Electrophysiological and Behavioral Effects of Alpha-Band Sensory Entrainment: Neural Mechanisms and Clinical Applications
Abstract
1. Alpha-Band Oscillations and Neural Entrainment Mechanisms
1.1. Alpha-Band Oscillations Shape Visual Perception
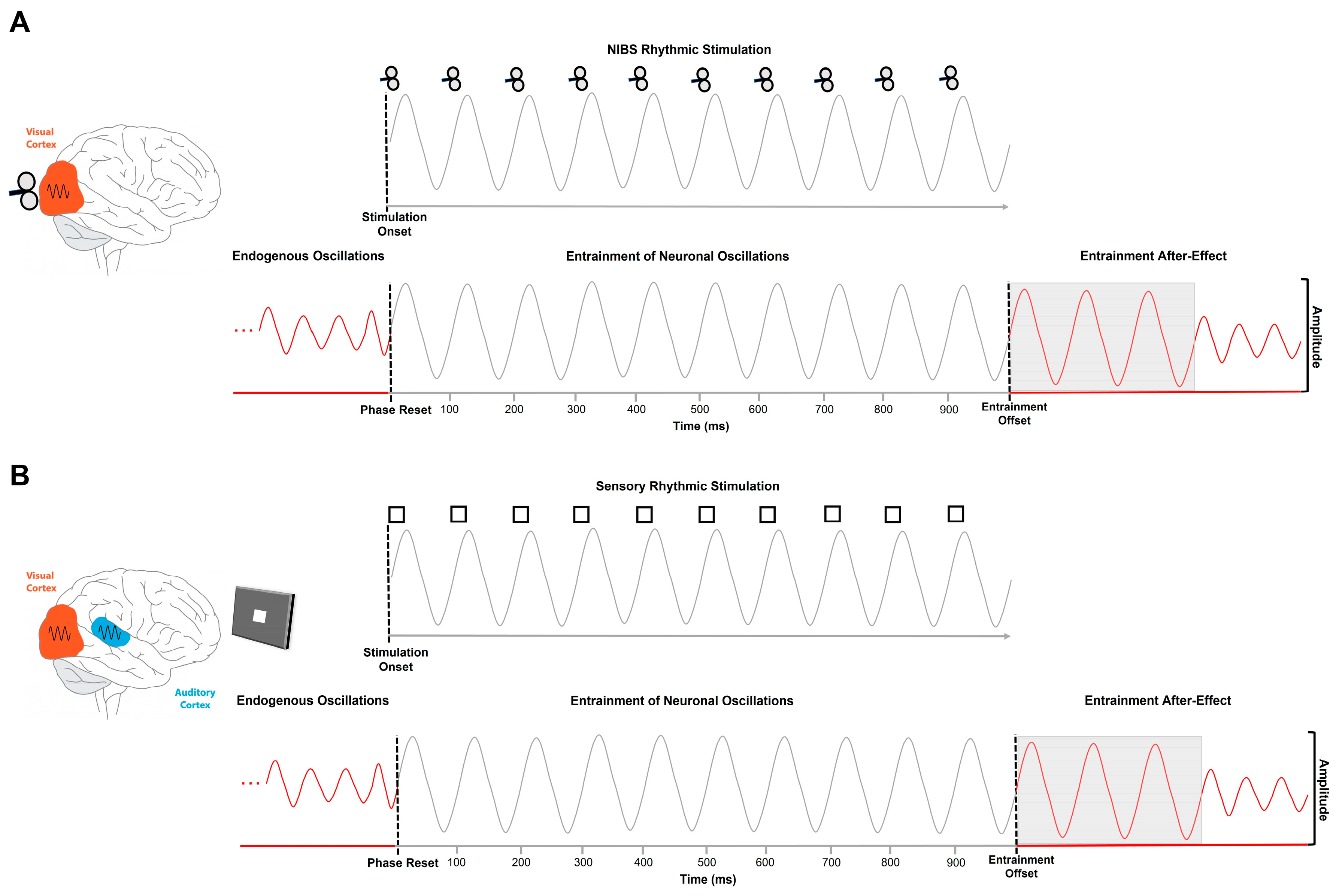
1.2. Entrainment Mechanisms of Alpha-Band Neuronal Oscillations
2. Physiological and Behavioral Effects of Alpha-Band Sensory Entrainment
2.1. Alpha-Band Visual Entrainment
2.2. Alpha-Band Auditory Entrainment
2.3. Alpha-Band Audiovisual Entrainment
2.4. Alpha-Band Sensory Entrainment: Current State-of-the-Art and Critical Aspects
3. Alpha-Band Sensory Entrainment: Long-Term Effects and Clinical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Osipova, D.; Hermes, D.; Jensen, O. Gamma Power Is Phase-Locked to Posterior Alpha Activity. PLoS ONE 2008, 3, e3990. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Mazaheri, A. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Voytek, B.; Canolty, R.T.; Shestyuk, A.; Crone, N.E.; Parvizi, J.; Knight, R.T. Shifts in gamma phase–amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Front. Hum. Neurosci. 2010, 4, 191. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Liu, Y.; Huang, H.; Ding, M. Coupling between visual alpha oscillations and default mode activity. Neuroimage 2013, 68, 112–118. [Google Scholar] [CrossRef]
- Jensen, O.; Gips, B.; Bergmann, T.O.; Bonnefond, M. Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci. 2014, 37, 357–369. [Google Scholar] [CrossRef]
- Van Diepen, R.M.; Miller, L.M.; Mazaheri, A.; Geng, J.J. The role of alpha activity in spatial and feature-based attention. eNeuro 2016, 3. [Google Scholar] [CrossRef]
- Limbach, K.; Corballis, P.M. Prestimulus alpha power influences response criterion in a detection task. Psychophysiology 2016, 53, 1154–1164. [Google Scholar] [CrossRef]
- Benwell, C.S.Y.; Keitel, C.; Harvey, M.; Gross, J.; Thut, G. Trial-by-trial co-variation of pre-stimulus EEG alpha power and visuospatial bias reflects a mixture of stochastic and deterministic effects. Eur. J. Neurosci. 2017, 48, 2566–2584. [Google Scholar] [CrossRef]
- Samaha, J.; Iemi, L.; Postle, B.R. Prestimulus alpha-band power biases visual discrimination confidence, but not accuracy. Conscious. Cogn. 2017, 54, 47–55. [Google Scholar] [CrossRef]
- Gallotto, S.; Duecker, F.; Oever, S.T.; Schuhmann, T.; de Graaf, T.A.; Sack, A. Relating alpha power modulations to competing visuospatial attention theories. Neuroimage 2019, 207, 116429. [Google Scholar] [CrossRef]
- Di Gregorio, F.; Trajkovic, J.; Roperti, C.; Marcantoni, E.; Di Luzio, P.; Avenanti, A.; Thut, G.; Romei, V. Tuning alpha rhythms to shape conscious visual perception. Curr. Biol. 2022, 32, 988–998.e6. [Google Scholar] [CrossRef]
- Trajkovic, J.; Di Gregorio, F.; Avenanti, A.; Thut, G.; Romei, V. Two oscillatory correlates of attention control in the alpha-band with distinct consequences on perceptual gain and metacognition. J. Neurosci. 2023, JN-RM-1827-22. [Google Scholar] [CrossRef]
- Romei, V.; Rihs, T.; Brodbeck, V.; Thut, G. Resting electroencephalogram alpha-power over posterior sites indexes baseline visual cortex excitability. Neuroreport 2008, 19, 203–208. [Google Scholar] [CrossRef]
- Mathewson, K.E.; Fabiani, M.; Gratton, G.; Beck, D.M.; Lleras, A. Rescuing stimuli from invisibility: Inducing a momentary release from visual masking with pre-target entrainment. Cognition 2010, 115, 186–191. [Google Scholar] [CrossRef]
- VanRullen, R. Perceptual cycles. Trends Cogn. Sci. 2016, 20, 723–735. [Google Scholar] [CrossRef]
- Sokoliuk, R.; VanRullen, R. The Flickering Wheel Illusion: When α Rhythms Make a Static Wheel Flicker. J. Neurosci. 2013, 33, 13498–13504. [Google Scholar] [CrossRef]
- Boncompte, G.; Villena-González, M.; Cosmelli, D.; López, V. Spontaneous alpha power lateralization predicts detection performance in an un-cued signal detection task. PLoS ONE 2016, 11, e0160347. [Google Scholar] [CrossRef]
- Sadaghiani, S.; Scheeringa, R.; Lehongre, K.; Morillon, B.; Giraud, A.L.; Kleinschmidt, A. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: A simultaneous electroencephalography/functional magnetic resonance imaging study. J. Neurosci. 2010, 30, 10243–10250. [Google Scholar] [CrossRef]
- Morillon, B.; Schroeder, C.E. Neuronal oscillations as a mechanistic substrate of auditory temporal prediction. Ann. N. Y. Acad. Sci. 2015, 1337, 26–31. [Google Scholar] [CrossRef]
- Iemi, L.; Chaumon, M.; Crouzet, S.M.; Busch, N.A. Spontaneous Neural Oscillations Bias Perception by Modulating Baseline Excitability. J. Neurosci. 2016, 37, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.; Noë, A. Neural Plasticity and Consciousness. Biol. Philos. 2003, 18, 131–168. [Google Scholar] [CrossRef]
- Espinosa, J.S.; Stryker, M.P. Development and Plasticity of the Primary Visual Cortex. Neuron 2012, 75, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Vossen, A.; Gross, J.; Thut, G. Alpha power increase after transcranial alternating current stimulation at alpha frequency (α-tACS) reflects plastic changes rather than entrainment. Brain Stimul. 2015, 8, 499–508. [Google Scholar] [CrossRef]
- Galuske, R.A.W.; Munk, M.H.J.; Singer, W. Relation between gamma oscillations and neuronal plasticity in the visual cortex. Proc. Natl. Acad. Sci. USA 2019, 116, 23317–23325. [Google Scholar] [CrossRef]
