Nicotine Administration Augments Abdominal Aortic Aneurysm Progression in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Experimental Animals, Housing, and Husbandry
2.3. Study Design
2.4. Porcine Pancreatic- Elastase-Induced AAA Model
2.5. Nicotine Preparation and Dosage
2.6. Measuring AAA Progression with Ultrasound Imaging
2.7. Euthanization and Tissue Preparation
2.8. Histological and Immunohistochemical Analyses
2.9. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
2.10. Zymography
2.11. Proteomic Analysis
2.12. Statistical Analyses
3. Results
3.1. Nicotine Treatment Promoted AAA Progression
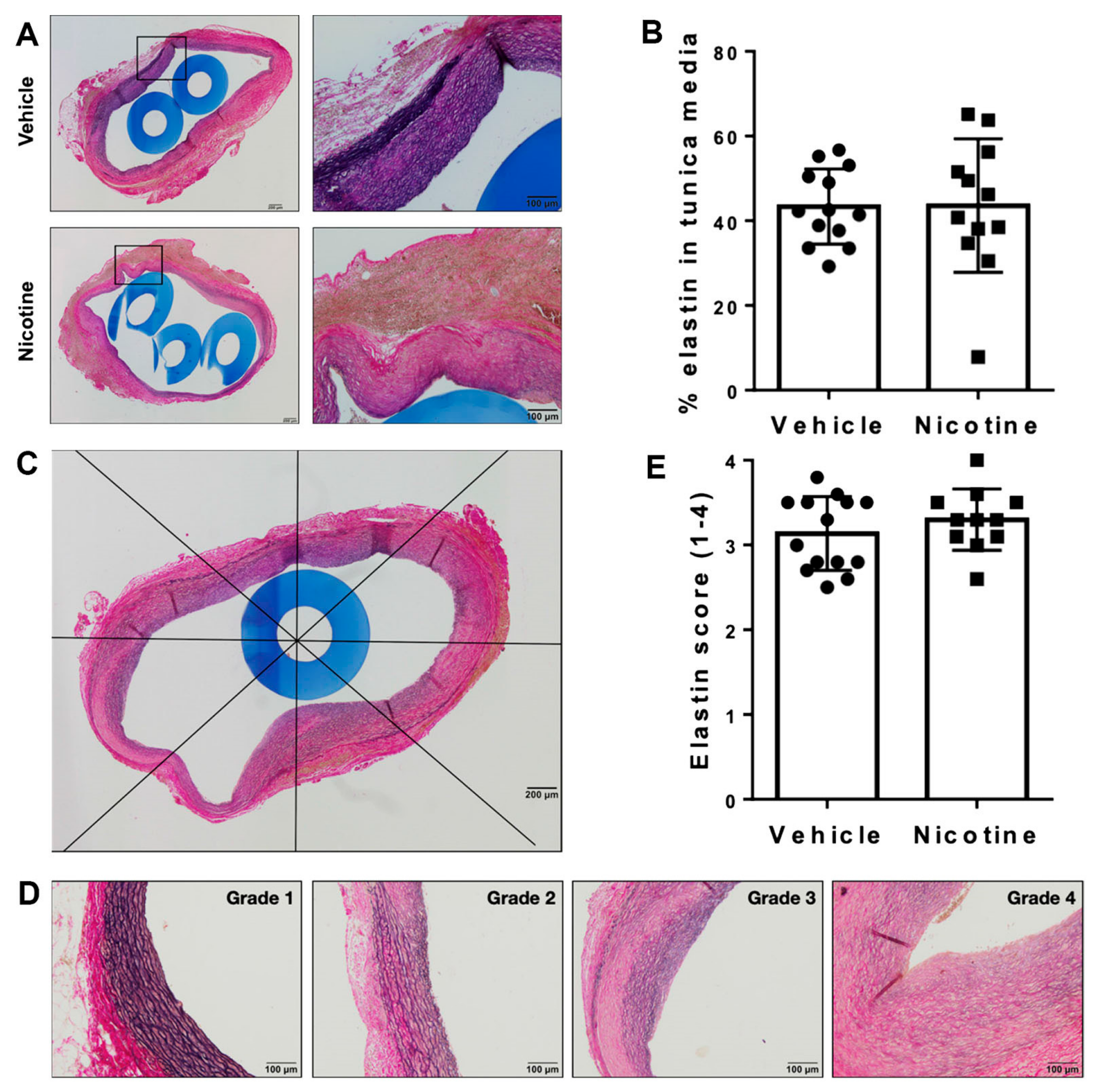
3.2. Nicotine Treatment Did Not Alter the Elastin Content or Architecture in Tunica Media
3.3. Nicotine Treatment Suppressed Pro-MMP2 and MMP9 Activity
3.4. Nicotine Treatment Failed to Exert Immunoregulatory Effects on Infiltration of Inflammatory Cells
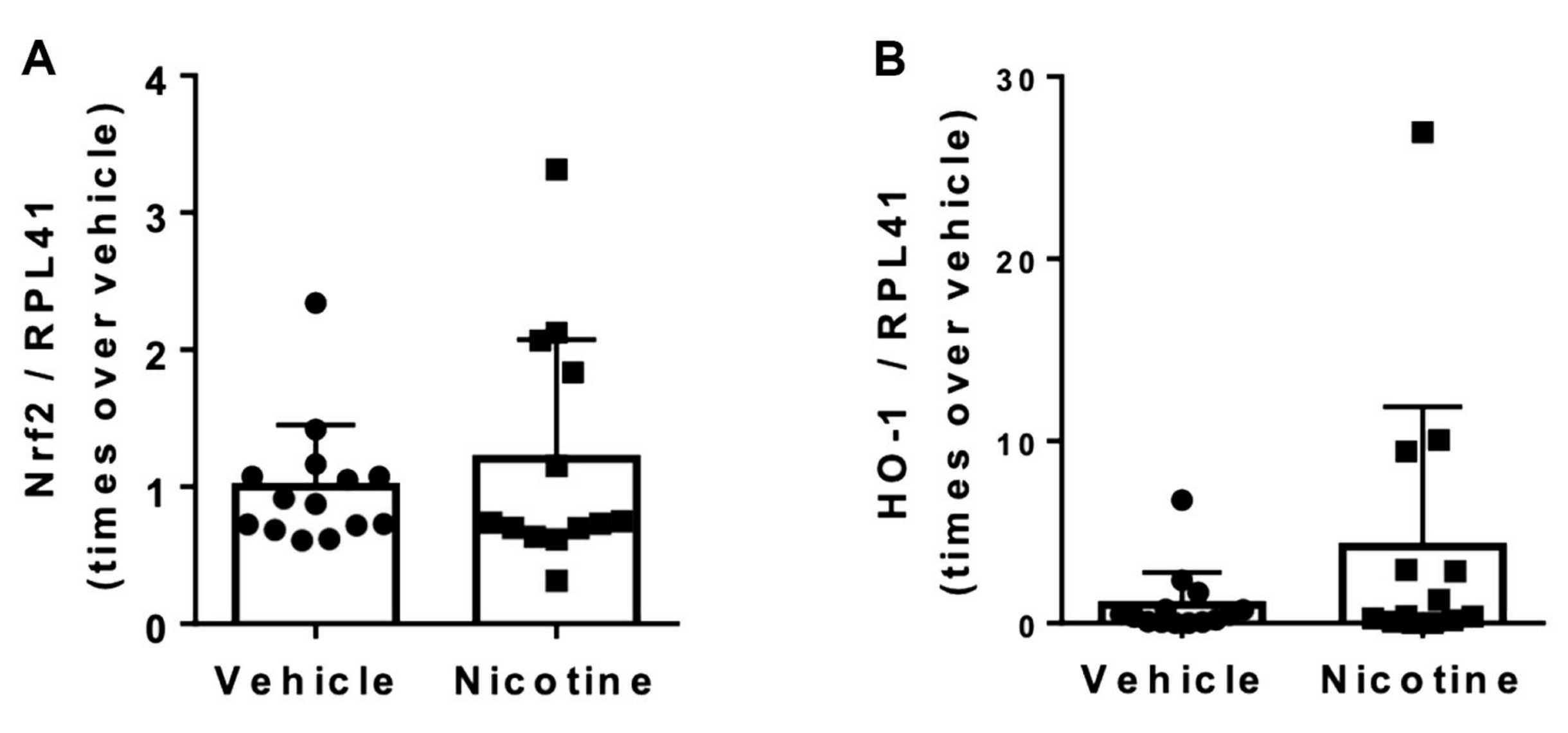
3.5. Nicotine Treatment Did Not Reduce Oxidative Stress
3.6. Nicotine Treatment Did Not Affect Vascular Smooth Muscle Cells
3.7. Proteomic Analyses of Abdominal Aorta Reveal That Nicotine Treatment Reduced Inflammatory Response and Cellular Response to Reactive Oxygen Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakalihasan, N.; Michel, J.B.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.O.; Nchimi, A.; Powell, J.T.; Yoshimura, K.; Hultgren, R. Abdominal aortic aneurysms. Nat. Rev. Dis. Prim. 2018, 4, 34. [Google Scholar] [CrossRef]
- Kvist, T.V.; Lindholt, J.S.; Rasmussen, L.M.; Søgaard, R.; Lambrechtsen, J.; Steffensen, F.H.; Frost, L.; Olsen, M.H.; Mickley, H.; Hallas, J.; et al. The DanCavas Pilot Study of Multifaceted Screening for Subclinical Cardiovascular Disease in Men and Women Aged 65–74 Years. Eur. J. Vasc. EndoVasc. Surg. 2017, 53, 123–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, L.; Bu, X.; Wang, X.; Liu, J.; Ma, A.; Wang, T. Global Burden of Aortic Aneurysm and Attributable Risk Factors from 1990 to 2017. Glob. Heart 2021, 16, 35. [Google Scholar] [CrossRef]
- Kent, K.C. Clinical practice. Abdominal aortic aneurysms. N. Engl. J. Med. 2014, 371, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ulug, P.; Powell, J.T.; Martinez, M.A.; Ballard, D.J.; Filardo, G. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst. Rev. 2020, 7, CD001835. [Google Scholar] [CrossRef]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef]
