Biomarkers of Neurodegeneration in Post-Traumatic Stress Disorder: An Integrative Review
Abstract
1. Introduction
- (a)
- to summarize the existing research on biological markers associated with neurodegenerative disorders in individuals suffering from PTSD;
- (b)
- to critically examine the contributions of possible confounding factors;
- (c)
- to synthesize this information in a manner that would be useful to future researchers in this field.
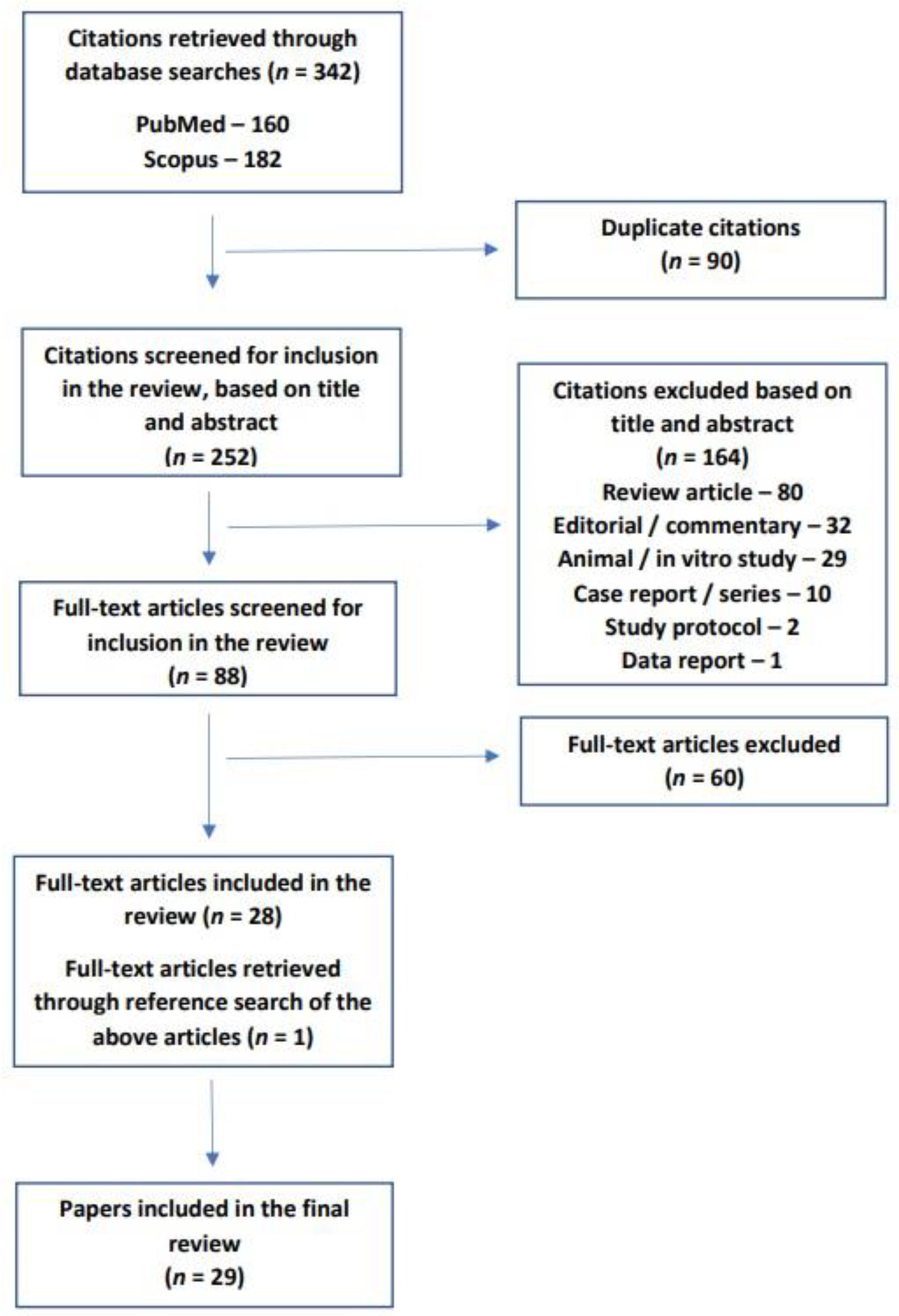
2. Study Selection and Search Strategy
- The study population should consist of patients with a diagnosis of PTSD, established using standard diagnostic criteria or rating scales, with or without a control or comparator group;
- Study participants should not currently fulfill the criteria for dementia or other neurodegenerative disorders;
- The study should measure one or more biomarkers that are actually or potentially linked to neurodegeneration, and this should be stated by the authors in the study methodology or protocol;
- Only human studies were included to ensure the specificity of any identified biomarkers for human subjects with PTSD;
- Only original research was included.
3. Characteristics of the Included Studies
4. Brain Imaging Studies
5. Genetic, Epigenetic, and Gene Expression-Related Markers
6. Biochemical Marker Studies
7. Immune and Inflammatory Marker Studies
8. Sleep-Related Studies
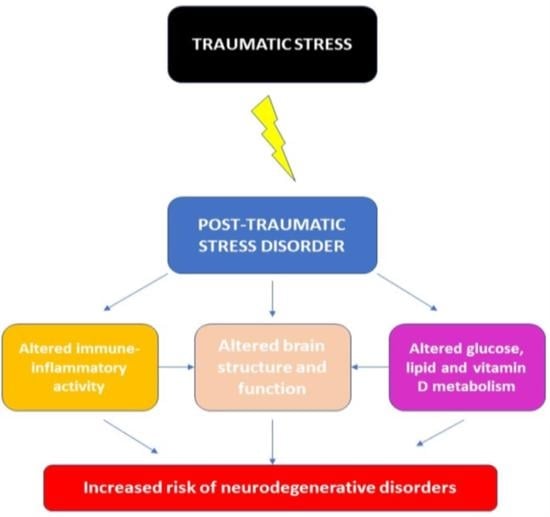
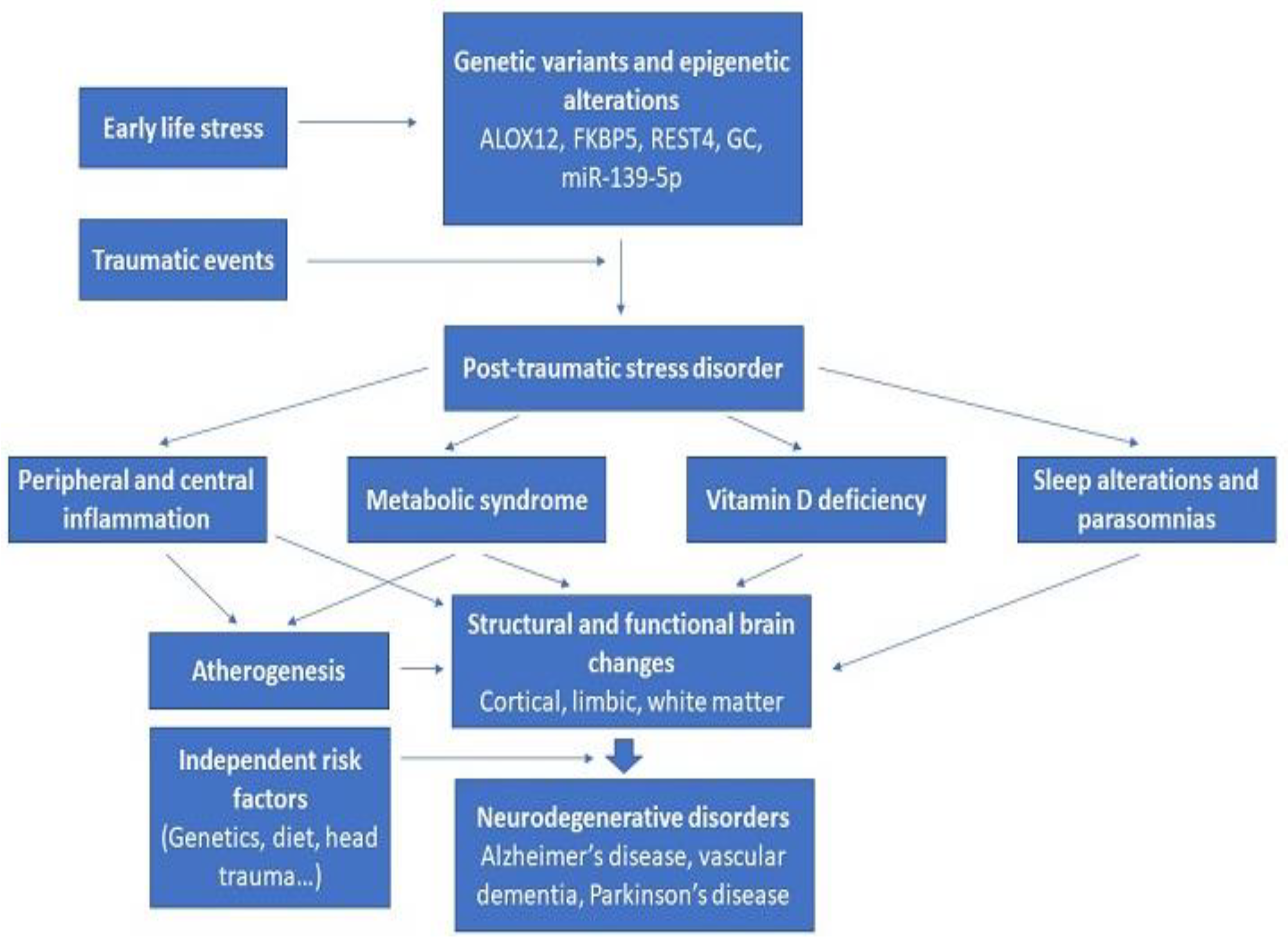
9. Integration of Existing Results on Biomarkers of Neurodegeneration in PTSD
10. Limitations of the Existing Data
11. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-beta protein |
| AD | Alzheimer’s disease |
| ALOX12 | Arachidonate 12-lipoxygenase |
| ALOX15 | Arachidonate 15-lipoxygenase |
| BOLD | Blood oxygenation level-dependent |
| CRP | C-reactive protein |
| DTI | Diffusion tensor imaging |
| FDA | Food and Drug Administration (United States) |
| FKBP5 | FK-506 binding protein 5 |
| GC | Vitamin D-binding protein |
| HPA | Hypothalamic–pituitary–adrenal axis |
| IL-6 | Interleukin-6 |
| miR | microRNA |
| MRI | Magnetic resonance imaging |
| NIH | National Institutes of Health (United States) |
| PET | Positron emission tomography |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PTSD | Post-Traumatic Stress Disorder |
| REST | RE1-silencing transcription factor gene |
| RSBD | Rapid Eye Movement (REM) Sleep Behavior Disorder |
| SEPT-4 | Septin-4 |
| SNP | Single nucleotide polymorphism |
| SPECT | Single photon emission computerized tomography |
| TBI | Traumatic brain injury |
| TNF-α | Tumor necrosis factor alpha |
| WTC | World Trade Center |
References
- Kirkpatrick, H.A.; Heller, G.M. Post-traumatic stress disorder: Theory and treatment update. Int. J. Psychiatry Med. 2014, 47, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, N.; Brooks, S.; Dunn, R. Latest developments in post-traumatic stress disorder: Diagnosis and treatment. Br. Med. Bull. 2015, 114, 147–155. [Google Scholar] [CrossRef]
- Yehuda, R.; Hoge, C.W.; McFarlane, A.C.; Vermetten, E.; Lanius, R.A.; Nievergelt, C.A.; Hobfoll, S.E.; Koenen, K.C.; Neylan, T.C.; Hyman, S.E. Post-traumatic stress disorder. Nat. Rev. Dis. Prim. 2015, 1, 15057. [Google Scholar] [CrossRef] [PubMed]
- Hoppen, T.H.; Priebe, S.; Vetter, I.; Morina, N. Global burden of post-traumatic stress disorder and major depression in countries affected by war between 1989 and 2019: A systematic review and meta-analysis. BMJ Glob. Health 2021, 6, e006303. [Google Scholar] [CrossRef] [PubMed]
- Dewar, M.; Paradis, A.; Fortin, C.A. Identifying trajectories and predictors of response to psychotherapy for post-traumatic stress disorder in adults: A systematic review of the literature. Can. J. Psychiatry 2020, 65, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Phillips, N.J.; Stein, D.J.; Ipser, J.C. Pharmacotherapy for post traumatic stress disorder. Cochrane Database Syst. Rev. 2022, 3, CD002795. [Google Scholar] [CrossRef] [PubMed]
- Morina, N.; Wicherts, J.M.; Lobbrecht, J.; Priebe, S. Remission from post-traumatic stress disorder in adults: A systematic review and meta-analysis of long term outcome studies. Clin. Psychol. Rev. 2014, 34, 249–255. [Google Scholar] [CrossRef]
- Benedict, T.M.; Keenan, P.G.; Nitz, A.J.; Moeller-Bertram, T. Post-traumatic stress disorder symptoms contribute to worse pain and health outcomes in veterans with PTSD compared to those without: A systematic review with meta-analysis. Mil. Med. 2020, 185, e1481–e1491. [Google Scholar] [CrossRef]
- Al Jowf, G.I.; Ahmed, Z.T.; An, N.; Reijnders, R.A.; Ambrosino, E.; Rutten, B.P.F.; de Nijs, L.; Eijssen, L.M.T. A public health perspective of post-traumatic stress disorder. Int. J. Environ. Res. Public Health 2022, 19, 6474. [Google Scholar] [CrossRef]