- Grasso, P.A.; Gallina, J.; Bertini, C. Shaping the visual system: Cortical and subcortical plasticity in the intact and the lesioned brain. Neuropsychologia 2020, 142, 107–464. [Google Scholar] [CrossRef]
- Vogeti, S.; Boetzel, C.; Herrmann, C.S. Entrainment and Spike-Timing Dependent Plasticity—A Review of Proposed Mechanisms of Transcranial Alternating Current Stimulation. Front. Syst. Neurosci. 2022, 16, 827353. [Google Scholar] [CrossRef]
- Thut, G.; Veniero, D.; Romei, V.; Miniussi, C.; Schyns, P.; Gross, J. Rhythmic TMS Causes Local Entrainment of Natural Oscillatory Signatures. Curr. Biol. 2011, 21, 1176–1185. [Google Scholar] [CrossRef]
- Lin, Y.J.; Shukla, L.; Dugué, L.; Valero-Cabré, A.; Carrasco, M. Transcranial magnetic stimulation entrains alpha oscillatory activity in occipital cortex. Sci. Rep. 2021, 11, 18562. [Google Scholar] [CrossRef]
- Coldea, A.; Veniero, D.; Morand, S.; Trajkovic, J.; Romei, V.; Harvey, M.; Thut, G. Effects of Rhythmic Transcranial Magnetic Stimulation in the Alpha-Band on Visual Perception Depend on Deviation from Alpha-Peak Frequency: Faster Relative Transcranial Magnetic Stimulation Alpha-Pace Improves Performance. Front. Neurosci. 2022, 16, 886342. [Google Scholar] [CrossRef]
- Zaehle, T.; Rach, S.; Herrmann, C.S. Transcranial Alternating Current Stimulation Enhances Individual Alpha Activity in Human EEG. PLoS ONE 2010, 5, e13766. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Schneider, T.R.; Rach, S.; Trautmann-Lengsfeld, S.A.; Engel, A.K.; Herrmann, C.S. Entrainment of Brain Oscillations by Transcranial Alternating Current Stimulation. Curr. Biol. 2014, 24, 333–339. [Google Scholar] [CrossRef]
- Minami, S.; Amano, K. Illusory Jitter Perceived at the Frequency of Alpha Oscillations. Curr. Biol. 2017, 27, 2344–2351.e4. [Google Scholar] [CrossRef]
- Borghini, G.; Candini, M.; Filannino, C.; Hussain, M.; Walsh, V.; Romei, V.; Zokaei, N.; Cappelletti, M. Alpha Oscillations Are Causally Linked to Inhibitory Abilities in Ageing. J. Neurosci. 2018, 38, 4418–4429. [Google Scholar] [CrossRef]
- Wolinski, N.; Cooper, N.R.; Sauseng, P.; Romei, V. The speed of parietal theta frequency drives visuospatial working memory capacity. PLoS Biol. 2018, 16, e2005348. [Google Scholar] [CrossRef]
- Bender, M.; Romei, V.; Sauseng, P. Slow Theta tACS of the Right Parietal Cortex Enhances Contralateral Visual Working Memory Capacity. Brain Topogr. 2019, 32, 477–481. [Google Scholar] [CrossRef]
- Huang, W.A.; Stitt, I.M.; Negahbani, E.; Passey, D.J.; Ahn, S.; Davey, M.; Dannhauer, M.; Doan, T.T.; Hoover, A.C.; Peterchev, A.V.; et al. Transcranial alternating current stimulation entrains alpha oscillations by preferential phase synchronization of fast-spiking cortical neurons to stimulation waveform. Nat. Commun. 2021, 12, 3151. [Google Scholar] [CrossRef]
- De Graaf, T.A.; Gross, J.; Paterson, G.; Rusch, T.; Sack, A.; Thut, G. Alpha-Band Rhythms in Visual Task Performance: Phase-Locking by Rhythmic Sensory Stimulation. PLoS ONE 2013, 8, e60035. [Google Scholar] [CrossRef]
- Spaak, E.; de Lange, F.P.; Jensen, O. Local Entrainment of Alpha Oscillations by Visual Stimuli Causes Cyclic Modulation of Perception. J. Neurosci. 2014, 34, 3536–3544. [Google Scholar] [CrossRef]
- Ronconi, L.; Pincham, H.L.; Szűcs, D.; Facoetti, A. Inducing attention not to blink: Auditory entrainment improves conscious visual processing. Psychol. Res. 2015, 80, 774–784. [Google Scholar] [CrossRef]
- Ronconi, L.; Pincham, H.L.; Cristoforetti, G.; Facoetti, A.; Szűcs, D. Shaping prestimulus neural activity with auditory rhythmic stimulation improves the temporal allocation of attention. Neuroreport 2016, 27, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Kizuk, S.A.D.; Mathewson, K.E. Power and Phase of Alpha Oscillations Reveal an Interaction between Spatial and Temporal Visual Attention. J. Cogn. Neurosci. 2017, 29, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Keitel, C.; Keitel, A.; Benwell, C.S.; Daube, C.; Thut, G.; Gross, J. Stimulus-Driven Brain Rhythms within the Alpha Band: The Attentional-Modulation Conundrum. J. Neurosci. 2019, 39, 3119–3129. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Melcher, D. The role of oscillatory phase in determining the temporal organization of perception: Evidence from sensory entrainment. J. Neurosci. 2017, 37, 10636–10644. [Google Scholar] [CrossRef]
- Ronconi, L.; Busch, N.A.; Melcher, D. Alpha-band sensory entrainment alters the duration of temporal windows in visual perception. Sci. Rep. 2018, 8, 11810. [Google Scholar] [CrossRef]
- Wiesman, A.; Wilson, T.W. Alpha Frequency Entrainment Reduces the Effect of Visual Distractors. J. Cogn. Neurosci. 2019, 31, 1392–1403. [Google Scholar] [CrossRef]
- Kawashima, T.; Shibusawa, S.; Amano, K. Frequency-and phase-dependent effects of auditory entrainment on attentional blink. Eur. J. Neurosci. 2022, 56, 4411–4424. [Google Scholar] [CrossRef]
- Romei, V.; Thut, G.; Mok, R.M.; Schyns, P.G.; Driver, J. Causal implication by rhythmic transcranial magnetic stimulation of alpha frequency in feature-based local vs. global attention. Eur. J. Neurosci. 2012, 35, 968–974. [Google Scholar] [CrossRef]
- Chanes, L.; Quentin, R.; Tallon-Baudry, C.; Valero-Cabré, A. Causal frequency-specific contributions of frontal spatiotemporal patterns induced by non-invasive neurostimulation to human visual performance. J. Neurosci. 2013, 33, 5000–5005. [Google Scholar] [CrossRef]