- Yuan, Z.; Lu, Y.; Wei, J.; Wu, J.; Yang, J.; Cai, Z. Abdominal Aortic Aneurysm: Roles of Inflammatory Cells. Front. Immunol. 2020, 11, 609161. [Google Scholar] [CrossRef]
- Liu, B.; Granville, D.J.; Golledge, J.; Kassiri, Z. Pathogenic mechanisms and the potential of drug therapies for aortic aneurysm. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H652–H670. [Google Scholar] [CrossRef]
- Eagleton, M.J. Inflammation in abdominal aortic aneurysms: Cellular infiltrate and cytokine profiles. Vascular 2012, 20, 278–283. [Google Scholar] [CrossRef]
- Emeto, T.I.; Moxon, J.V.; Au, M.; Golledge, J. Oxidative stress and abdominal aortic aneurysm: Potential treatment targets. Clin. Sci. 2016, 130, 301–315. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Aortic Wall Inflammation Predicts Abdominal Aortic Aneurysm Expansion, Rupture, and Need for Surgical Repair. Circulation 2017, 136, 787–797. [CrossRef]
- Ullery, B.W.; Hallett, R.L.; Fleischmann, D. Epidemiology and contemporary management of abdominal aortic aneurysms. Abdom. Radiol. 2018, 43, 1032–1043. [Google Scholar] [CrossRef]
- Han, X.; Li, W.; Li, P.; Zheng, Z.; Lin, B.; Zhou, B.; Guo, K.; He, P.; Yang, J. Stimulation of α7 Nicotinic Acetylcholine Receptor by Nicotine Suppresses Decidual M1 Macrophage Polarization Against Inflammation in Lipopolysaccharide-Induced Preeclampsia-Like Mouse Model. Front. Immunol. 2021, 12, 642071. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Q.; Gui, H.; Xu, D.P.; Yang, Y.L.; Su, D.F.; Liu, X. MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of pro-inflammatory cytokines. Cell Res. 2013, 23, 1270–1283. [Google Scholar] [CrossRef][Green Version]
- Qin, Z.; Xiang, K.; Su, D.F.; Sun, Y.; Liu, X. Activation of the Cholinergic Anti-Inflammatory Pathway as a Novel Therapeutic Strategy for COVID-19. Front. Immunol. 2020, 11, 595342. [Google Scholar] [CrossRef]
- Bencherif, M.; Lippiello, P.M.; Lucas, R.; Marrero, M.B. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol. Life Sci. 2011, 68, 931–949. [Google Scholar] [CrossRef][Green Version]
- Van Maanen, M.A.; Lebre, M.C.; van der Poll, T.; LaRosa, G.J.; Elbaum, D.; Vervoordeldonk, M.J.; Tak, P.P. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009, 60, 114–122. [Google Scholar] [CrossRef]
