The Interleukine-17 Cytokine Family: Role in Development and Progression of Spondyloarthritis, Current and Potential Therapeutic Inhibitors
Abstract
1. Introduction
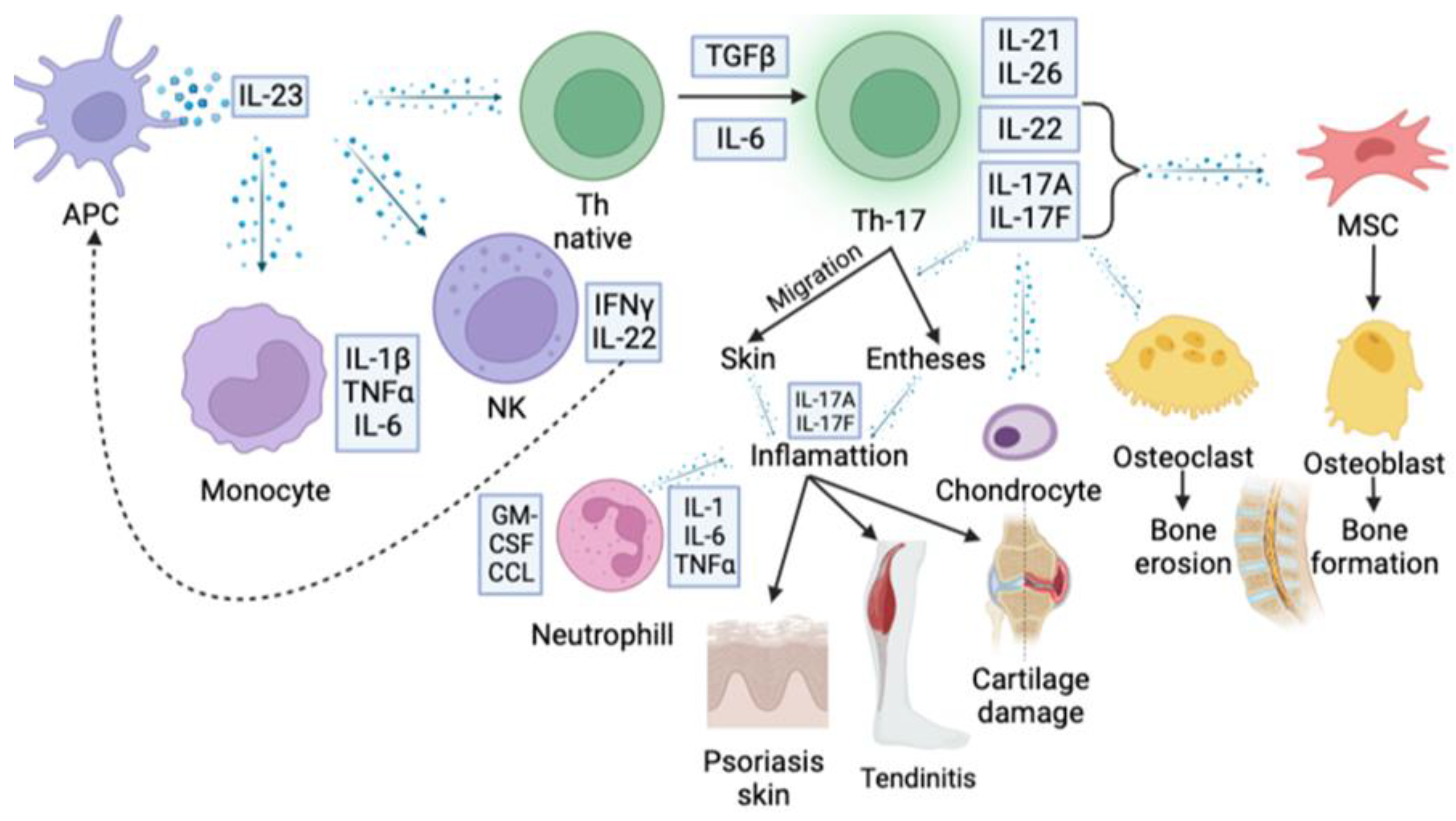
2. IL23/IL17 Axis in the Pathogenesis of Autoimmune Inflammation
3. IL-17 Family in the Pathogenesis of Rheumatic Diseases
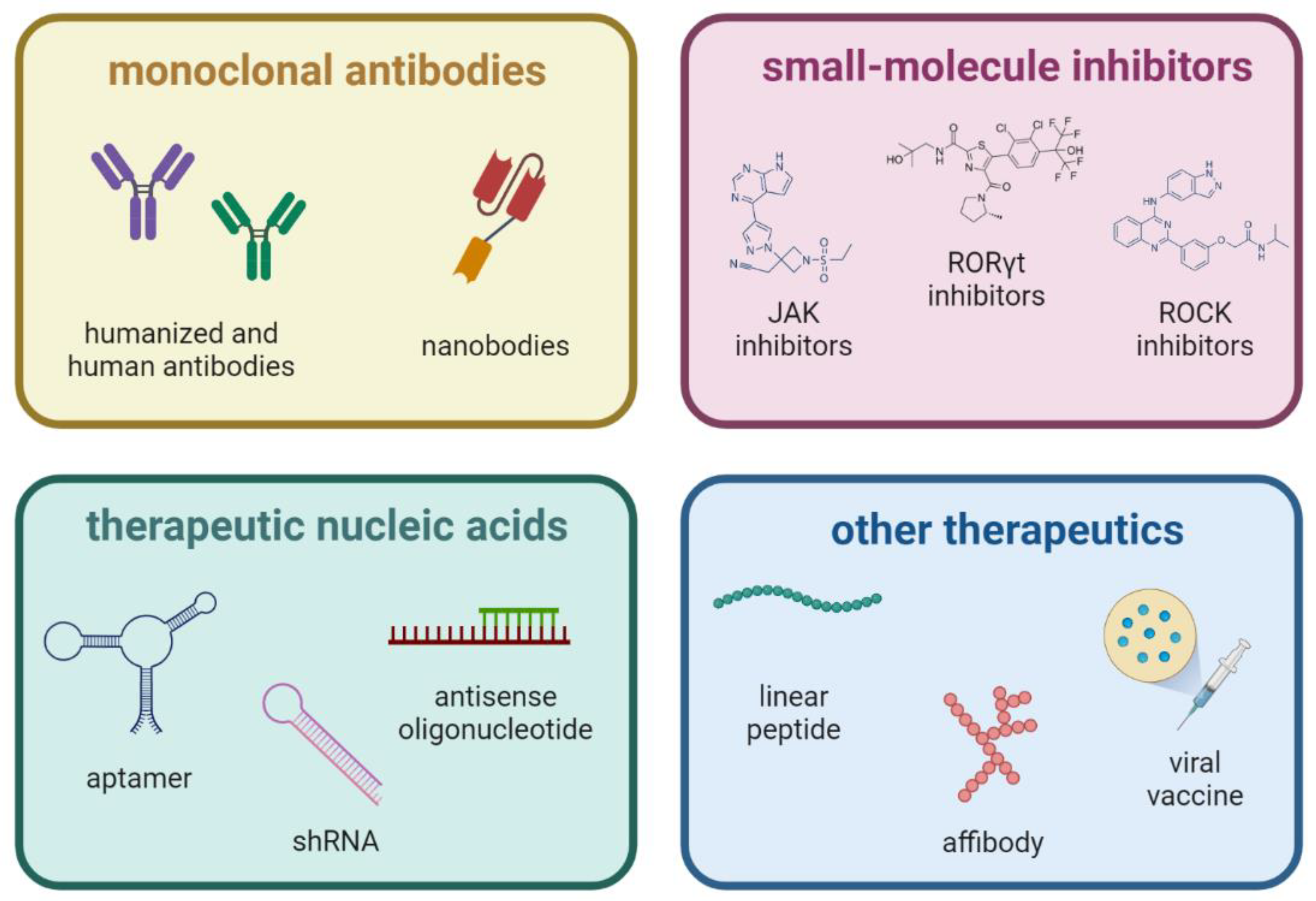
4. Selective Inhibition of IL17 Cytokine
4.1. Monoclonal Antibodies
4.2. Small-Molecule Inhibitors
4.3. Therapeutic Nucleic Acids
4.4. Other Aproaches to IL-17 Downregulation
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASAS/EULAR | European-based Assessment of SpondyloArthritis International Society/The European Alliance of Associations for Rheumatology |
| ASDAS | Ankylosing Spondylitis Disease Activity Score |
| CCL2 | C-C motif ligand 2 |
| CRP | C-reactive protein |
| DC | Dendritic cells |
| DNA | Deoxyribonucleic acid |
| EMA | European Medicines Agency |
| FDA | U.S. Food and Drug Administration |
| FOXP3 | Forkhead box P3 |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GRO-α | Growth-regulated protein alpha |
| HLA | Human Leukocyte Antigens |
| IgG | Immunoglobulin G |
| IL- | Interleukin-1 |
| IL-10 | Interleukin-10 |
| IL-17 | Interleukin-17 |
| IL-17R | Interleukin-17 receptor |
| IL-21 | Interleukin-21 |
| IL-23 | Interleukin-23 |
| IL-23R | Interleukin-23 receptor |
| IL-25 | Interleukin-25 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| ILC3 | Type 3 innate lymphoid cells |
| JAK | Janus kinase |
| LTBI | Latent tuberculosis infection |
| mAb | Monoclonal antibody |
| MAIT | Mucosal-associated invariant T cells |
| MCP-1 | Monocyte Chemoattractant Protein 1 |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger ribonucleic acid |
| mSASSS | Modified Stoke Ankylosing Spondylitis Spinal Score |
| NK-cells | Natural killer cells |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| PCR | polymerase chain reaction |
| RANKL | Receptor activator of nuclear factor kappa-Β ligand |
| ROCK | Rho-associated protein kinase |
| RORγt | RAR-related orphan receptor gamma |
| shRNA | Short hairpin ribonucleic acid |
| siRNA | Small interfering ribonucleic acid |
| SMAD3 | Mothers against decapentaplegic homolog 3 |
| SRMR RF | State Register of Medical Remedies Russian Federation |
| STAT | Signal transducer and activator of transcription |
| T-bet | T-box transcription factor |
| TGF-β | Transforming growth factor beta |
| TNF | Tumor necrosis factor |
| TYK | Tyrosine kinase |
References
- Winkler, A.E.; Miller, M. Update on Axial Spondyloarthritis. Mo. Med. 2022, 119, 79–83. [Google Scholar]
- Cardelli, C.; Monti, S.; Terenzi, R.; Carli, L. One year in review 2021: Axial spondyloarthritis. Clin. Exp. Rheumatol. 2021, 39, 1272–1281. [Google Scholar] [CrossRef]
- Reveille, J.D. Spondyloarthritis. In Clinical Immunology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 769–787. [Google Scholar]
- Nikiphorou, E.; Ramiro, S. Work Disability in Axial Spondyloarthritis. Curr. Rheumatol. Rep. 2020, 22, 55. [Google Scholar] [CrossRef]
- Wang, R.; Ward, M.M. Epidemiology of axial spondyloarthritis: An update. Curr. Opin. Rheumatol. 2018, 30, 137–143. [Google Scholar] [CrossRef]
- Scotti, L.; Franchi, M.; Marchesoni, A.; Corrao, G. Prevalence and incidence of psoriatic arthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2018, 48, 28–34. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Karreman, M.C.; Luime, J.J.; Hazes, J.M.W.; Weel, A.E.A.M. The prevalence and incidence of axial and peripheral spondyloarthritis in inflammatory bowel disease: A systematic review and meta-analysis. J. Crohn’s Colitis 2017, 11, 631–642. [Google Scholar] [CrossRef]
- Ogdie, A.; Hwang, M.; Veeranki, P.; Portelli, A.; Sison, S.; Shafrin, J.; Pedro, S.; Hass, S.; Hur, P.; Kim, N.; et al. Health care utilization and costs associated with functional status in patients with psoriatic arthritis. J. Manag. Care Spec. Pharm. 2022, 28, 997–1007. [Google Scholar] [CrossRef]
- Van Der Heijde, D.; Braun, J.; Rudwaleit, M.; Purcaru, O.; Kavanaugh, A.F. Improvements in workplace and household productivity with certolizumab pegol treatment in axial spondyloarthritis: Results to week 96 of a phase III study. RMD Open 2018, 4, e000659. [Google Scholar] [CrossRef]
- Walsh, J.A.; Magrey, M. Clinical Manifestations and Diagnosis of Axial Spondyloarthritis. JCR J. Clin. Rheumatol. 2021, 27, e547–e560. [Google Scholar] [CrossRef]
- Orbai, A.M.; Reddy, S.M.; Dennis, N.; Villacorta, R.; Peterson, S.; Mesana, L.; Chakravarty, S.D.; Lin, I.; Karyekar, C.S.; Wang, Y.; et al. Work absenteeism and disability associated with psoriasis and psoriatic arthritis in the USA—A retrospective study of claims data from 2009 TO 2020. Clin. Rheumatol. 2021, 40, 4933–4942. [Google Scholar] [CrossRef]
- Garrido-Cumbrera, M.; Poddubnyy, D.; Gossec, L.; Gálvez-Ruiz, D.; Bundy, C.; Mahapatra, R.; Makri, S.; Christen, L.; Delgado-Domínguez, C.J.; Sanz-Gómez, S.; et al. The European Map of Axial Spondyloarthritis: Capturing the Patient Perspective—An Analysis of 2846 Patients Across 13 Countries. Curr. Rheumatol. Rep. 2019, 21, 19. [Google Scholar] [CrossRef]
- Kiltz, U.; Hoeper, K.; Hammel, L.; Lieb, S.; Hähle, A.; Meyer-Olson, D. Work participation in patients with axial spondyloarthritis: High prevalence of negative workplace experiences and long-term work impairment. RMD Open 2023, 9, e002663. [Google Scholar] [CrossRef]
- Tillett, W.; de-Vries, C.; McHugh, N.J. Work disability in psoriatic arthritis: A systematic review. Rheumatology 2012, 51, 275–283. [Google Scholar] [CrossRef]
- Baraliakos, X.; Braun, J. Hip involvement in ankylosing spondylitis. Rheumatology 2010, 49, 3–4. [Google Scholar] [CrossRef]
- Vander Cruyssen, B.; Munoz-Gomariz, E.; Font, P.; Mulero, J.; de Vlam, K.; Boonen, A.; Vazquez-Mellado, J.; Flores, D.; Vastesaeger, N.; Collantes, E. Hip involvement in ankylosing spondylitis: Epidemiology and risk factors associated with hip replacement surgery. Rheumatology 2010, 49, 73–81. [Google Scholar] [CrossRef]
- López-Medina, C.; Molto, A.; Sieper, J.; Duruöz, T.; Kiltz, U.; Elzorkany, B.; Hajjaj-Hassouni, N.; Burgos-Vargas, R.; Maldonado-Cocco, J.; Ziade, N.; et al. Prevalence and distribution of peripheral musculoskeletal manifestations in spondyloarthritis including psoriatic arthritis: Results of the worldwide, cross-sectional ASAS-PerSpA study. RMD Open 2021, 7, e001450. [Google Scholar] [CrossRef]
- Day, M.; Nam, D.; Goodman, S.; Su, E.P.; Figgie, M. Psoriatic Arthritis. J. Acad. Orthop. Surg. 2012, 20, 28–37. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Wang, I.T.; Huang, W.F.; Tsai, Y.W.; Shiu, M.N.; Tsai, T.F. Increased risk of avascular necrosis in patients with psoriatic disease: A nationwide population-based matched cohort study. J. Am. Acad. Dermatol. 2017, 76, 903–910.e1. [Google Scholar] [CrossRef]
