Successful Treatment of a Rare Cholesterol Homeostasis Disorder Due to CYP27A1 Gene Mutation with Chenodeoxycholic Acid Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Examination and Treatment
2.3. Genomic Testing
2.4. Bioinformatics and Statistical Analysis
3. Results
3.1. Phenotypes of the Patients
3.2. Results of Genetic Testing
3.3. The Effect of Chenodeoxycholic Acid (CDCA) Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koyama, S.; Sekijima, Y.; Ogura, M.; Hori, M.; Matsuki, K.; Miida, T.; Harada-Shiba, M. Cerebrotendinous Xanthomatosis: Molecular Pathogenesis, Clinical Spectrum, Diagnosis, and Disease-Modifying Treatments. J. Atheroscler. Thromb. 2021, 28, 905–925. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I. Cerebrotendinous xanthomatosis. Curr. Opin. Lipidol. 2013, 24, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Leitersdorf, E.; Meiner, V. Cerebrotendinous xanthomatosis. Curr. Opin. Lipidol. 1994, 5, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Online Mendelian Inheritance in Man, Cerebrotendinous Xanthomatosis; CTX (#213700). Available online: https://www.omim.org/entry/213700 (accessed on 15 February 2023).
- Online Mendelian Inheritance in Man, Cytochrome P450, Subfamily XXVIIA, Polypeptide 1; CYP27A1 (*606530). Available online: https://www.omim.org/entry/606530 (accessed on 15 February 2023).
- Federico, A.; Gallus, G.N. Cerebrotendinous Xanthomatosis. In GeneReviews® [Internet]; Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1409/ (accessed on 1 February 2023).
- Islam, M.; Hoggard, N.; Hadjivassiliou, M. Cerebrotendinous Xanthomatosis: Diversity of presentation and refining treatment with chenodeoxycholic acid. Cerebellum Ataxias 2021, 8, 5. [Google Scholar] [CrossRef]
- Brlek, P.; Antičević, D.; Molnar, V.; Matišić, V.; Robinson, K.; Aradhya, S.; Krpan, D.; Primorac, D. X-Linked Osteogenesis Imperfecta Possibly Caused by a Novel Variant in PLS3. Genes 2021, 12, 1851. [Google Scholar] [CrossRef]
- Garuti, R.; Croce, M.A.; Tiozzo, R.; Dotti, M.T.; Federico, A.; Bertolini, S.; Calandra, S. Four novel mutations of sterol 27-hydroxylase gene in Italian patients with cerebrotendinous xanthomatosis. J. Lipid Res. 1997, 38, 2322–2334. [Google Scholar] [CrossRef]
- Nie, S.; Chen, G.; Cao, X.; Zhang, Y. Cerebrotendinous xanthomatosis: A comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet J. Rare Dis. 2014, 9, 179. [Google Scholar] [CrossRef]
- Li, Z.R.; Zhou, Y.L.; Jin, Q.; Xie, Y.Y.; Meng, H.M. CYP27A1 mutation in a case of cerebrotendinous xanthomatosis: A case report. World J. Clin. Cases 2022, 10, 6168–6174. [Google Scholar] [CrossRef]