- Bai, A.; Guo, Y.; Lu, N. The effect of the cholinergic anti-inflammatory pathway on experimental colitis. Scand. J. Immunol. 2007, 66, 538–545. [Google Scholar] [CrossRef]
- Parrish, W.R.; Rosas-Ballina, M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Yang, L.H.; Hudson, L.; Lin, X.; Patel, N.; Johnson, S.M.; et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med. 2008, 14, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Shen, Q.R.; Zhao, Y.; Ni, M.; Zhou, C.C.; Chen, J.K.; Chi, C.; Li, D.J.; Liang, G.; Shen, F.M. Activating α7nAChR ameliorates abdominal aortic aneurysm through inhibiting pyroptosis mediated by NLRP3 inflammasome. Acta Pharmacol. Sin. 2022, 43, 2585–2595. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Wang, L.; Ji, C.F.; Gu, S.F.; Yin, Q.; Zuo, J. The Role of α7nAChR-Mediated Cholinergic Anti-inflammatory Pathway in Immune Cells. Inflammation 2021, 44, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Yeh, H.I. Nicotine: A Double-Edged Sword in Atherosclerotic Disease. Acta Cardiol. Sin. 2014, 30, 108–113. [Google Scholar]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Kuivaniemi, H.; Ryer, E.J.; Elmore, J.R.; Tromp, G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev. CardioVasc. Ther. 2015, 13, 975–987. [Google Scholar] [CrossRef][Green Version]
- Howard, D.P.; Banerjee, A.; Fairhead, J.F.; Handa, A.; Silver, L.E.; Rothwell, P.M. Population-Based Study of Incidence of Acute Abdominal Aortic Aneurysms With Projected Impact of Screening Strategy. J. Am. Heart Assoc. 2015, 4, e001926. [Google Scholar] [CrossRef][Green Version]
- Johnston, W.F.; Salmon, M.; Su, G.; Lu, G.; Ailawadi, G.; Upchurch, G.R., Jr. Aromatase is required for female abdominal aortic aneurysm protection. J. Vasc. Surg. 2015, 61, 1565–1574.e4. [Google Scholar] [CrossRef][Green Version]
- Ailawadi, G.; Eliason, J.L.; Roelofs, K.J.; Sinha, I.; Hannawa, K.K.; Kaldjian, E.P.; Lu, G.; Henke, P.K.; Stanley, J.C.; Weiss, S.J.; et al. Gender differences in experimental aortic aneurysm formation. Arter. Thromb. Vasc. Biol. 2004, 24, 2116–2122. [Google Scholar] [CrossRef][Green Version]
- Melin, L.G.; Dall, J.H.; Lindholt, J.S.; Steffensen, L.B.; Beck, H.C.; Elkrog, S.L.; Clausen, P.D.; Rasmussen, L.M.; Stubbe, J. Cycloastragenol Inhibits Experimental Abdominal Aortic Aneurysm Progression. Biomedicines 2022, 10, 359. [Google Scholar] [CrossRef]