- Zammit, S.; Lewis, C.; Dawson, S.; Colley, H.; McCann, H.; Piekarski, A.; Rockliff, H.; Bisson, J. Undetected post-traumatic stress disorder in secondary-care mental health services: A systematic review. Br. J. Psychiatry 2018, 212, 11–18. [Google Scholar] [CrossRef]
- Maria-Rios, C.E.; Morrow, J.D. Mechanisms of shared vulnerability to post-traumatic stress disorder and substance use disorders. Front. Behav. Neurosci. 2020, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Britvic, D.; Anticevic, V.; Kaliterna, M.; Lusic, L.; Beg, A.; Brajevic-Gizdic, I.; Kudric, M.; Stupalo, Z.; Krolo, V.; Pivac, N. Comorbidities with posttraumatic stress disorder (PTSD) among combat veterans: 15 years postwar analysis. Int. J. Clin. Health Psychol. 2015, 15, 81–92. [Google Scholar] [CrossRef]
- Pietrzak, R.H.; Goldstein, R.B.; Southwick, S.M.; Grant, B.F. Physical health conditions associated with posttraumatic stress disorder in U.S. older adults: Results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J. Am. Geriatr. Soc. 2012, 60, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Krysinska, K.; Lester, D. Post-traumatic stress disorder and suicide risk: A systematic review. Arch. Suicide Res. 2010, 14, 1–23. [Google Scholar] [CrossRef]
- Hu, X.Y.; Wu, Y.L.; Cheng, C.H.; Liu, X.X.; Zhou, L. Association of Brain-Derived Neurotrophic Factor rs6265 G>A polymorphism and Post-traumatic Stress Disorder susceptibility: A systematic review and meta-analysis. Brain Behav. 2021, 11, e02118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shelton, R.C.; Dwivedi, Y. Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and post-traumatic stress disorder: A systematic review and meta-analysis. J. Affect. Disord. 2018, 225, 422–428. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Koenen, K.A.; Bromet, E.J.; Karam, E.G.; Liu, H.; Petukhova, M.; Ruscio, A.M.; Sampson, N.A.; Stein, D.J.; Aguilar-Gaxiola, S.; et al. Childhood adversities and post-traumatic stress disorder: Evidence for stress sensitization in the World Mental Health Surveys. Br. J. Psychiatry 2017, 211, 280–288. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Conron, K.J.; Koenen, K.C.; Gilman, S.E. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: A test of the stress sensitization hypothesis in a population-based sample of adults. Psychol. Med. 2010, 40, 1647–1658. [Google Scholar] [CrossRef]
- Stefanovic, M.; Ehring, T.; Wittekind, C.E.; Kleim, B.; Rohde, J.; Kruger-Gottschalk, A.; Knaevelsrud, C.; Rau, H.; Schafer, I.; Schellong, J.; et al. Comparing PTSD symptom networks in type I vs. type II trauma survivors. Eur. J. Psychotraumatol. 2022, 13, 2114260. [Google Scholar] [CrossRef]
- Hiscox, L.V.; Hiller, R.; Fraser, A.; Rabie, S.; Stewart, J.; Seedat, S.; Tomlinson, M.; Hallingan, S.L. Sex differences in post-traumatic stress disorder in a high adversity cohort of South African adolescents: An examination of depressive symptoms, age, and trauma type as explanatory factors. Eur. J. Psychotraumatol. 2021, 12, 1978669. [Google Scholar] [CrossRef]
- Panagou, C.; MacBeth, A. Deconstructing pathways to resilience: A systematic review of associations between psychosocial mechanisms and transdiagnostic adult mental health outcomes in the context of adverse childhood experiences. Clin. Psychol. Psychother. 2022, 29, 1626–1654. [Google Scholar] [CrossRef]
- Pankey, B.S.; Riedel, M.C.; Cowan, I.; Bartley, J.E.; Lobo, R.P.; Hill-Bowen, L.D.; Salo, T.; Musser, E.D.; Sutherland, M.T.; Laird, A.R. Extended functional connectivity of convergent structural alterations among individuals with PTSD: A neuroimaging meta-analysis. Behav. Brain Funct. 2022, 18, 9. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Z.; Wu, X.; Wen, S.W.; Liu, A. Salivary cortisol in post-traumatic stress disorder: A systematic review and meta-analysis. BMC Psychiatry 2018, 18, 324. [Google Scholar] [CrossRef] [PubMed]
- Dell’Oste, V.; Fantasia, S.; Gravina, D.; Palego, L.; Betti, L.; Dell’Osso, L.; Giannaccini, G.; Carmassi, C. Metabolic and inflammatory response in post-traumatic stress disorder (PTSD): A systematic review on peripheral neuroimmune biomarkers. Int. J. Environ. Res. Public Health 2023, 20, 2937. [Google Scholar] [CrossRef]