- Kumar, A.; Nagai, H.; Oakley, J.; Luu, B.; Hussain, M.M.; Gaba, R. Short to long term outcomes of 154 cemented total hip arthroplasties in ankylosing spondylitis. J. Clin. Orthop. Trauma 2021, 14, 34–39. [Google Scholar] [CrossRef]
- López-Medina, C.; Castro-Villegas, M.C.; Collantes-Estévez, E. Hip and Shoulder Involvement and Their Management in Axial Spondyloarthritis: A Current Review. Curr. Rheumatol. Rep. 2020, 22, 53. [Google Scholar] [CrossRef]
- Bengtsson, K.; Askling, J.; Lorentzon, M.; Rosengren, B.; Deminger, A.; Klingberg, E.; Jacobsson, L.; Forsblad-d’Elia, H. Occurrence and relative risks for non-vertebral fractures in patients with ankylosing spondylitis compared with the general population: A register-based study from Sweden. RMD Open 2023, 9, e002753. [Google Scholar] [CrossRef]
- Ramírez, J.; Nieto-González, J.C.; Curbelo Rodríguez, R.; Castañeda, S.; Carmona, L. Prevalence and risk factors for osteoporosis and fractures in axial spondyloarthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2018, 48, 44–52. [Google Scholar] [CrossRef]
- Yi, E.; Ahuja, A.; Rajput, T.; Thomas George, A.; Park, Y. Clinical, Economic, and Humanistic Burden Associated with Delayed Diagnosis of Axial Spondyloarthritis: A Systematic Review. Rheumatol. Ther. 2020, 7, 65–87. [Google Scholar] [CrossRef]
- Haroon, M.; Gallagher, P.; Fitzgerald, O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann. Rheum. Dis. 2015, 74, 1045–1050. [Google Scholar] [CrossRef]
- Redeker, I.; Callhoff, J.; Hoffmann, F.; Haibel, H.; Sieper, J.; Zink, A.; Poddubnyy, D. Determinants of diagnostic delay in axial spondyloarthritis: An analysis based on linked claims and patient-reported survey data. Rheumatology 2019, 58, 1634–1638. [Google Scholar] [CrossRef]
- Packham, J. Optimizing outcomes for ankylosing spondylitis and axial spondyloarthritis patients: A holistic approach to care. Rheumatology 2018, 57, vi29–vi34. [Google Scholar] [CrossRef]
- Molto, A.; López-Medina, C.; Van Den Bosch, F.E.; Boonen, A.; Webers, C.; Dernis, E.; Van Gaalen, F.A.; Soubrier, M.; Claudepierre, P.; Baillet, A.; et al. Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: Results of the open-label, pragmatic, cluster-randomised TICOSPA trial. Ann. Rheum. Dis. 2021, 80, 1436–1444. [Google Scholar] [CrossRef]
- Jo, S.; Wang, S.E.; Lee, Y.L.; Kang, S.; Lee, B.; Han, J.; Sung, I.-H.; Park, Y.-S.; Bae, S.-C.; Kim, T.-H. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res. Ther. 2018, 20, 115. [Google Scholar] [CrossRef]
- Reinhardt, A.; Yevsa, T.; Worbs, T.; Lienenklaus, S.; Sandrock, I.; Oberdörfer, L.; Korn, T.; Weiss, S.; Förster, R.; Prinz, I. Interleukin-23-Dependent γ/δ T Cells Produce Interleukin-17 and Accumulate in the Enthesis, Aortic Valve, and Ciliary Body in Mice. Arthritis Rheumatol. 2016, 68, 2476–2486. [Google Scholar] [CrossRef]
- Ramiro, S.; Nikiphorou, E.; Sepriano, A.; Ortolan, A.; Webers, C.; Baraliakos, X.; Landewé, R.B.M.; Van den Bosch, F.E.; Boteva, B.; Bremander, A.; et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 2022, 82, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, H. Human monoclonal antibodies: The benefits of humanization. Methods Mol. Biol. 2019, 1904, 1–10. [Google Scholar] [PubMed]
- Ling, W.-L.; Lua, W.-H.; Gan, S.K.-E. Sagacity in antibody humanization for therapeutics, diagnostics and research purposes: Considerations of antibody elements and their roles. Antib. Ther. 2020, 3, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front. Immunol. 2021, 12, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; Hafler, D.A.; et al. Induction and molecular signature of pathogenic T H 17 cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. IL-23-IL-17 immune axis: Discovery, Mechanistic Understanding, and Clinical Testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Gagliani, N.; Amezcua Vesely, M.C.; Iseppon, A.; Brockmann, L.; Xu, H.; Palm, N.W.; De Zoete, M.R.; Licona-Limón, P.; Paiva, R.S.; Ching, T.; et al. TH17 cells transdifferentiate into regulatory T cells uring resolution of inflammation. Nature 2015, 523, 221–225. [Google Scholar] [CrossRef]
- Downs-Canner, S.; Berkey, S.; Delgoffe, G.M.; Edwards, R.P.; Curiel, T.; Odunsi, K.; Bartlett, D.L.; Obermajer, N. Suppressive IL-17A+ Foxp3+ and ex-Th17 IL-17Aneg Foxp3+ Treg cells are a source of tumour-associated Treg cells. Nat. Commun. 2017, 8, 14649. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Zhu, Y.; Liu, X.; Gu, Y.; Dai, X.; Li, B. Transcriptional and posttranslational regulation of Th17/Treg balance in health and disease. Eur. J. Immunol. 2021, 51, 2137–2150. [Google Scholar] [CrossRef]
- Eberl, G. RORγt, a multitask nuclear receptor at mucosal surfaces. Mucosal Immunol. 2017, 10, 27–34. [Google Scholar] [CrossRef]
- Kannan, A.K.; Su, Z.; Gauvin, D.M.; Paulsboe, S.E.; Duggan, R.; Lasko, L.M.; Honore, P.; Kort, M.E.; McGaraughty, S.P.; Scott, V.E.; et al. IL-23 induces regulatory T cell plasticity with implications for inflammatory skin diseases. Sci. Rep. 2019, 9, 17675. [Google Scholar] [CrossRef]
- Kotake, S.; Udagawa, N.; Takahashi, N.; Matsuzaki, K.; Itoh, K.; Ishiyama, S.; Saito, S.; Inoue, K.; Kamatani, N.; Gillespie, M.T.; et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Investig. 1999, 103, 1345–1352. [Google Scholar] [CrossRef]
- Torres, T.; Romanelli, M.; Chiricozzi, A. A revolutionary therapeutic approach for psoriasis: Bispecific biological agents. Expert Opin. Investig. Drugs 2016, 25, 751–754. [Google Scholar] [CrossRef]
- Conti, H.R.; Gaffen, S.L. IL-17–Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J. Immunol. 2015, 195, 780–788. [Google Scholar] [CrossRef]
- Li, J.; Casanova, J.L.; Puel, A. Mucocutaneous IL-17 immunity in mice and humans: Host defense vs. excessive inflammation. Mucosal Immunol. 2018, 11, 581–589. [Google Scholar] [CrossRef]
- Kumar, P.; Monin, L.; Castillo, P.; Elsegeiny, W.; Horne, W.; Eddens, T.; Vikram, A.; Good, M.; Schoenborn, A.A.; Bibby, K.; et al. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity 2016, 44, 659–671. [Google Scholar] [CrossRef]
- Liu, T.; Han, S.; Dai, Q.; Zheng, J.; Liu, C.; Li, S.; Li, J.; Ceribelli, A.; Motta, F.; Vecellio, M.; et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity 2018, 12, 624–631. [Google Scholar] [CrossRef]
- Naik, S.; Larsen, S.B.; Gomez, N.C.; Alaverdyan, K.; Sendoel, A.; Yuan, S.; Polak, L.; Kulukian, A.; Chai, S.; Fuchs, E. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 2017, 550, 475–480. [Google Scholar] [CrossRef]
- Ramani, K.; Jawale, C.V.; Verma, A.H.; Coleman, B.M.; Kolls, J.K.; Biswas, P.S. Unexpected kidney-restricted role for IL-17 receptor signaling in defense against systemic Candida albicans infection. JCI Insight 2018, 3, e98241. [Google Scholar] [CrossRef]