- Abelson, K.S.; Jacobsen, K.R.; Sundbom, R.; Kalliokoski, O.; Hau, J. Voluntary ingestion of nut paste for administration of buprenorphine in rats and mice. Lab. Anim. 2012, 46, 349–351. [Google Scholar] [CrossRef][Green Version]
- Matta, S.G.; Balfour, D.J.; Benowitz, N.L.; Boyd, R.T.; Buccafusco, J.J.; Caggiula, A.R.; Craig, C.R.; Collins, A.C.; Damaj, M.I.; Donny, E.C.; et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 2007, 190, 269–319. [Google Scholar] [CrossRef]
- Steffensen, L.B.; Stubbe, J.; Lindholt, J.S.; Beck, H.C.; Overgaard, M.; Bloksgaard, M.; Genovese, F.; Holm Nielsen, S.; Tha, M.L.T.; Bang-Moeller, S.K.; et al. Basement membrane collagen IV deficiency promotes abdominal aortic aneurysm formation. Sci. Rep. 2021, 11, 12903. [Google Scholar] [CrossRef]
- Mulorz, J.; Spin, J.M.; Beck, H.C.; Tha Thi, M.L.; Wagenhäuser, M.U.; Rasmussen, L.M.; Lindholt, J.S.; Tsao, P.S.C.; Steffensen, L.B. Hyperlipidemia does not affect development of elastase-induced abdominal aortic aneurysm in mice. Atherosclerosis 2020, 311, 73–83. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Omotade, O.F.; Rui, Y.; Lei, W.; Yu, K.; Hartzell, H.C.; Fowler, V.M.; Zheng, J.Q. Tropomodulin Isoform-Specific Regulation of Dendrite Development and Synapse Formation. J. Neurosci. 2018, 38, 10271–10285. [Google Scholar] [CrossRef][Green Version]
- De Jonge, W.J.; Ulloa, L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 2007, 151, 915–929. [Google Scholar] [CrossRef][Green Version]
- Watanabe, A.; Ichiki, T.; Kojima, H.; Takahara, Y.; Hurt-Camejo, E.; Michaëlsson, E.; Sankoda, C.; Ikeda, J.; Inoue, E.; Tokunou, T.; et al. Suppression of abdominal aortic aneurysm formation by AR-R17779, an agonist for the α7 nicotinic acetylcholine receptor. Atherosclerosis 2016, 244, 113–120. [Google Scholar] [CrossRef]
- Obel, L.M.; Diederichsen, A.C.; Steffensen, F.H.; Frost, L.; Lambrechtsen, J.; Busk, M.; Urbonaviciene, G.; Egstrup, K.; Karon, M.; Rasmussen, L.M.; et al. Population-Based Risk Factors for Ascending, Arch, Descending, and Abdominal Aortic Dilations for 60-74-Year-Old Individuals. J. Am. Coll. Cardiol. 2021, 78, 201–211. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Kurokawa, M.; Ozaki, N.; Nara, K.; Atou, K.; Takada, E.; Kamochi, H.; Suzuki, N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin. Exp. Immunol. 2006, 146, 116–123. [Google Scholar] [CrossRef]