- Schiavone, S.; Jaquet, V.; Trabace, L.; Krause, K.H. Severe life stress and oxidative stress in the brain: From animal models to human pathology. Antioxid. Redox Signal. 2013, 18, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Krantz, D.S.; Shank, L.M.; Goodie, J.L. Post-traumatic stress disorder (PTSD) as a systemic disorder: Pathways to cardiovascular disease. Health Psychol. 2022, 41, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Polyak, H.; Galla, Z.; Nanasi, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabo, A.; Klivenyi, P.; Tanaka, M.; et al. The tryptophan-kynurenine metabolic system is suppressed in cuprizone-induced model of demyelination simulating progressive multiple sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Szok, D.; Csati, A.; Szabo, A.; Tanaka, M.; Vecsei, L. Exploring novel therapeutic targets in the common pathogenic factors in migraine and neuropathic pain. Int. J. Mol. Sci. 2023, 24, 4114. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, G.; Bertaccini, R.; Tarasi, L.; Di Gregorio, F.; Trajkovic, J.; Battaglia, S.; Romei, V. The role of alpha oscillations among the main neuropsychiatric disorders in the adult and developing human brain: Evidence from the last 10 years of research. Biomedicines 2022, 10, 3189. [Google Scholar] [CrossRef]
- Di Gregorio, F.; La Porta, F.; Petrone, V.; Battaglia, S.; Orlandi, S.; Ippolito, G.; Romei, V.; Piperno, R.; Lullini, G. Accuracy of EEG biomarkers in the detection of clinical outcome in disorders of consciousness after severe acquired brain injury: Preliminary results of a pilot study using a machine learning approach. Biomedicines 2022, 10, 1897. [Google Scholar] [CrossRef]
- Harrison, B.J.; Fullana, M.A.; Via, E.; Soriano-Mas, C.; Vervliet, B.; Martinez-Zalacan, I.; Pujol, J.; Davey, C.G.; Kircher, T.; Straube, B.; et al. Human ventromedial prefrontal cortex and the positive affective processing of safety signals. NeuroImage 2017, 152, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. Stopping in (e)motion: Reactive action inhibition when facing valence-independent emotional stimuli. Front. Behav. Neurosci. 2022, 16, 998714. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabo, A.; Vecsei, L. Integrating armchair, bench, and bedside research for behavioral neurology and neuropsychiatry: Editorial. Biomedicines 2022, 10, 2999. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Fear-induced bradycardia in mental disorders: Foundations, current advances, future perspectives. Neurosci. Biobehav. Rev. 2023, 149, 105163. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Di Fazio, C.; Vicario, C.M.; Avenanti, A. Neuropharmacological modulation of N-methyl-D-aspartate, noradrenaline and endocannabinoid receptors in fear extinction learning: Synaptic transmission and plasticity. Int. J. Mol. Sci. 2023, 24, 5926. [Google Scholar] [CrossRef]
- Tanaka, M.; Torok, M.; Vecsei, L. Novel pharmaceutical approaches in dementia. In Neuropsychopharmacotherapy; Riederer, P., Laux, G., Nagatsu, T., Le, W., Riederer, C., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Rafferty, L.A.; Cawkill, P.E.; Stevelink, S.A.M.; Greenberg, K.; Greenberg, N. Dementia, post-traumatic stress disorder and major depressive disorder: A review of the mental health risk factors for dementia in the military veteran population. Psychol. Med. 2018, 48, 1400–1409. [Google Scholar] [CrossRef]
- Gunak, M.M.; Billings, J.; Carratu, E.; Marchant, N.L.; Favarato, G.; Orgeta, V. Post-traumatic stress disorder as a risk factor for dementia: Systematic review and meta-analysis. Br. J. Psychiatry 2020, 217, 600–608. [Google Scholar] [CrossRef]
- Bergman, B.P.; Mackay, D.F.; Pell, J.P. Dementia in Scottish military veterans: Early evidence from a retrospective cohort study. Psychol. Med. 2021, 53, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sieurin, J.; Wirdefeldt, K.; Pedersen, N.L.; Almqvist, C.; Larsson, H.; Valdimarsdottir, U.A.; Fang, F. Association of stress-related disorders with subsequent neurodegenerative disorders. JAMA Neurol. 2020, 77, 700–709. [Google Scholar] [CrossRef]
- Chan, Y.E.; Bai, Y.M.; Hsu, J.W.; Huang, K.L.; Su, T.P.; Li, C.T.; Lin, W.C.; Pan, T.L.; Chen, T.J.; Tsai, S.J.; et al. Post-traumatic stress disorder and risk of Parkinson disease: A nationwide longitudinal study. Am. J. Geriatr. Psychiatry 2017, 25, 917–923. [Google Scholar] [CrossRef]
- White, D.L.; Kunik, M.E.; Yu, H.; Lin, H.L.; Richardson, P.A.; Moore, S.; Sarwar, A.I.; Marsh, L.; Jorge, R.E. Post-traumatic stress disorder is associated with further increased Parkinson’s disease in veterans with traumatic brain injury. Ann. Neurol. 2020, 88, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.B.; Palmer, B.W.; Eidt, C.A.; Aailaboyina, S.; Mausbach, B.T.; Wolkowitz, O.M.; Thorp, S.R.; Jeste, D.V. Is post-traumatic stress disorder associated with premature senescence? A review of the literature. Am. J. Geriatr. Psychiatry 2015, 23, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Novellino, F.; Sacca, V.; Donato, A.; Zaffino, P.; Spadea, M.F.; Vismara, M.; Arcidiacono, B.; Malara, N.; Presta, I.; Donato, G. Innate immunity: A common denominator between neurodegenerative and neuropsychiatric diseases. Int. J. Mol. Sci. 2020, 21, 1115. [Google Scholar] [CrossRef] [PubMed]