- Zúñiga, L.A.; Shen, W.-J.; Joyce-Shaikh, B.; Pyatnova, E.A.; Richards, A.G.; Thom, C.; Andrade, S.M.; Cua, D.J.; Kraemer, F.B.; Butcher, E.C. IL-17 Regulates Adipogenesis, Glucose Homeostasis, and Obesity. J. Immunol. 2010, 185, 6947–6959. [Google Scholar] [CrossRef]
- Albeltagy, E.S.; Elaziz, S.Y.A.; Abozaid, S.Y.; El Zomor, H.M.; Elhamed, S.S.A. Interleukin 6, interleukin 17, disease-related and contextual factor association with depression, and its severity in patients with rheumatoid arthritis. Clin. Rheumatol. 2021, 40, 895–904. [Google Scholar] [CrossRef]
- Ceribelli, A.; Motta, F.; Vecellio, M.; Isailovic, N.; Ciccia, F.; Selmi, C. Clinical Trials Supporting the Role of the IL-17/IL-23 Axis in Axial Spondyloarthritis. Front. Immunol. 2021, 12, 622770. [Google Scholar] [CrossRef]
- Liu, T.; Han, S.; Dai, Q.; Zheng, J.; Liu, C.; Li, S.; Li, J. IL-17A-Mediated Excessive Autophagy Aggravated Neuronal Ischemic Injuries via Src-PP2B-mTOR Pathway. Front. Immunol. 2019, 10, 2952. [Google Scholar] [CrossRef]
- Ni, P.; Dong, H.; Wang, Y.; Zhou, Q.; Xu, M.; Qian, Y.; Sun, J. IL-17A contributes to perioperative neurocognitive disorders through blood-brain barrier disruption in aged mice 11 Medical and Health Sciences 1103 Clinical Sciences. J. Neuroinflamm. 2018, 15, 332. [Google Scholar] [CrossRef]
- Krueger, J.G.; Wharton, K.A.; Schlitt, T.; Suprun, M.; Torene, R.I.; Jiang, X.; Wang, C.Q.; Fuentes-Duculan, J.; Hartmann, N.; Peters, T.; et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J. Allergy Clin. Immunol. 2019, 144, 750–763. [Google Scholar] [CrossRef]
- Fabre, T.; Molina, M.F.; Soucy, G.; Goulet, J.P.; Willems, B.; Villeneuve, J.P.; Bilodeau, M.; Shoukry, N.H. Type 3 cytokines IL-17A and IL-22 drive TGF–dependent liver fibrosis. Sci. Immunol. 2018, 3, eaar7754. [Google Scholar] [CrossRef]
- Reynolds, J.M.; Lee, Y.H.; Shi, Y.; Wang, X.; Angkasekwinai, P.; Nallaparaju, K.C.; Flaherty, S.; Chang, S.H.; Watarai, H.; Dong, C. Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity 2015, 42, 692–703. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Fujio, K.; Shoda, H.; Okamoto, A.; Tsuno, N.H.; Takahashi, K.; Yamamoto, K. IL-17B and IL-17C Are Associated with TNF-α Production and Contribute to the Exacerbation of Inflammatory Arthritis. J. Immunol. 2007, 179, 7128–7136. [Google Scholar] [CrossRef]
- You, Z.; DuRaine, G.; Tien, J.Y.L.; Lee, C.; Moseley, T.A.; Reddi, A.H. Expression of interleukin-17B in mouse embryonic limb buds and regulation by BMP-7 and bFGF. Biochem. Biophys. Res. Commun. 2005, 326, 624–631. [Google Scholar] [CrossRef]
- Kokubu, T.; Haudenschild, D.R.; Moseley, T.A.; Rose, L.; Reddi, A.H. Immunolocalization of IL-17A, IL-17B, and their receptors in chondrocytes during fracture healing. J. Histochem. Cytochem. 2008, 56, 89–95. [Google Scholar] [CrossRef]
- Ichinohe, N.; Ishii, M.; Tanimizu, N.; Kon, J.; Yoshioka, Y.; Ochiya, T.; Mizuguchi, T.; Hirata, K.; Mitaka, T. Transplantation of Thy1+ Cells Accelerates Liver Regeneration by Enhancing the Growth of Small Hepatocyte-Like Progenitor Cells via IL17RB Signaling. Stem Cells 2017, 35, 920–931. [Google Scholar] [CrossRef]
- Bie, Q.; Jin, C.; Zhang, B.; Dong, H. IL-17B: A new area of study in the IL-17 family. Mol. Immunol. 2017, 90, 50–56. [Google Scholar] [CrossRef]
- Bastid, J.; Dejou, C.; Docquier, A.; Bonnefoy, N. The Emerging Role of the IL-17B/IL-17RB Pathway in Cancer. Front. Immunol. 2020, 11, 718. [Google Scholar] [CrossRef]
- Huang, C.K.; Yang, C.Y.; Jeng, Y.M.; Chen, C.L.; Wu, H.H.; Chang, Y.C.; Ma, C.; Kuo, W.H.; Chang, K.J.; Shew, J.Y.; et al. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-κB-mediated antiapoptotic pathway. Oncogene 2014, 33, 2968–2977. [Google Scholar] [CrossRef]
- Shi, Y.; Ullrich, S.J.; Zhang, J.; Connolly, K.; Grzegorzewski, K.J.; Barber, M.C.; Wang, W.; Wathen, K.; Hodge, V.; Fisher, C.L.; et al. A novel cytokine receptor-ligand pair: Identification, molecular characterization, and in vivo immunomodutory activity. J. Biol. Chem. 2000, 275, 19167–19176. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional Specialization of Interleukin-17 Family Members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef]
- Robak, E.; Kulczycka-Siennicka, L.; Gerlicz, Z.; Kierstan, M.; Korycka-Wolowiec, A.; Sysa-Jedrzejowska, A. Correlations between concentrations of interleukin (IL)-17A, IL-17B and IL-17F, and endothelial cells and proangiogenic cytokines in systemic lupus erythematosus patients. Eur. Cytokine Netw. 2013, 24, 60–68. [Google Scholar] [CrossRef]
- Ramirez-Carrozzi, V.; Sambandam, A.; Luis, E.; Lin, Z.; Jeet, S.; Lesch, J.; Hackney, J.; Kim, J.; Zhou, M.; Lai, J.; et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011, 12, 1159–1166. [Google Scholar] [CrossRef]
- Peng, T.; Chanthaphavong, R.S.; Sun, S.; Trigilio, J.A.; Phasouk, K.; Jin, L.; Layton, E.D.; Li, A.Z.; Correnti, C.E.; De van der Schueren, W.; et al. Keratinocytes produce IL-17c to protect peripheral nervous systems during human HSV-2 reactivation. J. Exp. Med. 2017, 214, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Fritz, Y.; Dawes, S.M.; Diaconu, D.; Al-Attar, P.M.; Guzman, A.M.; Chen, C.S.; Fu, W.; Gudjonsson, J.E.; McCormick, T.S.; et al. Keratinocyte Overexpression of IL-17C Promotes Psoriasiform Skin Inflammation. J. Immunol. 2013, 190, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Vandeghinste, N.; Klattig, J.; Jagerschmidt, C.; Lavazais, S.; Marsais, F.; Haas, J.D.; Auberval, M.; Lauffer, F.; Moran, T.; Ongenaert, M.; et al. Neutralization of IL-17C Reduces Skin Inflammation in Mouse Models of Psoriasis and Atopic Dermatitis. J. Investig. Dermatol. 2018, 138, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Reynolds, J.M.; Pappu, B.P.; Chen, G.; Martinez, G.J.; Dong, C. Interleukin-17C Promotes Th17 Cell Responses and Autoimmune Disease via Interleukin-17 Receptor E. Immunity 2011, 35, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Krohn, S.; Nies, J.F.; Kapffer, S.; Schmidt, T.; Riedel, J.H.; Kaffke, A.; Peters, A.; Borchers, A.; Steinmetz, O.M.; Krebs, C.F.; et al. IL-17C/IL-17 receptor E signaling in CD4 + T cells promotes T H 17 cell-driven glomerular inflammation. J. Am. Soc. Nephrol. 2018, 29, 1210–1222. [Google Scholar] [CrossRef]
- O’Sullivan, T.; Saddawi-Konefka, R.; Gross, E.; Tran, M.; Mayfield, S.P.; Ikeda, H.; Bui, J.D. Interleukin-17D mediates tumor rejection through recruitment of natural killer cells. Cell Rep. 2014, 7, 989–998. [Google Scholar] [CrossRef]
- Starnes, T.; Broxmeyer, H.E.; Robertson, M.J.; Hromas, R. Cutting Edge: IL-17D, a Novel Member of the IL-17 Family, Stimulates Cytokine Production and Inhibits Hemopoiesis. J. Immunol. 2002, 169, 642–646. [Google Scholar] [CrossRef]