- Sharma, A.K.; Lu, G.; Jester, A.; Johnston, W.F.; Zhao, Y.; Hajzus, V.A.; Saadatzadeh, M.R.; Su, G.; Bhamidipati, C.M.; Mehta, G.S.; et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation 2012, 126, S38–S45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Y.; Liu, J.; Li, L.; Liu, L.; Wu, L. Role of Ras/PKCzeta/MEK/ERK1/2 signaling pathway in angiotensin II-induced vascular smooth muscle cell proliferation. Regul. Pept. 2005, 128, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, S.; Chen, Z.; Okagaki, T.; Kohama, K.; Nasu-Kawaharada, R.; Izumi, T.; Ohshima, N.; Nagai, T.; Nakamura, A. Nicotine exposure alters human vascular smooth muscle cell phenotype from a contractile to a synthetic type. Atherosclerosis 2014, 237, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Makkar, G.; Strickland, D.K.; Blanpied, T.A.; Stumpo, D.J.; Blackshear, P.J.; Sarkar, R.; Monahan, T.S. Myristoylated Alanine-Rich Protein Kinase Substrate (MARCKS) Regulates Small GTPase Rac1 and Cdc42 Activity and Is a Critical Mediator of Vascular Smooth Muscle Cell Migration in Intimal Hyperplasia Formation. J. Am. Heart Assoc. 2015, 4, e002255. [Google Scholar] [CrossRef][Green Version]
- Yu, D.; Gernapudi, R.; Drucker, C.; Sarkar, R.; Ucuzian, A.; Monahan, T.S. The myristoylated alanine-rich C kinase substrate differentially regulates kinase interacting with stathmin in vascular smooth muscle and endothelial cells and potentiates intimal hyperplasia formation. J. Vasc. Surg. 2019, 70, 2021–2031.e2021. [Google Scholar] [CrossRef]
- Albrecht, I.; Bieri, R.; Leu, A.; Granacher, P.; Hagmann, J.; Kilimann, M.W.; Christofori, G. Paralemmin-1 is expressed in lymphatic endothelial cells and modulates cell migration, cell maturation and tumor lymphangiogenesis. Angiogenesis 2013, 16, 795–807. [Google Scholar] [CrossRef][Green Version]
- Csányi, G.; Singla, B. Arterial Lymphatics in Atherosclerosis: Old Questions, New Insights, and Remaining Challenges. J. Clin. Med. 2019, 8, 495. [Google Scholar] [CrossRef][Green Version]
- Ackers-Johnson, M.; Talasila, A.; Sage, A.P.; Long, X.; Bot, I.; Morrell, N.W.; Bennett, M.R.; Miano, J.M.; Sinha, S. Myocardin regulates vascular smooth muscle cell inflammatory activation and disease. Arter. Thromb. Vasc. Biol. 2015, 35, 817–828. [Google Scholar] [CrossRef][Green Version]