- Antonelli-Salgado, T.; Ramos-Lima, L.F.; Machado, C.S.; Cassidy, R.M.; Cardoso, T.A.; Kapczinski, F.; Passos, I.C. Neuroprogression in post-traumatic stress disorder: A systematic review. Trends Psychiatry Psychother. 2021, 43, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Desmarais, P.; Weidman, D.; Wassef, A.; Bruneau, M.A.; Friedland, J.; Bajsarowicz, P.; Thibodeau, M.P.; Herrmann, N.; Nguyen, Q.D. The interplay between post-traumatic stress disorder and dementia: A systematic review. Am. J. Geriatr. Psychiatry 2020, 28, 48–60. [Google Scholar] [CrossRef]
- Elias, A.; Rowe, C.; Hopwood, M. Risk of dementia in posttraumatic stress disorder. J. Geriatr. Psychiatry Neurol. 2021, 34, 555–564. [Google Scholar] [CrossRef]
- Tanaka, M.; Vecsei, L. Editorial of special issue ‘Dissecting neurological and neuropsychiatric diseases: Neurodegeneration and neuroprotection’. Int. J. Mol. Sci. 2022, 23, 6991. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Int. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Chao, L.L.; Yaffe, K.; Samuelson, K.; Neylan, T.C. Hippocampal volume is inversely related to PTSD duration. Psychiatry Res. Neuroimaging 2014, 222, 119–123. [Google Scholar] [CrossRef]
- Mueller, S.G.; Ng, P.; Neylan, T.; Mackin, S.; Wolkowitz, O.; Mellon, S.; Yan, X.; Flory, J.; Yehuda, R.; Marmar, C.R.; et al. Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatry Res. Neuroimaging 2015, 234, 194–201. [Google Scholar] [CrossRef]
- Main, K.L.; Soman, S.; Pestilli, F.; Furst, A.; Noda, A.; Hernandez, B.; Kong, J.; Cheng, J.; Fairchild, J.K.; Taylor, J.; et al. DTI measures identify mild and moderate TBI cases among patients with complex health problems: A receiver operating characteristic analysis of U.S. veterans. NeuroImage Clin. 2017, 16, 1–16. [Google Scholar] [CrossRef]
- Basavaraju, R.; France, J.; Maas, B.; Brickman, A.M.; Flory, J.D.; Szeszko, P.R.; Yehuda, R.; Rutherford, B.R.; Provenzano, F.A. Right hippocampal volume deficit in an older population with posttraumatic stress disorder. J Psychiatr. Res. 2021, 17, 368–375. [Google Scholar] [CrossRef]
- Olivé, I.; Makris, N.; Densmore, M.; McKinnon, M.C.; Lanius, R.A. Altered basal forebrain BOLD signal variability at rest in posttraumatic stress disorder: A potential candidate vulnerability mechanism for neurodegeneration in PTSD. Hum. Brain Mapp. 2021, 42, 3561–3575. [Google Scholar] [CrossRef]
- Brown, E.M.; Salat, D.H.; Milberg, W.P.; Fortier, C.B.; McGlinchey, R.E. Accelerated longitudinal cortical atrophy in OEF/OIF/OND veterans with severe PTSD and the impact of comorbid TBI. Hum. Brain Mapp. 2022, 43, 3694–3705. [Google Scholar] [CrossRef]
- Kritikos, M.; Huang, C.; Clouston, S.A.P.; Pellecchia, A.C.; Mejia-Santiago, S.; Carr, M.A.; Hagan, T.; Kotov, R.; Gandy, S.; Sano, M.; et al. DTI connectometry analysis reveals white matter changes in cognitively impaired World Trade Center responders at midlife. J. Alzheimers Dis. 2022, 89, 1075–1089. [Google Scholar] [CrossRef]
- Kuan, P.F.; Yang, X.; Clouston, S.; Ren, X.; Kotov, R.; Waszczuk, M.; Singh, P.K.; Glenn, S.T.; Gomez, E.C.; Wang, J.; et al. Cell type-specific gene expression patterns associated with posttraumatic stress disorder in World Trade Center responders. Transl. Psychiatry 2021, 9, 1. [Google Scholar] [CrossRef]
- Sragovich, S.; Gershovits, M.; Lam, J.C.K.; Li, V.O.K.; Gozes, I. Putative somatic blood mutations in post-traumatic stress disorder-symptomatic soldiers: High impact of cytoskeletal and inflammatory proteins. J. Alzheimers Dis. 2021, 79, 1723–1734. [Google Scholar] [CrossRef]
- Wolf, E.J.; Zhao, X.; Hawn, S.E.; Morrison, F.G.; Zhou, Z.; Fein-Schaffer, D.; Huber, B.; Miller, M.W.; Logue, M.W. Gene expression correlates of advanced epigenetic age and psychopathology in postmortem cortical tissue. Neurobiol. Stress 2021, 15, 100371. [Google Scholar] [CrossRef]
- Clouston, S.A.P.; Deri, Y.; Diminich, E.; Kew, R.; Kotov, R.; Stewart, C.; Yang, X.; Gandy, S.; Sano, M.; Bromet, E.J.; et al. Posttraumatic stress disorder and total amyloid burden and amyloid-β 42/40 ratios in plasma: Results from a pilot study of World Trade Center responders. Alzheimers Dement. 2019, 11, 216–220. [Google Scholar] [CrossRef]
- Cimino, N.; Kang, M.S.; Honig, L.S.; Rutherford, B.R. Blood-based biomarkers for Alzheimer’s disease in older adults with posttraumatic stress disorder. J. Alzheimers Dis. Rep. 2022, 6, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rosen, R.; Reibman, J.; Shao, Y. Posttraumatic stress disorder mediates the association between traumatic World Trade Center dust cloud exposure and ongoing systemic inflammation in community members. Int. J. Environ. Res. Public Health 2022, 19, 8622. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.E.; Opel, R.A.; Weymann, K.B.; Chau, A.Q.; Papesh, M.A.; Callahan, M.L.; Storzbach, D.; Lim, M.M. Sleep disturbances in traumatic brain injury: Association with sensory sensitivity. J. Clin. Sleep Med. 2018, 14, 1177–1186. [Google Scholar] [CrossRef]