- Saddawi-Konefka, R.; Seelige, R.; Gross, E.T.E.; Levy, E.; Searles, S.C.; Washington, A.; Santosa, E.K.; Liu, B.; O’Sullivan, T.E.; Harismendy, O.; et al. Nrf2 Induces IL-17D to Mediate Tumor and Virus Surveillance. Cell Rep. 2016, 16, 2348–2358. [Google Scholar] [CrossRef]
- Huang, J.; Lee, H.Y.; Zhao, X.; Han, J.; Su, Y.; Sun, Q.; Shao, J.; Ge, J.; Zhao, Y.; Bai, X.; et al. Interleukin-17D regulates group 3 innate lymphoid cell function through its receptor CD93. Immunity 2021, 54, 673–686.e4. [Google Scholar] [CrossRef]
- Washington, A.; Varki, N.; Valderrama, J.A.; Nizet, V.; Bui, J.D. Evaluation of IL-17D in Host Immunity to Group A Streptococcus Infection. J. Immunol. 2020, 205, 3122–3129. [Google Scholar] [CrossRef]
- Yan, X.; Tu, H.; Liu, Y.; Chen, T.; Cao, J. Interleukin-17D Aggravates Sepsis by Inhibiting Macrophage Phagocytosis. Crit. Care Med. 2020, 48, e58–e65. [Google Scholar] [CrossRef]
- Fallon, P.G.; Ballantyne, S.J.; Mangan, N.E.; Barlow, J.L.; Dasvarma, A.; Hewett, D.R.; McIlgorm, A.; Jolin, H.E.; McKenzie, A.N.J. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 2006, 203, 1105–1116. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef]
- Mchenga, S.S.S.; Wang, D.; Li, C.; Shan, F.; Lu, C. Inhibitory effect of recombinant IL-25 on the development of dextran sulfate sodium-induced experimental colitis in mice. Cell. Mol. Immunol. 2008, 5, 425–431. [Google Scholar] [CrossRef]
- Kleinschek, M.A.; Owyang, A.M.; Joyce-Shaikh, B.; Langrish, C.L.; Chen, Y.; Gorman, D.M.; Blumenschein, W.M.; McClanahan, T.; Brombacher, F.; Hurst, S.D.; et al. IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 2007, 204, 161–170. [Google Scholar] [CrossRef]
- Miller, C.N.; Proekt, I.; von Moltke, J.; Wells, K.L.; Rajpurkar, A.R.; Wang, H.; Rattay, K.; Khan, I.S.; Metzger, T.C.; Pollack, J.L.; et al. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature 2018, 559, 627–631. [Google Scholar] [CrossRef]
- Borowczyk, J.; Shutova, M.; Brembilla, N.C.; Boehncke, W.H. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J. Allergy Clin. Immunol. 2021, 148, 40–52. [Google Scholar] [CrossRef]
- Fort, M.M.; Cheung, J.; Yen, D.; Li, J.; Zurawski, S.M.; Lo, S.; Menon, S.; Clifford, T.; Hunte, B.; Lesley, R.; et al. IL-25 Induces IL-4, IL-5, and IL-13 and Th2-Associated Pathologies In Vivo. Immunity 2001, 15, 985–995. [Google Scholar] [CrossRef]
- Beale, J.; Jayaraman, A.; Jackson, D.J.; Macintyre, J.D.R.; Edwards, M.R.; Walton, R.P.; Zhu, J.; Ching, Y.M.; Shamji, B.; Edwards, M.; et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci. Transl. Med. 2014, 6, 256ra134. [Google Scholar] [CrossRef]
- McMahon, D.F.; Gern, J.E. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Pediatrics 2011, 128. [Google Scholar] [CrossRef]
- Glatt, S.; Baeten, D.; Baker, T.; Griffiths, M.; Ionescu, L.; Lawson, A.D.G.; Maroof, A.; Oliver, R.; Popa, S.; Strimenopoulou, F.; et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: Evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann. Rheum. Dis. 2018, 77, 523–532. [Google Scholar] [CrossRef]
- Chen, Y.; Thai, P.; Zhao, Y.H.; Ho, Y.S.; DeSouza, M.M.; Wu, R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 2003, 278, 17036–17043. [Google Scholar] [CrossRef]
- Linden, S.V.D.; Valkenburg, H.A.; Cats, A. Evaluation of Diagnostic Criteria for Ankylosing Spondylitis. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Landewe, R.; van der Heijde, D.; Listing, J.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Collantes-Estevez, E.; Davis, J.; Dijkmans, B.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): Classification of paper patients by expert opinion including uncertainty appraisal. Ann. Rheum. Dis. 2009, 68, 770–776. [Google Scholar] [CrossRef]
- Rudwaleit, M.; van der Heijde, D.; Landewe, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Jurik, A.G.; Hermann, K.G.A.; Landewé, R.; Van Der Heijde, D.; Baraliakos, X.; Marzo-Ortega, H.; Østergaard, M.; Braun, J.; Sieper, J. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: A consensual approach by the ASAS/OMERACT MRI group. Ann. Rheum. Dis. 2009, 68, 1520–1527. [Google Scholar] [CrossRef]
- Faham, M.; Carlton, V.; Moorhead, M.; Zheng, J.; Klinger, M.; Pepin, F.; Asbury, T.; Vignali, M.; Emerson, R.O.; Robins, H.S.; et al. Discovery of T Cell Receptor β Motifs Specific to HLA–B27–Positive Ankylosing Spondylitis by Deep Repertoire Sequence Analysis. Arthritis Rheumatol. 2017, 69, 774–784. [Google Scholar] [CrossRef]
- Ranganathan, V.; Gracey, E.; Brown, M.A.; Inman, R.D.; Haroon, N. Pathogenesis of ankylosing spondylitis—Recent advances and future directions. Nat. Rev. Rheumatol. 2017, 13, 359–367. [Google Scholar] [CrossRef]
- Ke, D.; Fu, X.; Xue, Y.; Wu, H.; Zhang, Y.; Chen, X.; Hou, J. IL-17A regulates the autophagic activity of osteoclast precursors through RANKL-JNK1 signaling during osteoclastogenesis in vitro. Biochem. Biophys. Res. Commun. 2018, 497, 890–896. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, Y.; Du, F.; Chen, M.; Dong, X.; Chen, Y.; Huang, W. IL-17A Inhibits Osteogenic Differentiation of Bone Mesenchymal Stem Cells via Wnt Signaling Pathway. Med. Sci. Monit. 2017, 23, 4095–4101. [Google Scholar] [CrossRef]
- Croes, M.; Öner, F.C.; van Neerven, D.; Sabir, E.; Kruyt, M.C.; Blokhuis, T.J.; Dhert, W.J.A.; Alblas, J. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone 2016, 84, 262–270. [Google Scholar] [CrossRef]
- Schett, G.; Lories, R.J.; D’Agostino, M.-A.; Elewaut, D.; Kirkham, B.; Soriano, E.R.; McGonagle, D. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 2017, 13, 731–741. [Google Scholar] [CrossRef]
- Weinstein, J.E.; Pepple, K.L. Cytokines in uveitis. Curr. Opin. Ophthalmol. 2018, 29, 267–274. [Google Scholar] [CrossRef]
- Haroon, M.; Winchester, R.; Giles, J.T.; Heffernan, E.; FitzGerald, O. Certain class I HLA alleles and haplotypes implicated in susceptibility play a role in determining specific features of the psoriatic arthritis phenotype. Ann. Rheum. Dis. 2016, 75, 155–162. [Google Scholar] [CrossRef]
- Eder, L.; Chandran, V.; Pellet, F.; Shanmugarajah, S.; Rosen, C.F.; Bull, S.B.; Gladman, D.D. Human leucocyte antigen risk alleles for psoriatic arthritis among patients with psoriasis. Ann. Rheum. Dis. 2012, 71, 50–55. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Ocampo, D.V.; Gladman, D. Psoriatic arthritis. F1000Research 2019, 8, 1665. [Google Scholar] [CrossRef]
- Cuthbert, R.J.; Watad, A.; Fragkakis, E.M.; Dunsmuir, R.; Loughenbury, P.; Khan, A.; Millner, P.A.; Davison, A.; Marzo-Ortega, H.; Newton, D.; et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann. Rheum. Dis. 2019, 78, 1559–1565. [Google Scholar] [CrossRef]