- Ruzzoli, M.; Soto-Faraco, S. Alpha Stimulation of the Human Parietal Cortex Attunes Tactile Perception to External Space. Curr. Biol. 2014, 24, 329–332. [Google Scholar] [CrossRef]
- Quentin, R.; Chanes, L.; Vernet, M.; Valero-Cabré, A. Fronto-Parietal Anatomical Connections Influence the Modulation of Conscious Visual Perception by High-Beta Frontal Oscillatory Activity. Cereb. Cortex 2014, 25, 2095–2101. [Google Scholar] [CrossRef]
- Romei, V.; Thut, G.; Silvanto, J. Information-Based Approaches of Noninvasive Transcranial Brain Stimulation. Trends Neurosci. 2016, 39, 782–795. [Google Scholar] [CrossRef]
- Albouy, P.; Weiss, A.; Baillet, S.; Zatorre, R.J. Selective Entrainment of Theta Oscillations in the Dorsal Stream Causally Enhances Auditory Working Memory Performance. Neuron 2017, 94, 193–206.e5. [Google Scholar] [CrossRef]
- Amengual, J.L.; Vernet, M.; Adam, C.; Valero-Cabré, A. Local entrainment of oscillatory activity induced by direct brain stimulation in humans. Sci. Rep. 2017, 7, srep41908. [Google Scholar] [CrossRef]
- Albouy, P.; Baillet, S.; Zatorre, R.J. Driving working memory with frequency-tuned noninvasive brain stimulation. Ann. N. Y. Acad. Sci. 2018, 1423, 126–137. [Google Scholar] [CrossRef]
- Vernet, M.; Stengel, C.; Quentin, R.; Amengual, J.L.; Valero-Cabré, A. Entrainment of local synchrony reveals a causal role for high-beta right frontal oscillations in human visual consciousness. Sci. Rep. 2019, 9, 14510. [Google Scholar] [CrossRef]
- Lakatos, P.; Gross, J.; Thut, G. A New Unifying Account of the Roles of Neuronal Entrainment. Curr. Biol. 2019, 29, R890–R905. [Google Scholar] [CrossRef]
- Mathewson, K.E.; Prudhomme, C.; Fabiani, M.; Beck, D.M.; Lleras, A.; Gratton, G. Making Waves in the Stream of Consciousness: Entraining Oscillations in EEG Alpha and Fluctuations in Visual Awareness with Rhythmic Visual Stimulation. J. Cogn. Neurosci. 2012, 24, 2321–2333. [Google Scholar] [CrossRef]
- Thut, G.; Schyns, P.G.; Gross, J. Entrainment of Perceptually Relevant Brain Oscillations by Non-Invasive Rhythmic Stimulation of the Human Brain. Front. Psychol. 2011, 2, 170. [Google Scholar] [CrossRef]
- Regan, D. Comparison of transient and steady-state methods. Ann. N. Y. Acad. Sci. 1982, 388, 45–71. [Google Scholar] [CrossRef]
- Pikovsky, A.; Rosenblum, M.; Kurths, J.; Strogatz, S. Books-Synchronization: A Universal Concept in Nonlinear Sciences. Phys. Today 2003, 56, 47. [Google Scholar]
- Thut, G.; Miniussi, C.; Gross, J. The Functional Importance of Rhythmic Activity in the Brain. Curr. Biol. 2012, 22, R658–R663. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, J.; Di Gregorio, F.; Marcantoni, E.; Thut, G.; Romei, V. A TMS/EEG protocol for the causal assessment of the functions of the oscillatory brain rhythms in perceptual and cognitive processes. STAR Protoc. 2022, 3, 101435. [Google Scholar] [CrossRef] [PubMed]
- Romei, V.; Gross, J.; Thut, G. On the Role of Prestimulus Alpha Rhythms over Occipito-Parietal Areas in Visual Input Regulation: Correlation or Causation? J. Neurosci. 2010, 30, 8692–8697. [Google Scholar] [CrossRef]
- Notbohm, A.; Kurths, J.; Herrmann, C.S. Modification of Brain Oscillations via Rhythmic Light Stimulation Provides Evidence for Entrainment but Not for Superposition of Event-Related Responses. Front. Hum. Neurosci. 2016, 10, 10. [Google Scholar] [CrossRef]
- Ghazanfar, A.; Schroeder, C. Is neocortex essentially multisensory? Trends Cogn. Sci. 2006, 10, 278–285. [Google Scholar] [CrossRef]
- Senkowski, D.; Schneider, T.R.; Foxe, J.J.; Engel, A.K. Crossmodal binding through neural coherence: Implications for multisensory processing. Trends Neurosci. 2008, 31, 401–409. [Google Scholar] [CrossRef]
- Lakatos, P.; O’Connell, M.N.; Barczak, A.; Mills, A.; Javitt, D.C.; Schroeder, C.E. The Leading Sense: Supramodal Control of Neurophysiological Context by Attention. Neuron 2009, 64, 419–430. [Google Scholar] [CrossRef]
- Landau, A.N.; Fries, P. Attention Samples Stimuli Rhythmically. Curr. Biol. 2012, 22, 1000–1004. [Google Scholar] [CrossRef]
- Fiebelkorn, I.C.; Saalmann, Y.B.; Kastner, S. Rhythmic Sampling within and between Objects despite Sustained Attention at a Cued Location. Curr. Biol. 2013, 23, 2553–2558. [Google Scholar] [CrossRef]
- Bauer, A.-K.R.; Debener, S.; Nobre, A.C. Synchronisation of Neural Oscillations and Cross-modal Influences. Trends Cogn. Sci. 2020, 24, 481–495. [Google Scholar] [CrossRef]
- de Graaf, T.A.; Duecker, F. No effects of rhythmic visual stimulation on target discrimination: An online alpha entrainment experiment. Eur. J. Neurosci. 2022, 55, 3340–3351. [Google Scholar] [CrossRef]
- Gray, M.J.; Emmanouil, T.A. Individual alpha frequency increases during a task but is unchanged by alpha-band flicker. Psychophysiology 2019, 57, e13480. [Google Scholar] [CrossRef]
- Lakatos, P.; Karmos, G.; Mehta, A.D.; Ulbert, I.; Schroeder, C.E. Entrainment of Neuronal Oscillations as a Mechanism of Attentional Selection. Science 2008, 320, 110–113. [Google Scholar] [CrossRef]
- Naue, N.; Rach, S.; Strüber, D.; Huster, R.J.; Zaehle, T.; Körner, U.; Herrmann, C.S. Auditory Event-Related Response in Visual Cortex Modulates Subsequent Visual Responses in Humans. J. Neurosci. 2011, 31, 7729–7736. [Google Scholar] [CrossRef]