- Iser, J.H.; Sali, A. Chenodeoxycholic acid: A review of its pharmacological properties and therapeutic use. Drugs 1981, 21, 90–119. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 2020, 4, 47–63. [Google Scholar] [CrossRef]
- Zurkinden, L.; Sviridov, D.; Vogt, B.; Escher, G. Downregulation of Cyp7a1 by Cholic Acid and Chenodeoxycholic Acid in Cyp27a1/ApoE Double Knockout Mice: Differential Cardiovascular Outcome. Front. Endocrinol. 2020, 11, 586980. [Google Scholar] [CrossRef]
- Tint, G.S.; Ginsberg, H.; Salen, G.; Le, N.A.; Shefer, S. Chenodeoxycholic acid normalizes elevated lipoprotein secretion and catabolism in cerebrotendinous xanthomatosis. J. Lipid Res. 1989, 30, 633–640. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Vega, G.L.; East, C.; Richards, G.; Grundy, S.M. Low-density lipoprotein metabolism in cerebrotendinous xanthomatosis. Metabolism 1987, 36, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kuwabara, S.; Sakakibara, R.; Oki, T.; Arai, H.; Oda, S.; Hattori, T. Combined treatment with LDL-apheresis, chenodeoxycholic acid and HMG-CoA reductase inhibitor for cerebrotendinous xanthomatosis. J. Neurol. Sci. 2003, 216, 179–182. [Google Scholar] [CrossRef]
- Lumbreras, S.; Ricobaraza, A.; Baila-Rueda, L.; Gonzalez-Aparicio, M.; Mora-Jimenez, L.; Uriarte, I.; Bunuales, M.; Avila, M.A.; Monte, M.J.; Marin, J.J.; et al. Gene supplementation of CYP27A1 in the liver restores bile acid metabolism in a mouse model of cerebrotendinous xanthomatosis. Mol. Ther.-Methods Clin. Dev. 2021, 22, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Shen, Q.; Zhang, L.H.; Okumura, M.; Kawakami, A.; Ambrose, J.; Sigoillot, F.; Miller, H.R.; Gleim, S.; Cobos-Correa, A.; et al. CYP27A1-dependent anti-melanoma activity of limonoid natural products targets mitochondrial metabolism. Cell Chem. Biol. 2021, 28, 1407–1419.e6. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Jiao, W.; Wang, L.; Chen, Y.; Li, D.; Zhang, Z.; Zhang, Z.; Liang, Y.; Niu, H. CYP27A1 inhibits proliferation and migration of clear cell renal cell carcinoma via activation of LXRs/ABCA1. Exp. Cell Res. 2022, 419, 113279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, X.; Dai, J.; Sun, G.; Zhang, H.; Liang, J.; Zhao, J.; Zhu, S.; Chen, J.; Zhu, X.; et al. The tumor-repressing effect of CYP27A1 on renal cell carcinoma by 27-HC arising from cholesterol metabolism. FASEB J. 2022, 36, e22499. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, Y.; Wang, L.; Li, D.; Yang, X.; Ma, G.; Wang, Y.; Li, Y.; Zhao, H.; Liang, Y.; et al. CYP27A1 inhibits bladder cancer cells proliferation by regulating cholesterol homeostasis. Cell Cycle. 2019, 18, 34–45. [Google Scholar] [CrossRef]