- McInnes, I.B.; Mease, P.J.; Kirkham, B.; Kavanaugh, A.; Ritchlin, C.T.; Rahman, P.; Van Der Heijde, D.; Landewé, R.; Conaghan, P.G.; Gottlieb, A.B.; et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1137–1146. [Google Scholar] [CrossRef]
- Deodhar, A.; Helliwell, P.S.; Boehncke, W.-H.; Kollmeier, A.P.; Hsia, E.C.; Subramanian, R.A.; Xu, X.L.; Sheng, S.; Agarwal, P.; Zhou, B.; et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1115–1125. [Google Scholar] [CrossRef]
- Kopylov, U.; Starr, M.; Watts, C.; Dionne, S.; Girardin, M.; Seidman, E.G. Detection of Crohn Disease in Patients with Spondyloarthropathy: The SpACE Capsule Study. J. Rheumatol. 2018, 45, 498–505. [Google Scholar] [CrossRef]
- Shrestha, S.; Brand, J.S.; Järås, J.; Schoultz, I.; Montgomery, S.; Askling, J.; Ludvigsson, J.F.; Olen, O.; Halfvarson, J.; Olsson, M.; et al. Association Between Inflammatory Bowel Disease and Spondyloarthritis: Findings from a Nationwide Study in Sweden. J. Crohn’s Colitis 2022, 16, 1540–1550. [Google Scholar] [CrossRef]
- Parkes, M.; Cortes, A.; van Heel, D.A.; Brown, M.A. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat. Rev. Genet. 2013, 14, 661–673. [Google Scholar] [CrossRef]
- Gracey, E.; Dumas, E.; Yerushalmi, M.; Qaiyum, Z.; Inman, R.D.; Elewaut, D. The ties that bind: Skin, gut and spondyloarthritis. Curr. Opin. Rheumatol. 2019, 31, 62–69. [Google Scholar] [CrossRef]
- Lécuyer, E.; Rakotobe, S.; Lengliné-Garnier, H.; Lebreton, C.; Picard, M.; Juste, C.; Fritzen, R.; Eberl, G.; McCoy, K.D.; Macpherson, A.J.; et al. Segmented Filamentous Bacterium Uses Secondary and Tertiary Lymphoid Tissues to Induce Gut IgA and Specific T Helper 17 Cell Responses. Immunity 2014, 40, 608–620. [Google Scholar] [CrossRef]
- Goodall, J.C.; Wu, C.; Zhang, Y.; McNeill, L.; Ellis, L.; Saudek, V.; Gaston, J.S.H. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc. Natl. Acad. Sci. USA 2010, 107, 17698–17703. [Google Scholar] [CrossRef]
- Ditto, M.C.; Parisi, S.; Landolfi, G.; Borrelli, R.; Realmuto, C.; Finucci, A.; Caviglia, G.P.; Ribaldone, D.G.; Astegiano, M.; Zanetti, A.; et al. Intestinal microbiota changes induced by TNF-inhibitors in IBD-related spondyloarthritis. RMD Open 2021, 7, e001755. [Google Scholar] [CrossRef]
- Park, Y.E.; Moon, H.S.; Yong, D.; Seo, H.; Yang, J.; Shin, T.-S.; Kim, Y.-K.; Kim, J.R.; Lee, Y.N.; Kim, Y.-H.; et al. Microbial changes in stool, saliva, serum, and urine before and after anti-TNF-α therapy in patients with inflammatory bowel diseases. Sci. Rep. 2022, 12, 6359. [Google Scholar] [CrossRef]
- Hueber, W.; Sands, B.E.; Lewitzky, S.; Vandemeulebroecke, M.; Reinisch, W.; Higgins, P.D.R.; Wehkamp, J.; Feagan, B.G.; Yao, M.D.; Karczewski, M.; et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012, 61, 1693–1700. [Google Scholar] [CrossRef]
- Targan, S.R.; Feagan, B.; Vermeire, S.; Panaccione, R.; Melmed, G.Y.; Landers, C.; Li, D.; Russell, C.; Newmark, R.; Zhang, N.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of Brodalumab in Patients with Moderate-to-Severe Crohn’s Disease. Am. J. Gastroenterol. 2016, 111, 1599–1607. [Google Scholar] [CrossRef]
- Wang, J.; Bhatia, A.; Cleveland, N.K.; Gupta, N.; Dalal, S.; Rubin, D.T.; Sakuraba, A. Rapid Onset of Inflammatory Bowel Disease after Receiving Secukinumab Infusion. ACG Case Rep. J. 2018, 5, e56. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sendid, B.; Jouault, T.; Poulain, D. Secukinumab failure in Crohn’s disease: The yeast connection? Gut 2013, 62, 800–801. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- Sharip, A.; Kunz, J. Understanding the Pathogenesis of Spondyloarthritis. Biomolecules 2020, 10, 1461. [Google Scholar] [CrossRef]
- Noto Llana, M.; Sarnacki, S.H.; Morales, A.L.; Aya Castañeda, M.d.R.; Giacomodonato, M.N.; Blanco, G.; Cerquetti, M.C. Activation of iNKT Cells Prevents Salmonella-Enterocolitis and Salmonella-Induced Reactive Arthritis by Downregulating IL-17-Producing γδT Cells. Front. Cell. Infect. Microbiol. 2017, 7, 398. [Google Scholar] [CrossRef]
- Díaz-Peña, R.; Blanco-Gelaz, M.A.; Njobvu, P.; López-Vazquez, A.; Suárez-Alvarez, B.; López-Larrea, C. Influence of HLA-B*5703 and HLA-B*1403 on Susceptibility to Spondyloarthropathies in the Zambian Population. J. Rheumatol. 2008, 35, 2236–2240. [Google Scholar] [CrossRef]
- Ge, S.; He, Q.; Granfors, K. HLA-B27 Modulates Intracellular Growth of Salmonella Pathogenicity Island 2 Mutants and Production of Cytokines in Infected Monocytic U937 Cells. PLoS ONE 2012, 7, e34093. [Google Scholar] [CrossRef]
- Zeng, H.; Luo, B.; Zhang, Y.; Xie, Z.; Ye, Z. Treatment of reactive arthritis with biological agents: A review. Biosci. Rep. 2020, 40, BSR20191927. [Google Scholar] [CrossRef]
- Deodhar, A.; Miossec, P.; Baraliakos, X. Is undifferentiated spondyloarthritis a discrete entity? A debate. Autoimmun. Rev. 2018, 17, 29–32. [Google Scholar] [CrossRef]
- Xia, Q.; Fan, D.; Yang, X.; Li, X.; Zhang, X.; Wang, M.; Xu, S.; Pan, F. Progression rate of ankylosing spondylitis in patients with undifferentiated spondyloarthritis. Medicine 2017, 96, e5960. [Google Scholar] [CrossRef]
- Zaripova, L.N.; Midgley, A.; Christmas, S.E.; Beresford, M.W.; Baildam, E.M.; Oldershaw, R.A. Juvenile idiopathic arthritis: From aetiopathogenesis to therapeutic approaches. Pediatr. Rheumatol. 2021, 19, 135. [Google Scholar] [CrossRef]
- Paroli, M.; Spadea, L.; Caccavale, R.; Spadea, L.; Paroli, M.P.; Nante, N. The Role of Interleukin-17 in Juvenile Idiopathic Arthritis: From Pathogenesis to Treatment. Medicina 2022, 58, 1552. [Google Scholar] [CrossRef]
- Kiwalkar, S.; Beier, S.; Deodhar, A. Ixekizumab for treating ankylosing spondylitis. Immunotherapy 2019, 11, 1273–1282. [Google Scholar] [CrossRef]
- Baraliakos, X.; Kivitz, A.J.; Deodhar, A.A.; Braun, J.; Wei, J.C.; Delicha, E.M.; Talloczy, Z.; Porter, B. MEASURE 1 Study Group Long-term effects of interleukin-17A inhibition with secukinumab in active ankylosing spondylitis: 3-year efficacy and safety results from an extension of the Phase 3 MEASURE 1 trial. Clin. Exp. Rheumatol. 2018, 36, 50–55. [Google Scholar]
- Erdes, S.; Nasonov, E.; Kunder, E.; Pristrom, A.; Soroka, N.; Shesternya, P.; Dubinina, T.; Smakotina, S.; Raskina, T.; Krechikova, D.; et al. Primary efficacy of netakimab, a novel interleukin-17 inhibitor, in the treatment of active ankylosing spondylitis in adults. Clin. Exp. Rheumatol. 2020, 38, 27–34. [Google Scholar] [CrossRef]
- Kohler, G.; Milstein, C. Continous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Shepard, H.M.; Phillips, G.L.; Thanos, C.D.; Feldmann, M. Developments in therapy with monoclonal antibodies and related proteins. Clin. Med. J. R. Coll. Physicians Lond. 2017, 17, 220–232. [Google Scholar] [CrossRef]