- Elliott, J.E.; Opel, R.A.; Pleshakov, D.; Rachakonda, T.; Chau, A.Q.; Weymann, K.B.; Lim, M.M. Posttraumatic stress disorder increases the odds of REM sleep behavior disorder and other parasomnias in veterans with and without comorbid traumatic brain injury. Sleep 2020, 43, zsz237. [Google Scholar] [CrossRef]
- Feemster, J.C.; Steele, T.A.; Palermo, K.P.; Ralston, C.L.; Tao, Y.; Bauer, D.A.; Edgar, L.; Rivera, S.; Walters-Smith, M.; Gossard, T.R.; et al. Abnormal rapid eye movement sleep atonia control in chronic post-traumatic stress disorder. Sleep 2022, 45, zsab259. [Google Scholar] [CrossRef]
- Liu, Y.; Partinen, E.; Chan, N.Y.; Dauvilliers, Y.; Inoue, Y.; De Gennaro, L.; Piazzi, G.; Bolstad, C.J.; Nadorff, M.R.; Merikanto, I.; et al. Dream-enactment behaviours during the COVID-19 pandemic: An international COVID-19 sleep study. J. Sleep Res. 2023, 32, e13613. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.G.; Ekhator, N.N.; Kasckow, J.W.; Hill, K.K.; Zoumakis, E.; Dashevsky, B.A.; Chrousos, G.P.; Geracioti, T.D. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation 2021, 9, 209–217. [Google Scholar] [CrossRef]
- Mohlenhoff, B.S.; Chao, L.L.; Buckley, S.T.; Weiner, M.W.; Neylan, T.C. Are hippocampal size difference in posttraumatic stress disorder mediated by sleep pathology? Alzheimers Dement. 2014, 10, S146–S154. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Wolf, E.J.; Sadeh, N.; Logue, M.; Spielberg, J.M.; Hayes, J.P.; Sperbeck, E.; Schichman, S.A.; Stone, A.; Carter, W.C.; et al. A novel locus in the oxidative stress-related gene ALOX12 moderates the association between PTSD and thickness of the prefrontal cortex. Psychoneuroendocrinology 2015, 62, 359–365. [Google Scholar] [CrossRef]
- O’Donovan, A.; Chao, L.L.; Paulson, J.; Samuelson, K.W.; Shigenaga, J.K.; Grunfeld, C.; Weiner, M.W.; Neylan, T.C. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology 2015, 51, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.J.; Sadeh, N.; Leritz, E.C.; Logue, M.W.; Stoop, T.; McGlinchey, R.; Milberg, W.; Miller, M.W. PTSD as a catalyst for the association between metabolic syndrome and reduced cortical thickness. Biol. Psychiatry 2016, 80, 363–371. [Google Scholar] [CrossRef]
- Hayes, J.P.; Logue, M.W.; Sadeh, N.; Spielberg, J.M.; Verfaelle, M.; Hayes, S.M.; Reagan, A.; Salat, D.H.; Wolf, E.J.; McGlinchey, R.E.; et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain 2017, 140, 813–825. [Google Scholar] [CrossRef]
- Hayes, J.P.; Reagan, A.; Logue, M.W.; Hayes, S.M.; Sadeh, N.; Miller, D.R.; Verfaellie, M.; Wolf, E.J.; McGlinchey, R.E.; Milberg, W.P.; et al. BDNF genotype is associated with hippocampal volume in mild traumatic brain injury. Genes Brain Behav. 2018, 17, 107–117. [Google Scholar] [CrossRef]
- Kang, J.I.; Mueller, S.G.; Wu, G.W.Y.; Lin, J.; Ng, P.; Yehuda, R.; Flory, J.D.; Abu-Amara, D.; Reus, V.I.; Gautam, A.; et al. Effect of combat exposure and posttraumatic stress disorder on telomere length and amygdala volume. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Terock, J.; Hannemann, A.; Van der Auwera, S.; Janowitz, D.; Spitzer, C.; Bonk, S.; Volzke, H.; Grabe, H.J. Posttraumatic stress disorder is associated with reduced vitamin D levels and functional polymorphisms of the vitamin D-binding protein in a population-based sample. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 96, 109760. [Google Scholar] [CrossRef] [PubMed]
- Guedes, V.A.; Lai, C.; Devoto, C.; Edwards, K.A.; Mithani, S.; Sass, D.; Vorn, R.; Qu, B.X.; Rusch, H.L.; Martin, C.A.; et al. Extracellular vesicle proteins and microRNAs are linked to chronic post-traumatic stress disorder symptoms in service members and veterans with mild traumatic brain injury. Front. Pharmacol. 2021, 12, 745348. [Google Scholar] [CrossRef]
- Weiner, M.W.; Harvey, D.; Landau, S.M.; Veitch, D.P.; Neylan, T.C.; Grafman, J.H.; Aisen, P.S.; Petersen, R.C.; Jack, C.R.; Tosun, D.; et al. Traumatic brain injury and post-traumatic stress disorder are not associated with Alzheimer’s disease pathology measured with biomarkers. Alzheimers Dement. 2023, 19, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Kritikos, M.; Diminich, E.D.; Meliker, J.; Mielke, M.; Bennett, D.A.; Finch, C.E.; Gandy, S.E.; Carr, M.A.; Yang, X.; Kotov, R.; et al. Plasma amyloid beta 40/42, phosphorylated tau 181, and neurofilament light are associated with cognitive impairment and neuropathological changes among World Trade Center responders: A prospective cohort study of exposures and cognitive aging at midlife. Alzheimers Dement. 2023, 15, e12409. [Google Scholar] [CrossRef]
- STRING: Functional Protein Association Networks. Available online: https://string-db.org/ (accessed on 28 March 2023).