- Vieira-Alves, I.; Coimbra-Campos, L.M.C.; Sancho, M.; da Silva, R.F.; Cortes, S.F.; Lemos, V.S. Role of the α7 Nicotinic Acetylcholine Receptor in the Pathophysiology of Atherosclerosis. Front. Physiol. 2020, 11, 621769. [Google Scholar] [CrossRef]
- Fu, X.; Zong, T.; Yang, P.; Li, L.; Wang, S.; Wang, Z.; Li, M.; Li, X.; Zou, Y.; Zhang, Y.; et al. Nicotine: Regulatory roles and mechanisms in atherosclerosis progression. Food Chem. Toxicol. 2021, 151, 112154. [Google Scholar] [CrossRef]
- Ulleryd, M.A.; Mjörnstedt, F.; Panagaki, D.; Yang, L.J.; Engevall, K.; Gutiérrez, S.; Wang, Y.; Gan, L.M.; Nilsson, H.; Michaëlsson, E.; et al. Stimulation of alpha 7 nicotinic acetylcholine receptor (α7nAChR) inhibits atherosclerosis via immunomodulatory effects on myeloid cells. Atherosclerosis 2019, 287, 122–133. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ichiki, T.; Watanabe, A.; Hurt-Camejo, E.; Michaëlsson, E.; Ikeda, J.; Inoue, E.; Matsuura, H.; Tokunou, T.; Kitamoto, S.; et al. Stimulation of α7 nicotinic acetylcholine receptor by AR-R17779 suppresses atherosclerosis and aortic aneurysm formation in apolipoprotein E-deficient mice. Vasc. Pharmacol. 2014, 61, 49–55. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Zhu, W.; Xu, Y.; Liu, M.; Zhu, L.; Yang, F.; Zhang, L.; Liu, X.; Zhong, Z.; et al. Nicotine Accelerates Atherosclerosis in Apolipoprotein E-Deficient Mice by Activating α7 Nicotinic Acetylcholine Receptor on Mast Cells. Arter. Thromb. Vasc. Biol. 2017, 37, 53–65. [Google Scholar] [CrossRef][Green Version]
- Sénémaud, J.; Caligiuri, G.; Etienne, H.; Delbosc, S.; Michel, J.B.; Coscas, R. Translational Relevance and Recent Advances of Animal Models of Abdominal Aortic Aneurysm. Arter. Thromb. Vasc. Biol. 2017, 37, 401–410. [Google Scholar] [CrossRef][Green Version]
- Dale, M.A.; Xiong, W.; Carson, J.S.; Suh, M.K.; Karpisek, A.D.; Meisinger, T.M.; Casale, G.P.; Baxter, B.T. Elastin-Derived Peptides Promote Abdominal Aortic Aneurysm Formation by Modulating M1/M2 Macrophage Polarization. J. Immunol. 2016, 196, 4536–4543. [Google Scholar] [CrossRef][Green Version]
- Liu, C.; Zhou, M.S.; Li, Y.; Wang, A.; Chadipiralla, K.; Tian, R.; Raij, L. Oral nicotine aggravates endothelial dysfunction and vascular inflammation in diet-induced obese rats: Role of macrophage TNFα. PLoS ONE 2017, 12, e0188439. [Google Scholar] [CrossRef][Green Version]
