ± 14.8 | NR | NR | NR | VAT 45 mm threshold aOR = 8.9 (1.9–42.2) for pre-gravid Obese and threshold of 45 mm; aOR = 6.1 (0.7–55.3) for Pre-gravid Non-obese and threshold of 45 mm | Significantly different VAT means between GDM (VAT = 55.4 ± 11.4 mm) and non-GDM (VAT = 42.5 ± 11.4 mm). |
| De Souza et al., 2016 [33] | 32.9 ± 4.8 | 41 ± 17 | 19 ± 8 | TAT 59 ± 21 | BMI at 11–14 GW 25.1 ± 5.1 kg/m2 | SAT (highest quartile)
VAT- highest quartile
TAT- highest quartile
| The highest quartile of VAT and TAT were each associated with the composite outcome (GDM, IFG, IGT). |
| Gur et al., 2014 [34] | Normal glucose metabolism 47.5 IGT 53.9 GDM 43.4 | Normal glucose metabolism VAT max = 44.9 IGT VAT max = 48.1 GDM VAT max = 67.2 | Normal glucose metabolismSAT min = 44.9 IGTSAT min = 48.7 GDM SAT min = 66.7 | NR | Normal glucose metabolism
| VAT max p = 0.04 SAT min p = 0.06 | optimal cutoff points predicting disglycemia were VAT max = 19.5 mm (AUC = 0.66, p = 0.043), WC = 103.5 cm (AUC = 0.64, p = 0.079), and BMI = 34.5 (AUC = 0.64, p = 0.069). |
| De Souza et al., 2016 [35] | 32.9 ± 4.8 | NR | NR | hepatic fat NR TAT NR |
|
| Association was independently of maternal age, ethnicity, family history of type 2 DM or maternal BMI. |
| Saif Elnasr et al., 2021 [36] | 26.8 | GDM group 58.5 ± 4.7 Non-GDM group 23 ± 6 | GDM group 18 ± 5.7 Non-GDM group 16.6 ± 5.9 | NA | GDM group
| GDM vs non-GDM:
| No significant relationship between SAT and HOMA-IR. |
| Tunc et al., 2022 [37] | GDM group 29.5 ± 6.29 Non-GDM group 27.31 ± 5.38 | GDM group 24.75 ± 10.34 Non-GDM group 16.68 ± 6.73 | GDM group 26.33 ± 5.33 Non-GDM group 17.68 ± 4.86 | NR | GDM group
| GDM prediction:
| The mean VAT and TAT were significantly higher in the GDM group p < 0.001 |
| Gupta et al., 2022 [38] | 23.24 ± 2 | NR | NR | TAT | BMI kg/m2 20.67 | Association with GDM
| A logistic regression utilizing age, gestational age, TSH, VAT, SAT, TAT as predictors was statiscally significant (chi square = 56.311, df = 8, p = 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmen, B.-M.; Pietrosel, V.-A.; Durdu, C.-E.; Salmen, T.; Diaconu, C.T.; Bica, I.-C.; Potcovaru, C.G.; Gherghiceanu, F.; Stoica, R.-A.; Pantea Stoian, A. Evaluating the Adipose Tissue Depth as a Predictor Factor for Gestational Diabetes in Later Pregnancy—A Systematic Review. Biomedicines 2023, 11, 1492. https://doi.org/10.3390/biomedicines11051492
Salmen B-M, Pietrosel V-A, Durdu C-E, Salmen T, Diaconu CT, Bica I-C, Potcovaru CG, Gherghiceanu F, Stoica R-A, Pantea Stoian A. Evaluating the Adipose Tissue Depth as a Predictor Factor for Gestational Diabetes in Later Pregnancy—A Systematic Review. Biomedicines. 2023; 11(5):1492. https://doi.org/10.3390/biomedicines11051492
Chicago/Turabian StyleSalmen, Bianca-Margareta, Valeria-Anca Pietrosel, Cristiana-Elena Durdu, Teodor Salmen, Cosmina Theodora Diaconu, Ioana-Cristina Bica, Claudia Gabriela Potcovaru, Florentina Gherghiceanu, Roxana-Adriana Stoica, and Anca Pantea Stoian. 2023. "Evaluating the Adipose Tissue Depth as a Predictor Factor for Gestational Diabetes in Later Pregnancy—A Systematic Review" Biomedicines 11, no. 5: 1492. https://doi.org/10.3390/biomedicines11051492
APA StyleSalmen, B.-M., Pietrosel, V.-A., Durdu, C.-E., Salmen, T., Diaconu, C. T., Bica, I.-C., Potcovaru, C. G., Gherghiceanu, F., Stoica, R.-A., & Pantea Stoian, A. (2023). Evaluating the Adipose Tissue Depth as a Predictor Factor for Gestational Diabetes in Later Pregnancy—A Systematic Review. Biomedicines, 11(5), 1492. https://doi.org/10.3390/biomedicines11051492