- Lugli, G.; Cohen, A.M.; Bennett, D.A.; Shah, R.C.; Fields, C.J.; Hernandez, A.G.; Smalheiser, N.R. Plasma exosomal miRNAs in persons with and without Alzheimer disease: Altered expression and prospects for biomarkers. PLoS ONE 2015, 10, e0139233. [Google Scholar] [CrossRef]
- Zhuang, K.; Chen, X.; Cassady, K.E.; Baker, S.L.; Jagust, W.J. Metacognition, cortical thickness, and tauopathy in aging. Neurobiol. Aging 2022, 118, 44–54. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Chang, I.; Alzheimer’s Disease Neuroimaging Initiative. Development of quantitative and continuous measure for severity degree of Alzheimer’s disease evaluated from MRI images of 761 human brains. BMC Bioinform. 2022, 23, 357. [Google Scholar] [CrossRef]
- Sexton, C.E.; Kalu, U.G.; Fillippini, N.; Mackay, C.E.; Ebmeier, K.P. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2011, 32, e5–e2322. [Google Scholar] [CrossRef]
- Qin, L.; Guo, Z.; McClure, M.A.; Mu, Q. White matter changes from mild cognitive impairment to Alzheimer’s disease: A meta-analysis. Acta Neurol. Belg. 2021, 121, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Herdick, M.; Dyrba, M.; Fritz, H.C.J.; Altenstein, S.; Ballarini, T.; Brosseron, F.; Buerger, K.; Cetindag, A.C.; Dechent, P.; Dobisch, L.; et al. Multimodal MRI analysis of basal forebrain structure and function across the Alzheimer’s disease spectrum. Neuroimage Clin. 2020, 28, 102495. [Google Scholar] [CrossRef]
- Atti, A.R.; Valente, S.; Iodice, A.; Caramella, I.; Ferrari, B.; Albert, U.; Mandelli, L.; De Ronchi, D. Metabolic syndrome, mild cognitive impairment, and dementia: A meta-analysis of longitudinal studies. Am. J. Geriatr. Psychiatry 2019, 27, 625–637. [Google Scholar] [CrossRef]
- Zuin, M.; Roncon, L.; Passaro, A.; Cervellati, C.; Zuliani, G. Metabolic syndrome and the risk of late onset Alzheimer’s disease: An updated review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2244–2252. [Google Scholar] [CrossRef]
- Pillai, J.A.; Bena, J.; Bekris, L.; Kodur, N.; Kasumov, T.; Leverenz, J.B.; Kashyap, S.R.; Alzheimer’s Disease Neuroimaging Initiative. Metabolic syndrome biomarkers relate to rate of cognitive decline in MCI and dementia stages of Alzheimer’s disease. Alzheimers Res. Ther. 2023, 15, 54. [Google Scholar] [CrossRef]
- Sommer, I.; Griebler, U.; Kien, C.; Auer, S.; Klerings, I.; Hammer, R.; Holzer, P.; Gartlehner, G. Vitamin D deficiency as a risk factor for dementia: A systematic review and meta-analysis. BMC Geriatr. 2017, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Navale, S.S.; Mulugeta, A.; Zhou, A.; Llewellyn, D.J.; Hypponen, E. Vitamin D and brain health: An observational and Mendelian randomization study. Am. J. Clin. Nutr. 2022, 116, 531–540. [Google Scholar] [CrossRef]
- Ghahremani, M.; Smith, E.E.; Chen, H.Y.; Creese, B.; Goodarzi, Z.; Ismail, Z. Vitamin D supplementation and incident dementia: Effects of sex, APOE, and baseline cognitive status. Alzheimers Dement. 2023, 15, e12404. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Hinz, R.; Gentleman, S.; Hampshire, A.; Dani, M.; Brooks, D.J.; Edison, P. Neuroinflammation is independently associated with brain network dysfunction in Alzheimer’s disease. Mol. Psychiatry 2023, 28, 1303–1311. [Google Scholar] [CrossRef]
- Chu, M.; Wen, L.; Jiang, D.; Liu, L.; Nan, H.; Yue, A.; Wang, Y.; Wang, Y.; Qu, M.; Wang, N.; et al. Peripheral inflammation in behavioural variant frontotemporal dementia: Associations with central degeneration and clinical measures. J. Neuroinflammation 2023, 20, 65. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Cao, Z.; Liu, Q.; Cheng, Y. Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Front. Immunol. 2018, 9, 2122. [Google Scholar] [CrossRef]
- Renna, M.E.; O’Toole, M.S.; Spaeth, P.E.; Lekander, M.; Mennin, D.S. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: A systematic review and meta-analysis. Depress. Anxiety 2018, 35, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Chen, Y.; Meng, Y.; Yang, Z.; Wei, M.; Li, T.; Ni, J.; Shi, J.; Tian, J. Peripheral high levels of CRP predict progression from normal cognition to dementia: A systematic review and meta-analysis. J. Clin. Neurosci. 2023, 107, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Custodero, C.; Ciavarella, A.; Panza, F.; Gnocchi, D.; Lenato, G.M.; Lee, J.; Mazzocca, A.; Sabba, C.; Solfrizzi, V. Role of inflammatory markers in the diagnosis of vascular contributions to cognitive impairment and dementia: A systematic review and meta-analysis. Geroscience 2022, 44, 1373–1392. [Google Scholar] [CrossRef]
- Portilla-Fernandez, E.; Hwang, S.J.; Wilson, R.; Maddock, J.; Hill, D.W.; Teumer, A.; Mishra, P.P.; Brody, J.A.; Joehanes, R.; Litghart, S.; et al. Meta-analysis of epigenome-wide association studies of carotid intima-media thickness. Eur. J. Epidemiol. 2021, 36, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Tomimoto, H.; Kitayama, H.; Morioka, Y.; Akiguchi, I.; Shibasaki, H.; Noda, M.; Kinoshita, M. Association of the cytoskeletal GTP-binding protein Sept4/H5 with cytoplasmic inclusions found in Parkinson’s disease and other synucleinopathies. J. Biol. Chem. 2003, 278, 24095–24102. [Google Scholar] [CrossRef] [PubMed]
- Saba, R.; Goodman, C.D.; Huzarewich, R.L.; Robertson, C.; Booth, S.A. A miRNA signature of prion-induced neurodegeneration. PLoS ONE 2008, 3, e3652. [Google Scholar] [CrossRef]
- Lemche, E. Early life stress and epigenetics in late-onset Alzheimer’s Dementia: A systematic review. Curr. Genomics 2018, 19, 522–602. [Google Scholar] [CrossRef]
- Galbiati, A.; Verga, L.; Giora, E.; Zucconi, M.; Ferini-Strambi, L. The risk of neurodegeneration in REM sleep behavior disorder: A systematic review and meta-analysis of longitudinal studies. Sleep Med. Rev. 2019, 43, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Germain, A. Sleep disturbances as the hallmark of PTSD: Where are we now? Am. J. Psychiatry 2013, 170, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Barone, D.A. Dream enactment behavior-a real nightmare: A review of post-traumatic stress disorder, REM sleep behavior disorder, and trauma-associated sleep disorder. J. Clin. Sleep Med. 2020, 16, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Mysliwiec, V.; Brock, M.S.; Creamer, J.L.; O’Reilly, B.M.; Germain, A.; Roth, B.J. Trauma induced sleep disorder: A parasomnia induced by trauma. Sleep Med. Rev. 2018, 37, 94–104. [Google Scholar] [CrossRef]
- Eraly, S.A.; Nievergelt, C.A.; Maihofer, A.X.; Barkauskas, D.A.; Biswas, N.; Agorastos, A.; O’Connor, D.T.; Baker, D.G.; MRS Team. Assessment of plasma C-reactive protein as a biomarker of PTSD risk. JAMA Psychiatry 2014, 71, 423–431. [Google Scholar] [CrossRef]
- Stein, D.J.; Karam, E.G.; Shahly, V.; Hill, E.D.; King, A.; Petukhova, M.; Atwoli, L.; Bromet, E.J.; Florescu, S.; Haro, J.M.; et al. Post-traumatic stress disorder associated with life-threatening motor vehicle collisions in the WHO World Mental Health Surveys. BMC Psychiatry 2016, 16, 257. [Google Scholar] [CrossRef]
- Hawes, A.M.; Axinn, W.G.; Ghimire, D.J. Ethnicity and psychiatric disorders. Ann. Psychiatry Ment. Health 2016, 4, 1072. [Google Scholar]
- Dworkin, E.R.; Schumacher, J.A. Preventing posttraumatic stress related to sexual assault through early intervention: A systematic review. Trauma Violence Abus. 2018, 19, 459–472. [Google Scholar] [CrossRef]