- Li, Z.Z.; Dai, Q.Y. Pathogenesis of abdominal aortic aneurysms: Role of nicotine and nicotinic acetylcholine receptors. Mediat. Inflamm. 2012, 2012, 103120. [Google Scholar] [CrossRef][Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef][Green Version]
- Novotny, T.E.; Hardin, S.N.; Hovda, L.R.; Novotny, D.J.; McLean, M.K.; Khan, S. Tobacco and cigarette butt consumption in humans and animals. Tob. Control 2011, 20 (Suppl. S1), i17–i20. [Google Scholar] [CrossRef]
- Yeboah, M.M.; Xue, X.; Duan, B.; Ochani, M.; Tracey, K.J.; Susin, M.; Metz, C.N. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008, 74, 62–69. [Google Scholar] [CrossRef][Green Version]
- Park, J.; Kang, J.W.; Lee, S.M. Activation of the cholinergic anti-inflammatory pathway by nicotine attenuates hepatic ischemia/reperfusion injury via heme oxygenase-1 induction. Eur. J. Pharmacol. 2013, 707, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Burbank, A.D. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Báez-Pagán, C.A.; Delgado-Vélez, M.; Lasalde-Dominicci, J.A. Activation of the Macrophage α7 Nicotinic Acetylcholine Receptor and Control of Inflammation. J. Neuroimmune Pharmacol. 2015, 10, 468–476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grady, S.R.; Drenan, R.M.; Breining, S.R.; Yohannes, D.; Wageman, C.R.; Fedorov, N.B.; McKinney, S.; Whiteaker, P.; Bencherif, M.; Lester, H.A.; et al. Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors. Neuropharmacology 2010, 58, 1054–1066. [Google Scholar] [CrossRef][Green Version]
- Zou, Q.; Leung, S.W.; Vanhoutte, P.M. Activation of nicotinic receptors can contribute to endothelium-dependent relaxations to acetylcholine in the rat aorta. J. Pharmacol. Exp. Ther. 2012, 341, 756–763. [Google Scholar] [CrossRef][Green Version]
- Ren, A.; Wu, H.; Liu, L.; Guo, Z.; Cao, Q.; Dai, Q. Nicotine promotes atherosclerosis development in apolipoprotein E-deficient mice through α1-nAChR. J. Cell Physiol. 2018, 234, 14507–14518. [Google Scholar] [CrossRef]
- Wagenhäuser, M.U.; Schellinger, I.N.; Yoshino, T.; Toyama, K.; Kayama, Y.; Deng, A.; Guenther, S.P.; Petzold, A.; Mulorz, J.; Mulorz, P.; et al. Chronic Nicotine Exposure Induces Murine Aortic Remodeling and Stiffness Segmentation-Implications for Abdominal Aortic Aneurysm Susceptibility. Front. Physiol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Leite, P.E.; Lagrota-Candido, J.; Moraes, L.; D’Elia, L.; Pinheiro, D.F.; da Silva, R.F.; Yamasaki, E.N.; Quirico-Santos, T. Nicotinic acetylcholine receptor activation reduces skeletal muscle inflammation of mdx mice. J. NeuroImmunol. 2010, 227, 44–51. [Google Scholar] [CrossRef]