- Miller, J.E.; Carlson, L.A.; McAuley, J.D. When what you hear influences when you see: Listening to an auditory rhythm influences the temporal allocation of visual attention. Psychol. Sci. 2013, 24, 11–18. [Google Scholar] [CrossRef]
- Mercier, M.R.; Foxe, J.J.; Fiebelkorn, I.C.; Butler, J.S.; Schwartz, T.H.; Molholm, S. Auditory-driven phase reset in visual cortex: Human electrocorticography reveals mechanisms of early multisensory integration. Neuroimage 2013, 79, 19–29. [Google Scholar] [CrossRef]
- Shapiro, K.L.; Raymond, J.E.; Arnell, K.M. The attentional blink. Trends Cogn. Sci. 1997, 1, 291–296. [Google Scholar] [CrossRef]
- Enns, J.T.; Di Lollo, V. What’s new in visual masking? Trends Cogn. Sci. 2000, 4, 345–352. [Google Scholar] [CrossRef]
- Keysers, C.; Perrett, D.I. Visual masking and RSVP reveal neural competition. Trends Cogn. Sci. 2002, 6, 120–125. [Google Scholar] [CrossRef]
- Martens, S.; Wyble, B. The attentional blink: Past, present, and future of a blind spot in perceptual awareness. Neurosci. Biobehav. Rev. 2010, 34, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Glenberg, A.M.; Jona, M. Temporal coding in rhythm tasks revealed by modality effects. Mem. Cogn. 1991, 19, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Guttman, S.E.; Gilroy, L.A.; Blake, R. Hearing what the eyes see: Auditory encoding of visual temporal sequences. Psychol. Sci. 2005, 16, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Repp, B.; Penel, A. Rhythmic movement is attracted more strongly to auditory than to visual rhythms. Psychol. Res. 2003, 68, 252–270. [Google Scholar] [CrossRef]
- Grahn, J.A. See what I hear? Beat perception in auditory and visual rhythms. Exp. Brain Res. 2012, 220, 51–61. [Google Scholar] [CrossRef]
- Nozaradan, S. Exploring how musical rhythm entrains brain activity with electroencephalogram frequency-tagging. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130393. [Google Scholar] [CrossRef]
- Merchant, H.; Grahn, J.; Trainor, L.; Rohrmeier, M.; Fitch, W.T. Finding the beat: A neural perspective across humans and non-human primates. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140093. [Google Scholar] [CrossRef]
- Zoefel, B.; VanRullen, R. Oscillatory Mechanisms of Stimulus Processing and Selection in the Visual and Auditory Systems: State-of-the-Art, Speculations and Suggestions. Front. Neurosci. 2017, 11, 296. [Google Scholar] [CrossRef]
- Shipley, T. Auditory Flutter-Driving of Visual Flicker. Science 1964, 145, 1328–1330. [Google Scholar] [CrossRef]
- Escoffier, N.; Herrmann, C.S.; Schirmer, A. Auditory rhythms entrain visual processes in the human brain: Evidence from evoked oscillations and event-related potentials. Neuroimage 2015, 111, 267–276. [Google Scholar] [CrossRef]
- Samaha, J.; Postle, B.R. The Speed of Alpha-Band Oscillations Predicts the Temporal Resolution of Visual Perception. Curr. Biol. 2015, 25, 2985–2990. [Google Scholar] [CrossRef]
- Cooke, J.; Poch, C.; Gillmeister, H.; Costantini, M.; Romei, V. Oscillatory Properties of Functional Connections Between Sensory Areas Mediate Cross-Modal Illusory Perception. J. Neurosci. 2019, 39, 5711–5718. [Google Scholar] [CrossRef]
- Bastiaansen, M.; Berberyan, H.; Stekelenburg, J.J.; Schoffelen, J.M.; Vroomen, J. Are alpha oscillations instrumental in multisensory synchrony perception? Brain Res. 2020, 1734, 146744. [Google Scholar] [CrossRef]
- Migliorati, D.; Zappasodi, F.; Perrucci, M.G.; Donno, B.; Northoff, G.; Romei, V.; Costantini, M. Individual alpha frequency predicts perceived visuotactile simultaneity. J. Cogn. Neurosci. 2020, 32, 1–11. [Google Scholar] [CrossRef]
- Noguchi, Y. Audio–Visual Fission Illusion and Individual Alpha Frequency: Perspective on Buergers and Noppeney (2022). J. Cogn. Neurosci. 2023, 1–6. [Google Scholar] [CrossRef]
- Cecere, R.; Rees, G.; Romei, V. Individual Differences in Alpha Frequency Drive Crossmodal Illusory Perception. Curr. Biol. 2014, 25, 231–235. [Google Scholar] [CrossRef]
- Venskus, A.; Ferri, F.; Migliorati, D.; Spadone, S.; Costantini, M.; Hughes, G. Temporal binding window and sense of agency are related processes modifiable via occipital tACS. PLoS ONE 2021, 16, e0256987. [Google Scholar] [CrossRef]
- Ronconi, L.; Vitale, A.; Federici, A.; Mazzoni, N.; Battaglini, L.; Molteni, M.; Casartelli, L. Neural dynamics driving audio-visual integration in autism. Cereb. Cortex 2022, 33, 543–556. [Google Scholar] [CrossRef]
- Halbleib, A.; Gratkowski, M.; Schwab, K.; Ligges, C.; Witte, H.; Haueisen, J. Topographic analysis of engagement and disengagement of neural oscillators in photic driving: A combined electroencephalogram/magnetoencephalogram study. J. Clin. Neurophysiol. 2012, 29, 33–41. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Axmacher, N.; Inman, C.S. Modulating Human Memory via Entrainment of Brain Oscillations. Trends Neurosci. 2019, 42, 485–499. [Google Scholar] [CrossRef]
- Albouy, P.; Martinez-Moreno, Z.E.; Hoyer, R.S.; Zatorre, R.J.; Baillet, S. Supramodality of neural entrainment: Rhythmic visual stimulation causally enhances auditory working memory performance. Sci. Adv. 2022, 8, eabj9782. [Google Scholar] [CrossRef] [PubMed]