- Peterson, K.; Veazie, S.; Bourne, D.; Anderson, J. Association between traumatic brain injury and dementia in veterans: A rapid systematic review. J. Head Trauma Rehabil. 2020, 35, 198–208. [Google Scholar] [CrossRef]
- Islamoska, S.; Hansen, A.M.; Ishtiak-Ahmed, K.; Garde, A.H.; Andersen, P.K.; Garde, E.; Taudorf, L.; Waldemar, G.; Nabe-Nielsen, K. Stress diagnoses in midlife and risk of dementia: A register-based follow-up study. Aging Ment. Health 2021, 25, 1151–1160. [Google Scholar] [CrossRef]
- van Dongen, D.H.E.; Havermans, D.; Deckers, K.; Olff, M.; Verhey, F.; Sobczak, S. A first insight into the clinical manifestation of posttraumatic stress disorder in dementia: A systematic literature review. Psychogeriatrics 2022, 22, 509–520. [Google Scholar] [CrossRef] [PubMed]
| Scheme | Modality | Study Population | Design | Biomarker(s) Studied | Results |
|---|---|---|---|---|---|
| Brain imaging studies | |||||
| Chao et al., 2014 [51] | Structural MRI | Military veterans with PTSD as per DSM-IV criteria (n = 55) | Cross-sectional; association | Hippocampal volume as test region; caudate nucleus volume as a control region | Duration of PTSD significantly and negatively correlated with right hippocampal volume, even after adjusting for confounders. No association between PTSD duration and left hippocampal or caudate volumes. |
| Mueller et al., 2015 [52] | Structural MRI | Military veterans with (n = 40) and without (n = 45) significant PTSD symptoms as measured using CAPS | Cross-sectional; case-control | Cortical and hippocampal volumes; structural connectivity of the prefrontal-limbic network | PTSD significantly associated with reduced rostral cingulate and insular cortical thickness but no hippocampal volume loss. Evidence of reduced prefrontal-limbic structural connectivity in PTSD. |
| Main et al., 2017 [53] | DTI | Military veterans (n = 109); 71.6% PTSD as per DSM-IV criteria; 57.8% mild TBI; 9.2% moderate TBI | Cross-sectional; association | FA and diffusivity of white matter fiber tracts | Altered parameters in left cingulum and inferior frontal-occipital fasciculus and right anterior thalamic tract specifically associated with TBI. Altered white matter parameters in right cingulum and inferior longitudinal fasciculus and left anterior thalamic radiation associated with PTSD. |
| Basavaraju et al., 2021 [54] | Structural MRI | Older adults (age ≥ 50 years) with a history of trauma exposure with (n = 55) and without (n = 36) PTSD | Cross-sectional; case-control | Cortical volume | Significant reduction of right parahippocampal cortical volume, but not other cortical regions, in PTSD. |
| Olivé et al., 2021 [55] | Functional MRI | Patients with PTSD (n = 103; 38 with dissociative subtype of PTSD); healthy controls (n = 46) | Cross-sectional; case-control | Variability of BOLD signal in basal forebrain regions | Increased BOLD signal variability in extended amygdala and nucleus accumbens in dissociative PTSD compared to both PTSD and controls. |
| Brown et al., 2022 [56] | Structural MRI | Military veterans (n = 254); 59.8% PTSD as per DSM-IV-TR criteria; 34.4% severe PTSD; (CAPS ≥ 60); 45.7% mild TBI | Longitudinal (2-year follow-up) with group comparisons (severe vs. non-severe PTSD) | Changes in cortical thickness, area, and volume | Severe PTSD associated with reduced cortical thickness, area, and volume, especially in frontal regions. More marked reductions in severe PTSD with mild TBI. |
| Kritikos et al., 2022 [57] | DTI | World Trade Center responders (n = 99); 48.4% cognitive impairment not amounting to dementia; 47.5% PTSD as per DSM-IV criteria | Cross-sectional; association | Whole-brain FA of white matter tracts | Reduced FA in fornix, cingulum, forceps minor, and right uncinate fasciculus in subjects with PTSD and cognitive impairment. Reduced FA in superior thalamic radiation and cerebellum in PTSD regardless of cognitive impairment. |
| Genetic, epigenetic, and gene expression studies | |||||
| Kuan et al., 2019 [58] | Peripheral blood transcriptome | World Trade Center responders with (n = 20) and without (n = 19) PTSD as per DSM-IV criteria | Cross-sectional; case-control | Transcriptome-wide analysis of gene expression in four peripheral blood immune cell subtypes | FKBP5 and PI4KAP1 upregulated across all cell types in PTSD. REST and SEPT4 upregulated in monocytes in PTSD. |
| Sragovich et al., 2021 [59] | Somatic mutation | Military veterans with (n = 27) and without (n = 55) PTSD as per DSM-IV criteria | Cross-sectional; case-control | Rates of somatic mutations based on peripheral blood samples | Increased number of mutations related to cytoskeletal genes and inflammation in PTSD. |
| Wolf et al., 2021 [60] | Post-mortem gene expression | Post-mortem cortical brain tissue from military veterans (n = 97); 43.3% PTSD as per DSM-5 criteria; 30.9% alcohol use disorder | Post-mortem; association | DNA methylation-based estimates of cellular age in relation to chronological age (DNAm age residuals); gene expression in cortical tissue. | Specific interaction effects with age residuals identified for four genes (SNORA73B, COL6A3, GCNT1, and GPRIN3) specific to PTSD. |
| Biochemical marker studies | |||||
| Clouston et al., 2019 [61] | Plasma assay | World Trade Center responders with (n = 17) and without (n = 17) probable PTSD as per PCL-17 | Cross-sectional; case-control | Plasma total amyloid-beta, amyloid-beta 42/40 ratio, total tau, and NfL | PTSD associated with lower plasma amyloid-beta and higher amyloid-beta 42/40 ratio. |
| Cimino et al., 2022 [62] | Serum assay | Adults, age ≥ 50, with a history of trauma exposure with (n = 44) and without (n = 26) subsequent PTSD as per DSM-5 criteria | Cross-sectional; case-control | Serum amyloid-beta 42 and 40 levels and ratio; serum total tau | No significant differences in amyloid-beta levels, ratios, or total tau levels between groups. |
| Immune-inflammatory marker studies | |||||
| Zhang et al., 2022 [63] | Serum assay | Residents living near the World Trade Center; 43.2% probable PTSD as per PCL; 50.3% dust cloud exposure | Cross-sectional; association | Serum CRP | Total CRP level and “high” CRP (>3 mg/L) both associated with PTSD. PCL score was a significant predictor of serum CRP. |
| Sleep-related marker studies | |||||
| Elliott et al., 2018 [64] | Polysomnography, self-report | Military veterans with a history of TBI (n = 130); 37.7% PTSD as per DSM-5 criteria | Cross-sectional; association | Sleep EEG/EMG; self-reported sleep disturbance; sensory (noise and light) sensitivity | Sleep disturbance and sensory sensitivity associated with PTSD in veterans with TBI. |
| Elliott et al., 2020 [65] | Polysomnography | Military veterans (n = 394); 28.6% probable PTSD as per PCL-5; 19.2% mild TBI | Cross-sectional; association | RSBD and other parasomnias | Increased rates of RSBD both in veterans with mild TBI and PTSD and in those with PTSD alone. |
| Feemster et al., 2022 [66] | Polysomnography | Patients with PTSD (n = 36), idiopathic RSBD (n = 18), and healthy controls (n = 51) | Cross-sectional; case-control | REM sleep with atonia | Higher REM sleep with atonia associated with PTSD independent of dream enactment behavior. |
| Liu et al., 2023 [67] | Self-report | Adults from fifteen countries (n = 21,870) during the COVID-19 pandemic; 3% COVID-19 positive; PTSD symptoms assessed using abbreviated PCL | Cross-sectional; association | Dream enactment behavior, weekly and lifetime | Screening positive for PTSD associated with a 1.2 to 1.4-fold increase in dream enactment behavior. |