- Brlek, P.; Kafka, A.; Bukovac, A.; Pećina-Šlaus, N. Integrative cBioPortal Analysis Revealed Molecular Mechanisms That Regulate EGFR-PI3K-AKT-mTOR Pathway in Diffuse Gliomas of the Brain. Cancers 2021, 13, 3247. [Google Scholar] [CrossRef]
- DeBarber, A.E.; Luo, J.; Star-Weinstock, M.; Purkayastha, S.; Geraghty, M.T.; Merkens, L.S.; Pappu, A.S.; Steiner, R.D. A blood test for cerebrotendinous xanthomatosis with potential for disease detection in newborns. J. Lipid Res. 2014, 55, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Daiker, J.; Sadilek, M.; DeBarber, A.E.; Chiang, J.; Duan, J.; Bootsma, A.H.; Huidekoper, H.H.; Vaz, F.M.; Gelb, M.H. Toward newborn screening of cerebrotendinous xanthomatosis: Results of a biomarker research study using 32,000 newborn dried blood spots. Genet. Med. 2020, 22, 1606–1612. [Google Scholar] [CrossRef]
- Vaz, F.M.; Bootsma, A.H.; Kulik, W.; Verrips, A.; Wevers, R.A.; Schielen, P.C.; DeBarber, A.E.; Huidekoper, H.H. A newborn screening method for cerebrotendinous xanthomatosis using bile alcohol glucuronides and metabolite ratios. J. Lipid Res. 2017, 58, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.M. Genetic Counseling and Genome Sequencing in Pediatric Rare Disease. Cold Spring Harb. Perspect. Med. 2020, 10, a036632. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, N.; Thiruvadi, V.; Paranthakan, C.; Rekha, A.; Magesh, A.; Mahadevan, N.; Magesh, M.A., Sr. Cerebrotendinous Xanthomatosis: Report of Two Siblings with the Same Mutation but Variable Presentation. Cureus 2023, 15, e33378. [Google Scholar] [CrossRef] [PubMed]


| Time since Treatment Initiation | Before Treatment | 3 Months | 6 Months | |||
|---|---|---|---|---|---|---|
| Characteristic | Patient 1 | Patient 2 | Patient 1 | Patient 2 | Patient 1 | Patient 2 |
| Epilepsy | + | − | + | − | + | − |
| Bilateral early onset cataracts | + | + | NA | NA | NA | NA |
| Thoracic kyphosis | + | + | + | + | + | + |
| Ataxia | + | + | Improved | Improved | Significantly improved | Significantly improved |
| Paraparesis | + | + | Improved | Improved | Significantly improved | Significantly improved |
| Dysarthria | + | + | Improved | Improved | Improved | Improved |
| Poor sphincter control | − | + | − | Improved | − | Improved |
| Diarrhea | + | + | Improved | Improved | Significantly improved | Significantly improved |
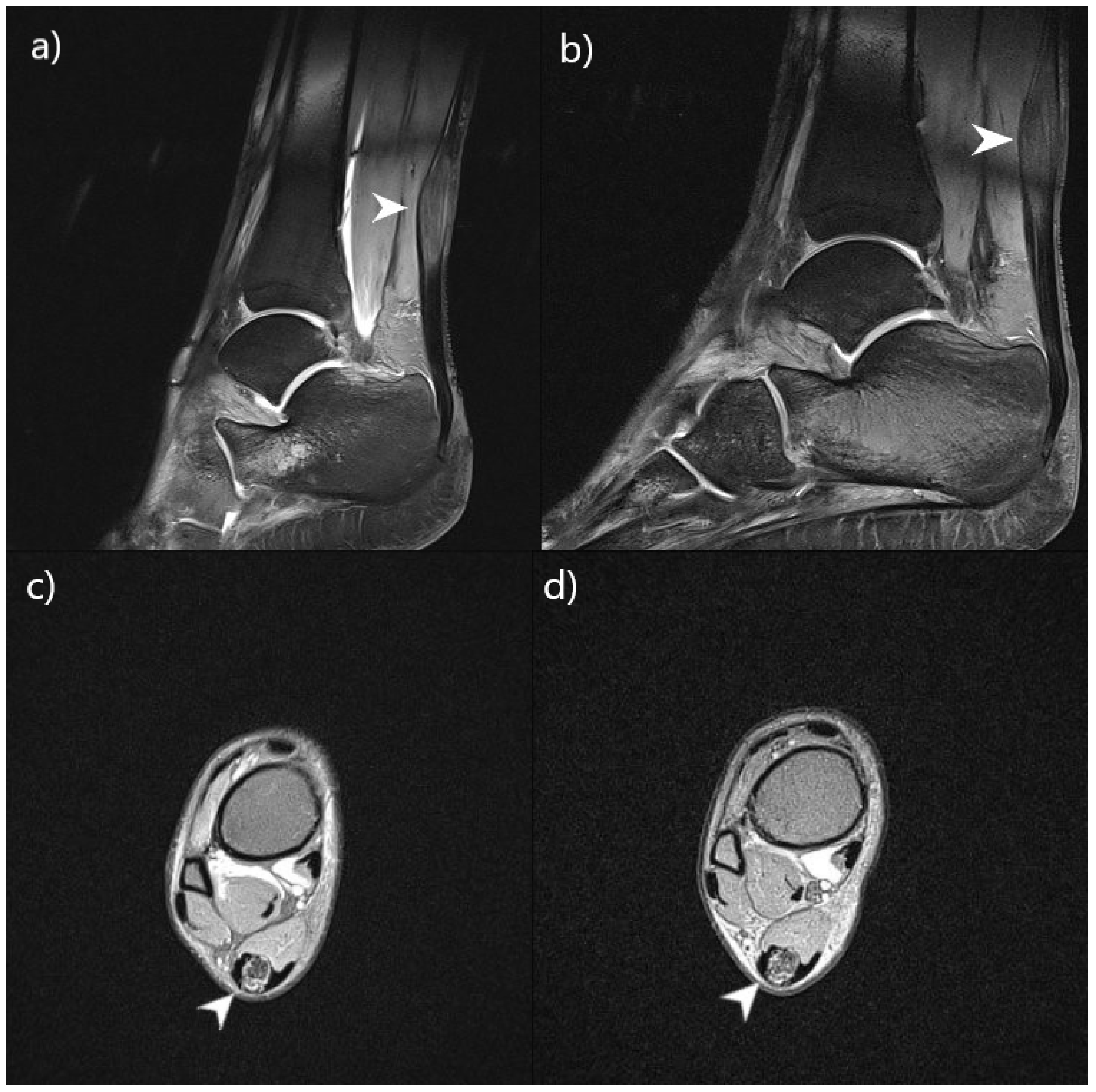
| Central nervous system abnormalities on MRI | + | + | / | / | Without progression | Without progression |
| Achilles tendon abnormalities on MRI | + | + | / | / | Without progression | Without progression |
| Cholestanol (<20.0 µmol/L) | 139.6 (H) | 140.1 (H) | 42.5 (H) | 51.2 (H) | 10.1 (N) | 8.8 (N) |