- Genovese, M.C.; Van den Bosch, F.; Roberson, S.A.; Bojin, S.; Biagini, I.M.; Ryan, P.; Sloan-Lancaster, J. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010, 62, 929–939. [Google Scholar] [CrossRef]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.M.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in Plaque Psoriasis—Results of Two Phase 3 Trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef]
- Navid, F.; Holt, V.; Colbert, R.A. The enigmatic role of HLA-B*27 in spondyloarthritis pathogenesis. Semin. Immunopathol. 2021, 43, 235–243. [Google Scholar] [CrossRef]
- Mease, P.J.; McInnes, I.B.; Kirkham, B.; Kavanaugh, A.; Rahman, P.; van der Heijde, D.; Landewé, R.; Nash, P.; Pricop, L.; Yuan, J.; et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 2015, 373, 1329–1339. [Google Scholar] [CrossRef]
- Baeten, D.; Sieper, J.; Braun, J.; Baraliakos, X.; Dougados, M.; Emery, P.; Deodhar, A.; Porter, B.; Martin, R.; Andersson, M.; et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. [Google Scholar] [CrossRef]
- Deodhar, A.; Blanco, R.; Dokoupilová, E.; Hall, S.; Kameda, H.; Kivitz, A.J.; Poddubnyy, D.; van de Sande, M.; Wiksten, A.S.; Porter, B.O.; et al. Improvement of Signs and Symptoms of Nonradiographic Axial Spondyloarthritis in Patients Treated with Secukinumab: Primary Results of a Randomized, Placebo-Controlled Phase III Study. Arthritis Rheumatol. 2021, 73, 110–120. [Google Scholar] [CrossRef]
- Pavelka, K.; Kivitz, A.; Dokoupilova, E.; Blanco, R.; Maradiaga, M.; Tahir, H.; Pricop, L.; Andersson, M.; Readie, A.; Porter, B. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: A randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res. Ther. 2017, 19, 285. [Google Scholar] [CrossRef]
- Braun, J.; Baraliakos, X.; Deodhar, A.; Poddubnyy, D.; Emery, P.; Delicha, E.M.; Talloczy, Z.; Porter, B. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatol 2019, 58, 859–868. [Google Scholar] [CrossRef]
- Kampylafka, E.; d’Oliveira, I.; Linz, C.; Lerchen, V.; Stemmler, F.; Simon, D.; Englbrecht, M.; Sticherling, M.; Rech, J.; Kleyer, A.; et al. Resolution of synovitis and arrest of catabolic and anabolic bone changes in patients with psoriatic arthritis by IL-17A blockade with secukinumab: Results from the prospective PSARTROS study. Arthritis Res. Ther. 2018, 20, 153. [Google Scholar] [CrossRef]
- McInnes, I.B.; Behrens, F.; Mease, P.J.; Kavanaugh, A.; Ritchlin, C.; Nash, P.; Masmitja, J.G.; Goupille, P.; Korotaeva, T.; Gottlieb, A.B.; et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): A double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 2020, 395, 1496–1505. [Google Scholar] [CrossRef]
- Baraliakos, X.; Østergaard, M.; Gensler, L.S.; Poddubnyy, D.; Lee, E.Y.; Kiltz, U.; Martin, R.; Sawata, H.; Readie, A.; Porter, B. Comparison of the Effects of Secukinumab and Adalimumab Biosimilar on Radiographic Progression in Patients with Ankylosing Spondylitis: Design of a Randomized, Phase IIIb Study (SURPASS). Clin. Drug Investig. 2020, 40, 269–278. [Google Scholar] [CrossRef]
- Khatri, A.; Klünder, B.; Peloso, P.M.; Othman, A.A. Exposure-response analyses demonstrate no evidence of interleukin 17A contribution to efficacy of ABT-122 in rheumatoid or psoriatic arthritis. Rheumatology 2019, 58, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Weinblatt, M.E.; Aelion, J.A.; Mansikka, H.T.; Peloso, P.M.; Chen, K.; Li, Y.; Othman, A.A.; Khatri, A.; Khan, N.S.; et al. ABT-122, a Bispecific Dual Variable Domain Immunoglobulin Targeting Tumor Necrosis Factor and Interleukin-17A, in Patients with Rheumatoid Arthritis with an Inadequate Response to Methotrexate: A Randomized, Double-Blind Study. Arthritis Rheumatol. 2018, 70, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Kampylafka, E.; Simon, D.; D’Oliveira, I.; Linz, C.; Lerchen, V.; Englbrecht, M.; Rech, J.; Kleyer, A.; Sticherling, M.; Schett, G.; et al. Disease interception with interleukin-17 inhibition in high-risk psoriasis patients with subclinical joint inflammation—Data from the prospective IVEPSA study. Arthritis Res. Ther. 2019, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- O’Rielly, D.D.; Rahman, P. A review of ixekizumab in the treatment of psoriatic arthritis. Expert Rev. Clin. Immunol. 2018, 14, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Dougados, M.; Wei, J.C.C.; Landewé, R.; Sieper, J.; Baraliakos, X.; Van Den Bosch, F.; Maksymowych, W.P.; Ermann, J.; Walsh, J.A.; Tomita, T.; et al. Efficacy and safety of ixekizumab through 52 weeks in two phase 3, randomised, controlled clinical trials in patients with active radiographic axial spondyloarthritis (COAST-V and COAST-W). Ann. Rheum. Dis. 2019, 79, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, A.; van der Heijde, D.; Gensler, L.S.; Kim, T.H.; Maksymowych, W.P.; Østergaard, M.; Poddubnyy, D.; Marzo-Ortega, H.; Bessette, L.; Tomita, T.; et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): A randomised, placebo-controlled trial. Lancet 2020, 395, 53–64. [Google Scholar] [CrossRef]
- Chandran, V.; Van Der Heijde, D.; Fleischmann, R.M.; Lespessailles, E.; Helliwell, P.S.; Kameda, H.; Burgos-Vargas, R.; Erickson, J.S.; Rathmann, S.S.; Sprabery, A.T.; et al. Ixekizumab treatment of biologic-naïve patients with active psoriatic arthritis: 3-year results from a phase III clinical trial (SPIRIT-P1). Rheumatology 2020, 59, 2774–2784. [Google Scholar] [CrossRef]
- Puig, L.; Bakulev, A.L.; Kokhan, M.M.; Samtsov, A.V.; Khairutdinov, V.R.; Morozova, M.A.; Zolkin, N.A.; Kuryshev, I.V.; Petrov, A.N.; Artemeva, A.V.; et al. Efficacy and Safety of Netakimab, A Novel Anti-IL-17 Monoclonal Antibody, in Patients with Moderate to Severe Plaque Psoriasis. Results of A 54-Week Randomized Double-Blind Placebo-Controlled PLANETA Clinical Trial. Dermatol. Ther. 2021, 11, 1319–1332. [Google Scholar] [CrossRef]
- Korotaeva, T.; Gaydukova, I.; Mazurov, V.; Samtsov, A.; Khayrutdinov, V.; Bakulev, A.; Kokhan, M.; Kundzer, A.; Soroka, N.; Dokukina, E.; et al. Netakimab decreases disease activity in patients with psoriatic arthritis: Results from a randomized double-blind phase 3 clinical trial (PATERA). Ann. Rheum. Dis. 2020, 79, 141–142. [Google Scholar] [CrossRef]
- Korotaeva, T.; Mazurov, V.; Lila, A.; Gaydukova, I.; Bakulev, A.; Samtsov, A.; Khairutdinov, V.; Zinkina-Orikhan, A.V.; Sevastyanova, Y.; Eremeeva, A. Efficacy of netakimab in key psoriatic arthritis domains: 54-week results from the phase III BCD-085-8/PATERA study. Rheumatol. Sci. Pract. 2021, 59, 47–55. (In Russia) [Google Scholar] [CrossRef]
- Mazurov, V.I.; Erdes, S.F.; Gaydukova, I.Z.; Dubinina, T.V.; Pristrom, A.M.; Kunder, E.V.; Soroka, N.F.; Kastanayan, A.A.; Povarova, T.V.; Zhugrova, E.S.; et al. Long-term efficacy and safety of netakimab in the treatment of ankylosing spondylitis: Results of Phase III international, multicenter, randomized double-blind clinical trial BCD-085-5/ASTERA. Mod. Rheumatol. J. 2020, 14, 39–49. (In Russia) [Google Scholar] [CrossRef]