| Multi-modality studies | |||||
| Baker et al., 2001 [68] | Plasma and CSF assays | Combat veterans (n = 11) with PTSD as per DSM-IV criteria; matched healthy controls (n = 8) | Cross-sectional; case-control | CSF levels of CRH, IL-6, norepinephrine; plasma levels of IL-6, ACTH, cortisol and norepinephrine | Increased CSF IL-6 in PTSD. Positive correlation between plasma IL-6 and norepinephrine in PTSD, but not in controls. |
| Mohlenhoff et al., 2014 [69] | Self-report (sleep); structural MRI (brain imaging) | Military veterans (n = 136); 7% PTSD as per DSM-IV criteria | Cross-sectional; association | Self-reported sleep disturbance; hippocampal volume | No association between PTSD and hippocampal volume. Possible association between sleep disturbance and left hippocampal volume. |
| Miller et al., 2015 [70] | Genetic association; structural MRI | Military veterans (n = 146); PTSD symptoms measured as a continuous variable using CAPS | Cross-sectional; interaction | Oxidative stress-related genes (ALOX12 and ALOX15); prefrontal cortex thickness | PTSD symptom severity negatively correlated with right, but not left, prefrontal volume. Two SNPs of ALOX12 moderated association between PTSD and right prefrontal volume. |
| O’Donovan et al., 2015 [71] | Serum assays; structural MRI | Military veterans with (n = 73) and without (n = 132) PTSD as per DSM-IV criteria | Cross-sectional; interaction | Serum IL-6 and sTNF-RII; hippocampal volume | sTNF-RII level negatively correlated with hippocampal volume regardless of PTSD diagnosis. PTSD severity associated with increased sTNF-RII and decreased IL-6. |
| Wolf et al., 2016 [72] | Plasma assays; structural MRI | Military veterans (n = 346); 77.2% PTSD as per DSM-IV criteria | Cross-sectional; interaction | Prevalence of metabolic syndrome as per NCEP-ATP III criteria; cortical thickness | Metabolic syndrome and its criteria more common in veterans with PTSD. Metabolic syndrome found to significantly mediate the association between PTSD and reduced cortical volume in precuneus, temporal cortex, rostral anterior cingulate cortex, and postcentral gyrus. |
| Hayes et al., 2017 [73] | Polygenic risk score; structural MRI | Military veterans (n = 160); 70% PTSD as per DSM-IV criteria; 65.6% mild TBI | Cross-sectional; interaction | Polygenic risk score for Alzheimer’s disease; cortical thickness | No significant association between cortical thickness and PTSD, either alone or in association with TBI or polygenic risk score. |
| Hayes et al., 2018 [74] | Genetic association; structural and functional MRI | Military veterans (n = 165); 66.7% mild TBI; 43% PTSD as per DSM-IV-TR criteria | Cross-sectional; interaction | BDNF genotype (9 SNPs); hippocampal volume; default mode network functional connectivity | No direct effect of PTSD on right or left hippocampal volume. TBI associated with reduced hippocampal volumes. Significant interaction between BDNF rs1157659 and TBI on hippocampal volume. No BDNF by PTSD interaction. |
| Kang et al., 2020 [75] | Peripheral blood and urine assays; structural MRI | Military veterans with (n = 102) and without (n = 113) PTSD as per DSM-IV criteria | Cross-sectional; interaction | Leukocyte telomere length; urinary catecholamines; amygdala volume | Shorter telomere length and increased amygdala volume associated with PTSD only in veterans exposed to high levels of trauma. Telomere shortening associated with increased urinary norepinephrine. |
| Terock et al., 2020 [76] | Genetic association; serum assay | 1653 adults with a history of trauma exposure; 3.8% PTSD as per DSM-IV criteria | Cross-sectional; interaction | Serum total vitamin D; two specific SNPs of the GC gene | Lower serum vitamin D associated with PTSD. Vitamin D deficiency more frequent in those with PTSD. CC genotype of rs4588 associated with lower risk of PTSD. T allele of rs7041 associated with increased risk of PTSD. |
| Guedes et al., 2021 [77] | Extracellular vesicle assay | Military veterans (n = 144); 31.3% PTSD as per PCL-5 screening; 80.6% mild TBI | Cross-sectional; association | Extracellular vesicle levels of 798 miRNAs; extracellular vesicle and plasma levels of NfL, amyloid-beta 42 and 40, tau, IL-10, IL-6, TNF-α, and VEGF | Elevated extracellular vesicle levels of NfL in patients with mTBI and PTSD. Significant association between miR-139-5p and PTSD symptom severity. |
| Weiner et al., 2022 [78] | CSF assay; structural MRI; PET | Military veterans (n = 289); 60.6% PTSD as per DSM-IV criteria; 47.8% moderate to severe TBI | Longitudinal (5-year follow-up) with group comparisons | CSF amyloid-beta 42, total tau, and p-tau181; PET measures of amyloid-beta and tau; cortical, hippocampal, and amygdala volume | No significant association of PTSD with biochemical or imaging markers, either cross-sectionally or at follow-up |
| Kritikos et al., 2023 [79] | Plasma assay; structural MRI | World Trade Center responders (n = 1173); 11.2% probable PTSD as per PCL-C; 16.4% mild cognitive impairment; 4.3% possible dementia | Cross-sectional; interaction | Plasma amyloid-beta 40/42 ratio, p-tau 181, NfL; hippocampal volume (only in 75 participants) | Significant intercorrelation between amyloid-beta 40/42, p-tau181, and NfL. PTSD associated with elevated amyloid-beta 40/42 ratio. Amyloid-beta 40/42 and p-tau 181 associated with reduced hippocampal volume. |
| Biomarker Type | Level of Evidence |
|---|---|
| Brain imaging | |
| Reduced cortical thickness | ++ |
| Reduced volume of specific right cortical regions | ++ |
| Reduced white matter tract integrity | ++ |
| Reduced structural grey matter connectivity | + |
| Reduced right hippocampal volume | ± |
| Increased amygdalar volume | ? |
| Increased BOLD variability in the basal forebrain | ? |
| Genetic and epigenetic | |
| Association between ALOX12 SNPs and reduced right prefrontal volume | + |
| Association between GC SNPs and PTSD risk | + |
| Upregulation of FKBP5, REST, SEPT4 in leukocytes | + |
| miR-139-5p | + |
| Reduced telomere length | ? |
| Post-mortem expression of COL6A3, GCNT1, GPRIN3 in brain | ? |
| Biochemical | |
| Reduced serum total vitamin D | + |
| Increased rate of metabolic syndrome | + |
| Elevated peripheral NfL | ± |
| Reduced peripheral Aβ 42/40 ratio | ± |
| Immune-inflammatory | |
| Serum CRP | + |
| Serum sTNF-RII | + |
| CSF IL-6 | + |
| Sleep-related | |
| Increased rates of RSBD or dream enactment behavior | ++ |
| Increased REM sleep with atonia | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkumar, R.P. Biomarkers of Neurodegeneration in Post-Traumatic Stress Disorder: An Integrative Review. Biomedicines 2023, 11, 1465. https://doi.org/10.3390/biomedicines11051465
Rajkumar RP. Biomarkers of Neurodegeneration in Post-Traumatic Stress Disorder: An Integrative Review. Biomedicines. 2023; 11(5):1465. https://doi.org/10.3390/biomedicines11051465
Chicago/Turabian StyleRajkumar, Ravi Philip. 2023. "Biomarkers of Neurodegeneration in Post-Traumatic Stress Disorder: An Integrative Review" Biomedicines 11, no. 5: 1465. https://doi.org/10.3390/biomedicines11051465
APA StyleRajkumar, R. P. (2023). Biomarkers of Neurodegeneration in Post-Traumatic Stress Disorder: An Integrative Review. Biomedicines, 11(5), 1465. https://doi.org/10.3390/biomedicines11051465