- Besle, J.; Schevon, C.A.; Mehta, A.D.; Lakatos, P.; Goodman, R.R.; McKhann, G.M.; Emerson, R.G.; Schroeder, C.E. Tuning of the Human Neocortex to the Temporal Dynamics of Attended Events. J. Neurosci. 2011, 31, 3176–3185. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ramirez, M.; Kelly, S.P.; Molholm, S.; Sehatpour, P.; Schwartz, T.H.; Foxe, J.J. Oscillatory Sensory Selection Mechanisms during Intersensory Attention to Rhythmic Auditory and Visual Inputs: A Human Electrocorticographic Investigation. J. Neurosci. 2011, 31, 18556–18567. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Nakazawa, K. Brain state-dependent abnormal LFP activity in the auditory cortex of a schizophrenia mouse model. Front. Neurosci. 2014, 8, 168. [Google Scholar] [CrossRef]
- Li, S.; Ma, L.; Wang, Y.; Wang, X.; Li, Y.; Qin, L. Auditory steady-state responses in primary and non-primary regions of the auditory cortex in neonatal ventral hippocampal lesion rats. PLoS ONE 2018, 13, e0192103. [Google Scholar] [CrossRef]
- Falchier, A.; Clavagnier, S.; Barone, P.; Kennedy, H. Anatomical Evidence of Multimodal Integration in Primate Striate Cortex. J. Neurosci. 2002, 22, 5749–5759. [Google Scholar] [CrossRef]
- Rockland, K.S.; Ojima, H. Multisensory convergence in calcarine visual areas in macaque monkey. Int. J. Psychophysiol. 2003, 50, 19–26. [Google Scholar] [CrossRef]
- Shaw, J.C. The Brain’s Alpha Rhythms and the Mind; Elsevier: Amsterdam, The Netherlands, 2003; pp. 15–32. [Google Scholar]
- Niedermeyer, E. The normal EEG of the waking adult. In Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Niedermeyer, E., Lopes da Silva, F., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 155–164. [Google Scholar]
- Buzsaki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Mathewson, K.J.; Jetha, M.K.; Drmic, I.E.; Bryson, S.E.; Goldberg, J.O.; Schmidt, L.A. Regional EEG alpha power, coherence, and behavioral symptomatology in autism spectrum disorder. Clin. Neurophysiol. 2012, 123, 1798–1809. [Google Scholar] [CrossRef]
- Otero, M.; Lea-Carnall, C.; Prado, P.; Escobar, M.-J.; El-Deredy, W. Modelling neural entrainment and its persistence: Influence of frequency of stimulation and phase at the stimulus offset. Biomed. Phys. Eng. Express 2022, 8, 045014. [Google Scholar] [CrossRef]
- Jansen, B.H.; Zouridakis, G.; Brandt, M.E. A neurophysiologically-based mathematical model of flash visual evoked potentials. Biol. Cybern. 1993, 68, 275–283. [Google Scholar] [CrossRef]
- Chota, S.; VanRullen, R. Visual Entrainment at 10 Hz Causes Periodic Modulation of the Flash Lag Illusion. Front. Neurosci. 2019, 13, 232. [Google Scholar] [CrossRef]
- Buergers, S.; Noppeney, U. The role of alpha oscillations in temporal binding within and across the senses. Nat. Hum. Behav. 2022, 6, 732–742. [Google Scholar] [CrossRef]
- Green, D.M.; Swets, J.A. Signal Detection Theory and Psychophysics; Wiley: New York, NY, USA, 1966; Volume 1, pp. 1969–2012. [Google Scholar]
- Tarasi, L.; Trajkovic, J.; Diciotti, S.; di Pellegrino, G.; Ferri, F.; Ursino, M.; Romei, V. Predictive waves in the autism-schizophrenia continuum: A novel biobehavioral model. Neurosci. Biobehav. Rev. 2022, 132, 1–22. [Google Scholar] [CrossRef]
- Prinzmetal, W.; McCool, C.; Park, S. Attention: Reaction Time and Accuracy Reveal Different Mechanisms. J. Exp. Psychol. Gen. 2005, 134, 73–92. [Google Scholar] [CrossRef]
- Rosenfeld, J.P.; Reinhart, A.M.; Srivastava, S. The Effects of Alpha (10-Hz) and Beta (22-Hz) “Entrainment” Stimulation on the Alpha and Beta EEG Bands: Individual Differences Are Critical to Prediction of Effects. Appl. Psychophysiol. Biofeedback 1997, 22, 3–20. [Google Scholar] [CrossRef]
- Pietrelli, M.; Zanon, M.; Làdavas, E.; Grasso, P.A.; Romei, V.; Bertini, C. Posterior brain lesions selectively alter alpha oscillatory activity and predict visual performance in hemianopic patients. Cortex 2019, 121, 347–361. [Google Scholar] [CrossRef]
- Gallina, J.; Pietrelli, M.; Zanon, M.; Bertini, C. Hemispheric differences in altered reactivity of brain oscillations at rest after posterior lesions. Brain Struct. Funct. 2021, 227, 709–723. [Google Scholar] [CrossRef]
- Gallina, J.; Zanon, M.; Mikulan, E.; Pietrelli, M.; Gambino, S.; Ibáñez, A.; Bertini, C. Alterations in resting-state functional connectivity after brain posterior lesions reflect the functionality of the visual system in hemianopic patients. Brain Struct. Funct. 2022, 227, 2939–2956. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010, 11, 100–113. [Google Scholar] [CrossRef]
- Ippolito, G.; Bertaccini, R.; Tarasi, L.; Di Gregorio, F.; Trajkovic, J.; Battaglia, S.; Romei, V. The Role of Alpha Oscillations among the Main Neuropsychiatric Disorders in the Adult and Developing Human Brain: Evidence from the Last 10 Years of Research. Biomedicines 2022, 10, 3189. [Google Scholar] [CrossRef]
- Tierney, A.L.; Gabard-Durnam, L.; Vogel-Farley, V.; Tager-Flusberg, H.; Nelson, C.A. Developmental Trajectories of Resting EEG Power: An Endophenotype of Autism Spectrum Disorder. PLoS ONE 2012, 7, e39127. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.; Estévez, M.; Leisman, G.; Melillo, R.; Rodriguez-Rojas, R.; DeFina, P.; Hernández, A.; Pérez-Nellar, J.; Naranjo, R.; Chinchilla, M.; et al. QEEG Spectral and Coherence Assessment of Autistic Children in Three Different Experimental Conditions. J. Autism Dev. Disord. 2013, 45, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zeng, K.; Kang, J.; Tong, Z.; Cai, E.; Chen, H.; Ding, M.; Gu, Y.; Ouyang, G.; Li, X. Development of Brain Network in Children with Autism from Early Childhood to Late Childhood. Neuroscience 2017, 367, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Palva, S.; Palva, J.M. Functional Roles of Alpha-Band Phase Synchronization in Local and Large-Scale Cortical Networks. Front. Psychol. 2011, 2, 204. [Google Scholar] [CrossRef]