- Reich, K.; Warren, R.B.; Lebwohl, M.; Gooderham, M.; Strober, B.; Langley, R.G.; Paul, C.; De Cuyper, D.; Vanvoorden, V.; Madden, C.; et al. Bimekizumab versus Secukinumab in Plaque Psoriasis. N. Engl. J. Med. 2021, 385, 142–152. [Google Scholar] [CrossRef]
- Mease, P.J.; Asahina, A.; Gladman, D.D.; Tanaka, Y.; Tillett, W.; Ink, B.; Assudani, D.; de la Loge, C.; Coarse, J.; Eells, J.; et al. Effect of bimekizumab on symptoms and impact of disease in patients with psoriatic arthritis over 3 years: Results from BE ACTIVE. Rheumatology 2022, 62, 617–628. [Google Scholar] [CrossRef]
- Van Der Heijde, D.; Gensler, L.S.; Deodhar, A.; Baraliakos, X.; Poddubnyy, D.; Kivitz, A.; Farmer, M.K.; Baeten, D.; Goldammer, N.; Coarse, J.; et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: Results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann. Rheum. Dis. 2020, 79, 595–604. [Google Scholar] [CrossRef]
- Van der Heijde, D.; Baraliakos, X.; Dougados, M.; Brown, M.; Poddubnyy, D.; Van den Bosch, F.; Haroon, N.; Xu, H.; Tomita, T.; Gensler, L.S.; et al. Bimekizumab in Patients with Active Ankylosing Spondylitis: 24-Week Efficacy & Safety from Be Mobile 2, a Phase 3, Multicentre, Randomised, Placebo-Controlled Study. Ann. Rheum. Dis. 2022, 81, 12–13. [Google Scholar] [CrossRef]
- Facheris, P.; Valenti, M.; Pavia, G.; Guanziroli, E.; Narcisi, A.; Borroni, R.G.; Costanzo, A. Brodalumab: A new way to inhibit IL-17 in psoriasis. Dermatol. Ther. 2020, 33, e13403. [Google Scholar] [CrossRef]
- Greig, S.L. Brodalumab: First Global Approval. Drugs 2016, 76, 1403–1412. [Google Scholar] [CrossRef]
- Blair, H.A. Brodalumab: A Review in Moderate to Severe Plaque Psoriasis. Drugs 2018, 78, 495–504. [Google Scholar] [CrossRef]
- Mease, P.J.; Helliwell, P.S.; Hjuler, K.F.; Raymond, K.; Mcinnes, I. Brodalumab in psoriatic arthritis: Results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann. Rheum. Dis. 2021, 80, 185–193. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, H.; Li, Q.; Chen, H.; Fang, M.; Yang, L.; Ding, Y. Pharmacokinetics, Pharmacodynamics, Safety, Tolerability, and Immunogenicity of the QX002N anti-IL-17 Monoclonal Antibody: A Phase I, Randomized, Double-Blind, Single Ascending Dose Study in Healthy Chinese Volunteers. Front. Pharmacol. 2022, 12, 3978. [Google Scholar] [CrossRef]
- Wang, J.; Kang, G.; Yuan, H.; Cao, X.; Huang, H.; de Marco, A. Research Progress and Applications of Multivalent, Multispecific and Modified Nanobodies for Disease Treatment. Front. Immunol. 2022, 12, 6013. [Google Scholar] [CrossRef]
- Papp, K.A.; Weinberg, M.A.; Morris, A.; Reich, K. IL17A/F nanobody sonelokimab in patients with plaque psoriasis: A multicentre, randomised, placebo-controlled, phase 2b study. Lancet 2021, 397, 1564–1575. [Google Scholar] [CrossRef]
- Svecova, D.; Lubell, M.W.; Casset-Semanaz, F.; Mackenzie, H.; Grenningloh, R.; Krueger, J.G. A randomized, double-blind, placebo-controlled phase 1 study of multiple ascending doses of subcutaneous M1095, an anti–interleukin 17A/F nanobody, in moderate-to-severe psoriasis. J. Am. Acad. Dermatol. 2019, 81, 196–203. [Google Scholar] [CrossRef]
- Kammüller, M.; Tsai, T.-F.; Griffiths, C.E.; Kapoor, N.; Kolattukudy, P.E.; Brees, D.; Chibout, S.-D.; Safi Jr, J.; Fox, T. Inhibition of IL-17A by secukinumab shows no evidence of increased Mycobacterium tuberculosis infections. Clin. Transl. Immunol. 2017, 6, e152. [Google Scholar] [CrossRef]
- Elewski, B.E.; Baddley, J.W.; Deodhar, A.A.; Magrey, M.; Rich, P.A.; Soriano, E.R.; Soung, J.; Bao, W.; Keininger, D.; Marfo, K.; et al. Association of Secukinumab Treatment with Tuberculosis Reactivation in Patients With Psoriasis, Psoriatic Arthritis, or Ankylosing Spondylitis. JAMA Dermatol. 2021, 157, 43. [Google Scholar] [CrossRef]
- Satoh, Y.; Nakano, K.; Yoshinari, H.; Nakayamada, S.; Iwata, S.; Kubo, S.; Miyagawa, I.; Yoshikawa, M.; Miyazaki, Y.; Saito, K.; et al. A case of refractory lupus nephritis complicated by psoriasis vulgaris that was controlled with secukinumab. Lupus 2018, 27, 1202–1206. [Google Scholar] [CrossRef]
- Fieldhouse, K.A.; Ukaibe, S.; Crowley, E.L.; Khanna, R.; O’Toole, A.; Gooderham, M.J. Inflammatory bowel disease in patients with psoriasis treated with interleukin-17 inhibitors. Drugs Context 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Gordon, K.B.; Langley, R.G.; Warren, R.B.; Okubo, Y.; Stein Gold, L.; Merola, J.F.; Peterson, L.; Wixted, K.; Cross, N.; Deherder, D.; et al. Bimekizumab Safety in Patients with Moderate to Severe Plaque Psoriasis. JAMA Dermatol. 2022, 158, 735. [Google Scholar] [CrossRef]
- Minnema, L.A.; Giezen, T.J.; Souverein, P.C.; Egberts, T.C.G.; Leufkens, H.G.M.; Gardarsdottir, H. Exploring the Association between Monoclonal Antibodies and Depression and Suicidal Ideation and Behavior: A VigiBase Study. Drug Saf. 2019, 42, 887–895. [Google Scholar] [CrossRef]
- Schmidt, C. Suicidal thoughts end Amgen’s blockbuster aspirations for psoriasis drug. Nat. Biotechnol. 2015, 33, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Talotta, R.; Salaffi, F.; Cassinotti, A.; Varisco, V.; Battellino, M.; Ardizzone, S.; Pace, F.; Sarzi-Puttini, P. Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun. Rev. 2013, 12, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Vaisman-Mentesh, A.; Gutierrez-Gonzalez, M.; DeKosky, B.J.; Wine, Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation Following Treatment With Monoclonal Antibodies. Front. Immunol. 2020, 11, 1951. [Google Scholar] [CrossRef] [PubMed]
- Talotta, R.; Rucci, F.; Canti, G.; Scaglione, F. Pros and cons of the immunogenicity of monoclonal antibodies in cancer treatment: A lesson from autoimmune diseases. Immunotherapy 2019, 11, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Damiani, A.; Li Gobbi, F.; Bandinelli, F.; Infantino, M.; Grossi, V.; Manfredi, M.; Noguier, G.; Meacci, F. Correlation between HLA haplotypes and the development of antidrug antibodies in a cohort of patients with rheumatic diseases. Biol. Targets Ther. 2018, 12, 37–41. [Google Scholar] [CrossRef]
- Sazonovs, A.; Kennedy, N.A.; Moutsianas, L.; Heap, G.A.; Rice, D.L.; Reppell, M.; Bewshea, C.M.; Chanchlani, N.; Walker, G.J.; Perry, M.H.; et al. HLA-DQA1*05 Carriage Associated with Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients With Crohn’s Disease. Gastroenterology 2020, 158, 189–199. [Google Scholar] [CrossRef]
- Bagel, J.; Lebwohl, M.; Israel, R.J.; Jacobson, A. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J. Am. Acad. Dermatol. 2020, 82, 344–351. [Google Scholar] [CrossRef]
- Mrowietz, U.; Leonardi, C.L.; Girolomoni, G.; Toth, D.; Morita, A.; Balki, S.A.; Szepietowski, J.C.; Regnault, P.; Thurston, H.; Papavassilis, C. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: A randomized, double-blind, noninferiority trial (SCULPTURE). J. Am. Acad. Dermatol. 2015, 73, 27–36.e1. [Google Scholar] [CrossRef]