| Total Serum cholesterol (2.0–5.0 mmol/L) | 4.5 (N) | 3.8 (N) | 6.5 (H) | 6.4 (H) | / | / |
| Lathosterol (<10.0 µmol/L) | 17.5 (H) | 21.6 (H) | 3.5 (N) | 4.6 (N) | / | / |
| 7-Dehydrocholesterol (<5.0 µmol/L) | 71.1 (H) | 58.3 (H) | 2.5 (N) | 2.3 (N) | / | / |
| ALT (12–48 U/L) | 16 (N) | 12 (N) | 22 (N) | 31 (N) | 25 (N) | 48 (N) |
| AST (11–38 U/L) | 18 (N) | 19 (N) | 20 (N) | 27 (N) | 22 (N) | 29 (N) |
| GGT (11–55 U/L) | 14 (N) | 10 (N) | 20 (N) | / | 19 (N) | 16 (N) |
| Gene | Variant | Zygosity | Variant Classification |
|---|---|---|---|
| PATIENT 1 | |||
| CYP27A1 | c.1184+1G>A (donor splice site) | Homozygous | Pathogenic |
| AGL | c.1028G>A (p.Arg343Gln) | Heterozygous | VUS |
| AMPD1 | c.133C>T (p.Gln45 *) | Heterozygous | VUS |
| CASQ1 | c.280-3_280-2del (splice site) | Heterozygous | VUS |
| CAV3 | c.449A>T (p.Glu150Val) | Heterozygous | VUS |
| COL12A1 | c.4049C>A (p.Pro1350His) | Heterozygous | VUS |
| ITGA7 | c.1601G>A (p.Arg534Gln) | Heterozygous | VUS |
| SYNE2 | c.6328C>T (p.Pro2110Ser) | Heterozygous | VUS |
| PATIENT 2 | |||
| CYP27A1 | c.1184+1G>A (donor splice site) | Homozygous | Pathogenic |
| AMPD1 | c.133C>T (p.Gln45 *) | Heterozygous | VUS |
| COL12A1 | c.4049C>A (p.Pro1350His) | Heterozygous | VUS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brlek, P.; Bulić, L.; Glavaš Weinberger, D.; Bošnjak, J.; Pavlović, T.; Tomić, S.; Krivdić Dupan, Z.; Borić, I.; Primorac, D. Successful Treatment of a Rare Cholesterol Homeostasis Disorder Due to CYP27A1 Gene Mutation with Chenodeoxycholic Acid Therapy. Biomedicines 2023, 11, 1430. https://doi.org/10.3390/biomedicines11051430
Brlek P, Bulić L, Glavaš Weinberger D, Bošnjak J, Pavlović T, Tomić S, Krivdić Dupan Z, Borić I, Primorac D. Successful Treatment of a Rare Cholesterol Homeostasis Disorder Due to CYP27A1 Gene Mutation with Chenodeoxycholic Acid Therapy. Biomedicines. 2023; 11(5):1430. https://doi.org/10.3390/biomedicines11051430
Chicago/Turabian StyleBrlek, Petar, Luka Bulić, David Glavaš Weinberger, Jelena Bošnjak, Tomislav Pavlović, Svetlana Tomić, Zdravka Krivdić Dupan, Igor Borić, and Dragan Primorac. 2023. "Successful Treatment of a Rare Cholesterol Homeostasis Disorder Due to CYP27A1 Gene Mutation with Chenodeoxycholic Acid Therapy" Biomedicines 11, no. 5: 1430. https://doi.org/10.3390/biomedicines11051430
APA StyleBrlek, P., Bulić, L., Glavaš Weinberger, D., Bošnjak, J., Pavlović, T., Tomić, S., Krivdić Dupan, Z., Borić, I., & Primorac, D. (2023). Successful Treatment of a Rare Cholesterol Homeostasis Disorder Due to CYP27A1 Gene Mutation with Chenodeoxycholic Acid Therapy. Biomedicines, 11(5), 1430. https://doi.org/10.3390/biomedicines11051430