- Ursino, M.; Serra, M.; Tarasi, L.; Ricci, G.; Magosso, E.; Romei, V. Bottom-up vs. top-down connectivity imbalance in individuals with high-autistic traits: An electroencephalographic study. Front. Syst. Neurosci. 2022, 16, 932128. [Google Scholar] [CrossRef]
- Murphy, M.; Öngür, D. Decreased peak alpha frequency and impaired visual evoked potentials in first episode psychosis. NeuroImage Clin. 2019, 22, 101693. [Google Scholar] [CrossRef]
- Tarasi, L.; Magosso, E.; Ricci, G.; Ursino, M.; Romei, V. The Directionality of Fronto-Posterior Brain Connectivity Is Associated with the Degree of Individual Autistic Traits. Brain Sci. 2021, 11, 1443. [Google Scholar] [CrossRef]
- Yeum, T.-S.; Kang, U.G. Reduction in Alpha Peak Frequency and Coherence on Quantitative Electroencephalography in Patients with Schizophrenia. J. Korean Med Sci. 2018, 33, e179. [Google Scholar] [CrossRef]
- Freche, D.; Naim-Feil, J.; Hess, S.; Peled, A.; Grinshpoon, A.; Moses, E.; Levit-Binnun, N. Phase-Amplitude Markers of Synchrony and Noise: A Resting-State and TMS-EEG Study of Schizophrenia. Cereb. Cortex Commun. 2020, 1, tgaa013. [Google Scholar] [CrossRef]
- Nakhnikian, A.; Oribe, N.; Hirano, S.; Hirano, Y.; Levin, M.; Spencer, K. Lower Peak Alpha Frequency Accounts for Elevated Theta-Alpha Power in Resting State EEG in Schizophrenia. Biol. Psychiatry 2020, 87, S411. [Google Scholar] [CrossRef]
- Koshiyama, D.; Miyakoshi, M.; Tanaka-Koshiyama, K.; Joshi, Y.B.; Sprock, J.; Braff, D.L.; Light, G.A. Abnormal phase discontinuity of alpha- and theta-frequency oscillations in schizophrenia. Schizophr. Res. 2021, 231, 73–81. [Google Scholar] [CrossRef]
- Ramsay, I.S.; Lynn, P.A.; Schermitzler, B.; Sponheim, S.R. Individual alpha peak frequency is slower in schizophrenia and related to deficits in visual perception and cognition. Sci. Rep. 2021, 11, 17852. [Google Scholar] [CrossRef]
- Başar, E.; Schmiedt-Fehr, C.; Mathes, B.; Femir, B.; Emek-Savaş, D.D.; Tülay, E.E.; Tan, D.; Düzgün, A.; Güntekin, B.; Özerdem, A.; et al. What does the broken brain say to the neuroscientist? Oscillations and connectivity in schizophrenia, Alzheimer’s disease, and bipolar disorder. Int. J. Psychophysiol. 2016, 103, 135–148. [Google Scholar] [CrossRef]
- Garakh, Z.; Zaytseva, Y.; Kapranova, A.; Fiala, O.; Horacek, J.; Shmukler, A.; Gurovich, I.Y.; Strelets, V.B. EEG correlates of a mental arithmetic task in patients with first episode schizophrenia and schizoaffective disorder. Clin. Neurophysiol. 2015, 126, 2090–2098. [Google Scholar] [CrossRef]
- Goldstein, M.R.; Peterson, M.J.; Sanguinetti, J.L.; Tononi, G.; Ferrarelli, F. Topographic deficits in alpha-range resting EEG activity and steady state visual evoked responses in schizophrenia. Schizophr. Res. 2015, 168, 145–152. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.S.; Han, D.H.; Min, K.J.; Lee, J.; Lee, K. Diagnostic utility of quantitative EEG in un-medicated schizophrenia. Neurosci. Lett. 2015, 589, 126–131. [Google Scholar] [CrossRef]
- Canali, P.; Sarasso, S.; Rosanova, M.; Casarotto, S.; Sferrazza-Papa, G.; Gosseries, O.; Fecchio, M.; Massimini, M.; Mariotti, M.; Cavallaro, R.; et al. Shared Reduction of Oscillatory Natural Frequencies in Bipolar Disorder, Major Depressive Disorder and Schizophrenia. J. Affect. Disord. 2015, 184, 111–115. [Google Scholar] [CrossRef]
- Candelaria-Cook, F.T.; Schendel, M.E.; Ojeda, C.J.; Bustillo, J.R.; Stephen, J.M. Reduced Parietal Alpha Power and Psychotic Symptoms: Test-Retest Reliability of Resting-State Magnetoencephalography in Schizophrenia and Healthy Controls. Schizophr. Res. 2020, 215, 229–240. [Google Scholar] [CrossRef]
- Mitra, S.; Nizamie, S.H.; Goyal, N.; Tikka, S.K. Electroencephalogram alpha-to-theta ratio over left fronto-temporal region correlates with negative symptoms in schizophrenia. Asian J. Psychiatry 2017, 26, 70–76. [Google Scholar]
- Trajkovic, J.; Di Gregorio, F.; Ferri, F.; Marzi, C.; Diciotti, S.; Romei, V. Resting state alpha oscillatory activity is a valid and reliable marker of schizotypy. Sci. Rep. 2021, 11, 10379. [Google Scholar] [CrossRef]
- Sanchez-Lopez, J.; Pedersini, C.A.; Di Russo, F.; Cardobi, N.; Fonte, C.; Varalta, V.; Prior, M.; Smania, N.; Savazzi, S.; Marzi, C.A. Visually evoked responses from the blind field of hemianopic patients. Neuropsychologia 2019, 128, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Grasso, P.A.; Pietrelli, M.; Zanon, M.; Làdavas, E.; Bertini, C. Alpha oscillations reveal implicit visual processing of motion in hemianopia. Cortex 2020, 122, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, F.; Petrone, V.; Casanova, E.; Lullini, G.; Romei, V.; Piperno, R.; La Porta, F. Hierarchical psychophysiological pathways subtend perceptual asymmetries in Neglect. Neuroimage 2023, 270, 119942. [Google Scholar] [CrossRef]