- Teng, P.; Liu, Y.; Dai, Y.; Zhang, H.; Liu, W.T.; Hu, J. Nicotine Attenuates Osteoarthritis Pain and Matrix Metalloproteinase-9 Expression via the α7 Nicotinic Acetylcholine Receptor. J. Immunol. 2019, 203, 485–492. [Google Scholar] [CrossRef]
- Lysgaard Poulsen, J.; Stubbe, J.; Lindholt, J.S. Animal Models Used to Explore Abdominal Aortic Aneurysms: A Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 487–499. [Google Scholar] [CrossRef][Green Version]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]






| Organ Weight/Body Weight (mg/g) | Vehicle (n = 14) | Nicotine (n = 13) | p-Value |
|---|---|---|---|
| Heart | 3.33 ± 0.38 | 3.27 ± 0.41 | 0.437 |
| Spleen | 2.75 ± 0.37 | 2.61 ± 0.64 | 0.375 |
| Lungs | 4.07 ±0.32 | 3.89 ± 0.16 | 0.099 |
| Kidneys | 6.16 ± 0.70 | 5.95 ± 0.67 | 0.437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadzikadunic, H.; Sjælland, T.B.; Lindholt, J.S.; Steffensen, L.B.; Beck, H.C.; Kavaliunaite, E.; Rasmussen, L.M.; Stubbe, J. Nicotine Administration Augments Abdominal Aortic Aneurysm Progression in Rats. Biomedicines 2023, 11, 1417. https://doi.org/10.3390/biomedicines11051417
Hadzikadunic H, Sjælland TB, Lindholt JS, Steffensen LB, Beck HC, Kavaliunaite E, Rasmussen LM, Stubbe J. Nicotine Administration Augments Abdominal Aortic Aneurysm Progression in Rats. Biomedicines. 2023; 11(5):1417. https://doi.org/10.3390/biomedicines11051417
Chicago/Turabian StyleHadzikadunic, Hana, Tea Bøvling Sjælland, Jes S. Lindholt, Lasse Bach Steffensen, Hans Christian Beck, Egle Kavaliunaite, Lars Melholt Rasmussen, and Jane Stubbe. 2023. "Nicotine Administration Augments Abdominal Aortic Aneurysm Progression in Rats" Biomedicines 11, no. 5: 1417. https://doi.org/10.3390/biomedicines11051417
APA StyleHadzikadunic, H., Sjælland, T. B., Lindholt, J. S., Steffensen, L. B., Beck, H. C., Kavaliunaite, E., Rasmussen, L. M., & Stubbe, J. (2023). Nicotine Administration Augments Abdominal Aortic Aneurysm Progression in Rats. Biomedicines, 11(5), 1417. https://doi.org/10.3390/biomedicines11051417