- Allaman, L.; Mottaz, A.; Guggisberg, A.G. Disrupted resting-state EEG alpha-band interactions as a novel marker for the severity of visual field deficits after brain lesion. Clin. Neurophysiol. 2021, 132, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Lasaponara, S.; Pinto, M.; Aiello, M.; Tomaiuolo, F.; Doricchi, F. The Hemispheric Distribution of α-Band EEG Activity During Orienting of Attention in Patients with Reduced Awareness of the Left Side of Space (Spatial Neglect). J. Neurosci. 2019, 39, 4332–4343. [Google Scholar] [CrossRef]
- Babiloni, C.; Ferri, R.; Moretti, D.V.; Strambi, A.; Binetti, G.; Forno, G.D.; Ferreri, F.; Lanuzza, B.; Bonato, C.; Nobili, F.; et al. Abnormal fronto-parietal coupling of brain rhythms in mild Alzheimer’s disease: A multicentric EEG study. Eur. J. Neurosci. 2004, 19, 2583–2590. [Google Scholar] [CrossRef]
- Meghdadi, A.H.; Stevanović Karić, M.; McConnell, M.; Rupp, G.; Richard, C.; Hamilton, J.; Salat, D.; Berka, C. Resting state EEG biomarkers of cognitive decline associated with Alzheimer’s disease and mild cognitive impairment. PLoS ONE 2021, 16, e0244180. [Google Scholar] [CrossRef]
- López-Sanz, D.; Bruña, R.; Garcés, P.; Camara, C.; Serrano, N.; Rodríguez-Rojo, I.C.; Delgado, M.L.; Montenegro, M.; López-Higes, R.; Yus, M.; et al. Alpha band disruption in the AD-continuum starts in the Subjective Cognitive Decline stage: A MEG study. Sci. Rep. 2016, 6, 37685. [Google Scholar] [CrossRef]
- Khan, S.; Gramfort, A.; Shetty, N.R.; Kitzbichler, M.G.; Ganesan, S.; Moran, J.M.; Lee, S.M.; Gabrieli, J.D.E.; Tager-Flusberg, H.B.; Joseph, R.M.; et al. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc. Natl. Acad. Sci. USA 2013, 110, 3107–3112. [Google Scholar] [CrossRef]
- Bhattacharya, B.S.; Coyle, D.; Maguire, L.P. Alpha and Theta Rhythm Abnormality in Alzheimer’s Disease: A Study Using a Computational Model. Adv. Exp. Med. Biol. 2011, 718, 57–73. [Google Scholar] [CrossRef]
- Brueggen, K.; Fiala, C.; Berger, C.; Ochmann, S.; Babiloni, C.; Teipel, S.J. Early Changes in Alpha Band Power and DMN BOLD Activity in Alzheimer’s Disease: A Simultaneous Resting State EEG-fMRI Study. Front. Aging Neurosci. 2017, 9, 319. [Google Scholar] [CrossRef]
- Garcés, P.; Vicente, R.; Wibral, M.; Pineda-Pardo, J.; López, M.E.; Aurtenetxe, S.; Marcos, A.; de Andrés, M.E.; Yus, M.; Sancho, M.; et al. Brain-wide slowing of spontaneous alpha rhythms in mild cognitive impairment. Front. Aging Neurosci. 2013, 5, 100. [Google Scholar] [CrossRef]
- Lopez-Diaz, K.; Henshaw, J.; Casson, A.J.; Brown, C.A.; Taylor, J.R.; Trujillo-Barreto, N.J.; Arendsen, L.J.; Jones, A.K.P.; Sivan, M. Alpha entrainment drives pain relief using visual stimulation in a sample of chronic pain patients: A proof-of-concept controlled study. Neuroreport 2021, 32, 394–398. [Google Scholar] [CrossRef]
- Lejko, N.; Larabi, D.I.; Herrmann, C.S.; Aleman, A.; Ćurčić-Blake, B. Alpha Power and Functional Connectivity in Cognitive Decline: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2020, 78, 1047–1088. [Google Scholar] [CrossRef]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef]
- Martorell, A.J.; Paulson, A.L.; Suk, H.-J.; Abdurrob, F.; Drummond, G.T.; Guan, W.; Young, J.Z.; Kim, D.N.-W.; Kritskiy, O.; Barker, S.J.; et al. Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition. Cell 2019, 177, 256–271.e22. [Google Scholar] [CrossRef]
- Ecsy, K.; Jones, A.; Brown, C. Alpha-range visual and auditory stimulation reduces the perception of pain. Eur. J. Pain 2016, 21, 562–572. [Google Scholar] [CrossRef]
- Ölçücü, M.T.; Yılmaz, K.; Karamık, K.; Okuducu, Y.; Özsoy, Ç.; Aktaş, Y.; Çakır, S.; Ateş, M. Effects of Listening to Binaural Beats on Anxiety Levels and Pain Scores in Male Patients Undergoing Cystoscopy and Ureteral Stent Removal: A Randomized Placebo-Controlled Trial. J. Endourol. 2021, 35, 54–61. [Google Scholar] [CrossRef]
- Maddison, R.; Nazar, H.; Obara, I.; Vuong, Q.C. The efficacy of sensory neural entrainment on acute and chronic pain: A systematic review and meta-analysis. Br. J. Pain 2022, 17, 126–141. [Google Scholar] [CrossRef]
- Foxe, J.J.; Snyder, A.C. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol. 2011, 2, 154. [Google Scholar] [CrossRef]
- Garland, E.L. Pain Processing in the Human Nervous System: A Selective Review of Nociceptive and Biobehavioral Pathways. Prim. Care Clin. Off. Pr. 2012, 39, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Furman, A.J.; Meeker, T.J.; Rietschel, J.C.; Yoo, S.; Muthulingam, J.; Prokhorenko, M.; Keaser, M.L.; Goodman, R.N.; Mazaheri, A.; Seminowicz, D.A. Cerebral peak alpha frequency predicts individual differences in pain sensitivity. Neuroimage 2018, 167, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Furman, A.J.; Prokhorenko, M.; Keaser, M.L.; Zhang, J.; Chen, S.; Mazaheri, A.; Seminowicz, D. Sensorimotor Peak Alpha Frequency Is a Reliable Biomarker of Prolonged Pain Sensitivity. Cereb. Cortex 2020, 30, 6069–6082. [Google Scholar] [CrossRef] [PubMed]
- Pino, O. A randomized controlled trial (RCT) to explore the effect of audio-visual entrainment among psychological disorders. Acta Biomed. 2022, 92, e2021408. [Google Scholar] [CrossRef]
| Authors, Year, Journal | Entrainment Modality | Entrainment Duration | Frequency | Visual Domain/Task | Behavioral Measures | Entrainment-Induced Effects |
|---|---|---|---|---|---|---|
| Mathewson et al., 2010; Cognition [15]; https://doi.org/10.1016/j.cognition.2009.11.010 | Unisensory: Visual | 0.800 s | 12 Hz | Spatial/Metacontrast Masking paradigm | Accuracy and Signal Detection Theory (d’) | Alpha Power increase and phase-locking lasting for ~200 ms. Increased perceptual sensitivity (d’) for the in-phase target. |
| Mathewson et al., 2012; Journal of Cognitive Neuroscience [58]; https://doi.org/10.1162/jocn_a_00288 | Unisensory: Visual | 0.576 s | 12 Hz | Spatial/Metacontrast Masking paradigm | Accuracy | Alpha Power increase and phase-locking lasting for ~200 ms. Increased detection accuracy for the in-phase target. |
| De Graaf et al., 2013; Plos One [38]; https://doi.org/10.1371/journal.pone.0060035 | Unisensory: Visual | 0.516 s | 10.6 Hz | Spatial/Cueing paradigm (Visual discrimination task) | Accuracy | Alpha phase-locking lasting for ~300 ms and improvments in discrimination rates for in-phase targets. |
| Spaak et al., 2014; Journal of Neuroscience [39]; https://doi.org/10.1523/JNEUROSCI.4385-13.2014 | Unisensory: Visual | 1.5 s | 10 Hz | Spatial/Visual detectiont task | Accuracy | Alpha power increase lasting for ~300 ms and improvements in detection rates for anti-phase targets. |
| Kizuk & Mathewson, 2017; Journal Of Cognitive Neuroscience [42]; https://doi.org/10.1162/jocn_a_01058 | Unisensory: Visual | 1 s | 12 Hz | Spatial/Metacontrast Masking paradigm | Accuracy | Alpha Power increase and phase-locking lasting for ~200 ms. Increased detection accuracy for the in-phase target. |
| Wiesman & Wilson, 2019 [46]; Journal of Cognitive Neuroscience; https://doi.org/10.1162/jocn_a_01422 | Unisensory: Visual | 1.5 s | 10 Hz | Spatial/Adapted version of the arrow-based Erikson “flanker” paradigm | RTs | Increased alpha ERS and ITPC lasting for ~550 ms, with reduced discrimination rates for distractor stimuli. |
| Gray & Emmanouil, 2020; Psychophysiology [73]; https://doi.org/10.1111/psyp.13480 | Unisensory: Visual | 1 s | Lower Alpha: 8.3 Hz; Upper Alpha: 12.5 Hz | Temporal/Two-flash fusion task | Accuracy and psychometric thresholds | No effects of alpha-band visual entrainment on the visual temporal integration/segregation task. Increased alpha power and ITPC during and following alpha entrainment. |
| De Graaf & Duecker, 2022; European Journal of Neuroscience [72]; https://doi.org/10.1111/ejn.15483 | Unisensory: Visual | 0.717 s | 10 Hz | Spatial/Cueing paradigm (Visual discrimination task) | RTs | No effects of alpha-band visual entrainment on visual detection performance (RTs). |
| Ronconi et al., 2016; Psych. Res. [40]; https://doi.org/10.1007/s00426-015-0691-8 | Unisensory: Visual and Auditory | 2 s | 10 Hz | Temporal/Attentional Blink | Accuracy | Only auditory—but not visual—alpha-band entrainment improved visual target detection accuracy, reducing the AB magnitude. |
| Ronconi et al., 2016; NeuroReport [41]; 10.1097/WNR.0000000000000565 | Unisensory: Auditory | 2 s | 10 Hz | Temporal/Attentional Blink | Accuracy | Increase in the alpha power during auditory entrainment, associated with improvements in the visual detection of the target. |
| Kawashima et al., 2022; European Journal of Neuroscience [47]; https://doi.org/10.1111/ejn.15760 | Unisensory: Auditory | 5 s | 10 Hz | Temporal/Attentional Blink | Accuracy | Auditory alpha-band entrainment improved visual target detection accuracy, reducing the AB magnitude. |
| Ronconi & Melcher, 2017; J. Neurosc. [44]; https://doi.org/10.1523/JNEUROSCI.1704-17.2017 | Multisensory: Audiovisual | 2 s | Lower Alpha: ~8.5 Hz; Upper Alpha: ~12 Hz | Temporal/Integration and Segregation task | Accuracy | Phase alignment of the perceptual oscillation, with two different power spectra that peaked toward the entrainment stimulation frequency. |
| Ronconi et al., 2018; Scientific Reports [45]; https://doi.org/10.1038/s41598-018-29671-5 | Multisensory: Audiovisual | 2 s | IAF − 2 Hz, IAF + 2 Hz | Temporal/Integration and Segregation task | Accuracy | IAF + 2 Hz entrainment improved the segregation performance of visual stimuli, whereas IAF − 2 Hz improved integration. Behavioral oscillations were almost in anti-phase between the two tasks: the highest integration performance corresponded to the lowest segregation performance. |
| Cognitive Impairment | Acquired Posterior Brain Lesion | Psychiatric Conditions |
|---|---|---|
AD
| Hemianopia
| ASD
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallina, J.; Marsicano, G.; Romei, V.; Bertini, C. Electrophysiological and Behavioral Effects of Alpha-Band Sensory Entrainment: Neural Mechanisms and Clinical Applications. Biomedicines 2023, 11, 1399. https://doi.org/10.3390/biomedicines11051399
Gallina J, Marsicano G, Romei V, Bertini C. Electrophysiological and Behavioral Effects of Alpha-Band Sensory Entrainment: Neural Mechanisms and Clinical Applications. Biomedicines. 2023; 11(5):1399. https://doi.org/10.3390/biomedicines11051399
Chicago/Turabian StyleGallina, Jessica, Gianluca Marsicano, Vincenzo Romei, and Caterina Bertini. 2023. "Electrophysiological and Behavioral Effects of Alpha-Band Sensory Entrainment: Neural Mechanisms and Clinical Applications" Biomedicines 11, no. 5: 1399. https://doi.org/10.3390/biomedicines11051399
APA StyleGallina, J., Marsicano, G., Romei, V., & Bertini, C. (2023). Electrophysiological and Behavioral Effects of Alpha-Band Sensory Entrainment: Neural Mechanisms and Clinical Applications. Biomedicines, 11(5), 1399. https://doi.org/10.3390/biomedicines11051399