- Cui, Y.; Cui, P.; Chen, B.; Li, S.; Guan, H. Monoclonal antibodies: Formulations of marketed products and recent advances in novel delivery system. Drug Dev. Ind. Pharm. 2017, 43, 519–530. [Google Scholar] [CrossRef]
- Elgundi, Z.; Reslan, M.; Cruz, E.; Sifniotis, V.; Kayser, V. The state-of-play and future of antibody therapeutics. Adv. Drug Deliv. Rev. 2017, 122, 2–19. [Google Scholar] [CrossRef]
- Keeling, S.; Maksymowych, W.P. JAK inhibitors, psoriatic arthritis, and axial spondyloarthritis: A critical review of clinical trials. Expert Rev. Clin. Immunol. 2021, 17, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, A.; Van den Bosch, F.; Poddubnyy, D.; Maksymowych, W.P.; van der Heijde, D.; Kim, T.-H.; Kishimoto, M.; Blanco, R.; Duan, Y.; Li, Y.; et al. Upadacitinib for the treatment of active non-radiographic axial spondyloarthritis (SELECT-AXIS 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2022, 400, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Compán, V.; Wei, J.C.C.; Van den Bosch, F.; Magrey, M.; Wang, L.; Fleishaker, D.; Cappelleri, J.C.; Wang, C.; Wu, J.; Dina, O.; et al. Effect of tofacitinib on pain, fatigue, health-related quality of life and work productivity in patients with active ankylosing spondylitis: Results from a phase III, randomised, double-blind, placebo-controlled trial. RMD Open 2022, 8, e002253. [Google Scholar] [CrossRef] [PubMed]
- van der Heijde, D.; Baraliakos, X.; Sieper, J.; Deodhar, A.; Inman, R.D.; Kameda, H.; Zeng, X.; Sui, Y.; Bu, X.; Pangan, A.L.; et al. Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: A double-blind, randomised, placebo-controlled phase 3 trial. Ann. Rheum. Dis. 2022, 81, 1515–1523. [Google Scholar] [CrossRef]
- Paroli, M.; Caccavale, R.; Paroli, M.P.; Spadea, L.; Accapezzato, D. Janus Kinase Inhibitors: A New Tool for the Treatment of Axial Spondyloarthritis. Int. J. Mol. Sci. 2023, 24, 1027. [Google Scholar] [CrossRef]
- Atzeni, F.; Popa, C.D.; Nucera, V.; Nurmohamed, M.T. Safety of JAK inhibitors: Focus on cardiovascular and thromboembolic events. Expert Rev. Clin. Immunol. 2022, 18, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.; Raine, T.; Rivett, L.; Roberts, J.; Dews, S.-A.; Choy, E.H. Herpes zoster and Janus kinase inhibition in rheumatology and gastroenterology patients: Managing risk and vaccination. Clin. Exp. Rheumatol. 2021, 40, 1432–1441. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Deodhar, A.; Sliwinska-Stanczyk, P.; Xu, H.; Baraliakos, X.; Gensler, L.S.; Fleishaker, D.; Wang, L.; Wu, J.; Menon, S.; Wang, C.; et al. Tofacitinib for the treatment of ankylosing spondylitis: A phase III, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2021, 80, 1004–1013. [Google Scholar] [CrossRef]
- Mease, P.; Hall, S.; FitzGerald, O.; van der Heijde, D.; Merola, J.F.; Avila-Zapata, F.; Cieślak, D.; Graham, D.; Wang, C.; Menon, S.; et al. Tofacitinib or Adalimumab versus Placebo for Psoriatic Arthritis. N. Engl. J. Med. 2017, 377, 1537–1550. [Google Scholar] [CrossRef]
- Gladman, D.; Rigby, W.; Azevedo, V.F.; Behrens, F.; Blanco, R.; Kaszuba, A.; Kudlacz, E.; Wang, C.; Menon, S.; Hendrikx, T.; et al. Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. N. Engl. J. Med. 2017, 377, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- van der Heijde, D.; Song, I.H.; Pangan, A.L.; Deodhar, A.; van den Bosch, F.; Maksymowych, W.P.; Kim, T.H.; Kishimoto, M.; Everding, A.; Sui, Y.; et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): A multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet 2019, 394, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Lertratanakul, A.; Anderson, J.K.; Papp, K.; Van Den Bosch, F.; Tsuji, S.; Dokoupilova, E.; Keiserman, M.; Wang, X.; Zhong, S.; et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann. Rheum. Dis. 2021, 80, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Menter, M.A.; Raman, M.; Disch, D.; Schlichting, D.E.; Gaich, C.; Macias, W.; Zhang, X.; Janes, J.M. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br. J. Dermatol. 2016, 174, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Luo, Y.; O’Shea, J.J.; Nakayamada, S. Janus kinase-targeting therapies in rheumatology: A mechanisms-based approach. Nat. Rev. Rheumatol. 2022, 18, 133–145. [Google Scholar] [CrossRef]
- van der Heijde, D.; Baraliakos, X.; Gensler, L.S.; Maksymowych, W.P.; Tseluyko, V.; Nadashkevich, O.; Abi-Saab, W.; Tasset, C.; Meuleners, L.; Besuyen, R.; et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): Results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018, 392, 2378–2387. [Google Scholar] [CrossRef]
- Mease, P.J.; Deodhar, A.A.; Van Der Heijde, D.; Behrens, F.; Kivitz, A.J.; Neal, J.; Kim, J.; Singhal, S.; Nowak, M.; Banerjee, S. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann. Rheum. Dis. 2022, 81, 815–822. [Google Scholar] [CrossRef]
- Zanin-Zhorov, A.; Weiss, J.M.; Trzeciak, A.; Chen, W.; Zhang, J.; Nyuydzefe, M.S.; Arencibia, C.; Polimera, S.; Schueller, O.; Fuentes-Duculan, J.; et al. Cutting Edge: Selective Oral ROCK2 Inhibitor Reduces Clinical Scores in Patients with Psoriasis Vulgaris and Normalizes Skin Pathology via Concurrent Regulation of IL-17 and IL-10. J. Immunol. 2017, 198, 3809–3814. [Google Scholar] [CrossRef]
- Ciofani, M.; Madar, A.; Galan, C.; Sellars, M.; MacE, K.; Pauli, F.; Agarwal, A.; Huang, W.; Parkurst, C.N.; Muratet, M.; et al. A validated regulatory network for Th17 cell specification. Cell 2012, 151, 289–303. [Google Scholar] [CrossRef]
- Leppkes, M.; Becker, C.; Ivanov, I.I.; Hirth, S.; Wirtz, S.; Neufert, C.; Pouly, S.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; et al. RORγ-Expressing Th17 Cells Induce Murine Chronic Intestinal Inflammation via Redundant Effects of IL-17A and IL-17F. Gastroenterology 2009, 136, 257–267. [Google Scholar] [CrossRef]
- Guendisch, U.; Weiss, J.; Ecoeur, F.; Riker, J.C.; Kaupmann, K.; Kallen, J.; Hintermann, S.; Orain, D.; Dawson, J.; Billich, A.; et al. Pharmacological inhibition of RORγt suppresses the Th17 pathway and alleviates arthritis in vivo. PLoS ONE 2017, 12, e0188391. [Google Scholar] [CrossRef] [PubMed]
- Venken, K.; Jacques, P.; Mortier, C.; Labadia, M.E.; Decruy, T.; Coudenys, J.; Hoyt, K.; Wayne, A.L.; Hughes, R.; Turner, M.; et al. RORγt inhibition selectively targets IL-17 producing iNKT and γδ-T cells enriched in Spondyloarthritis patients. Nat. Commun. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; De Leon-Tabaldo, A.; Luna-Roman, R.; Castro, G.; Albers, M.; Schoetens, F.; DePrimo, S.; Devineni, D.; Wilde, T.; Goldberg, S.; et al. Preclinical and clinical characterization of the RORγt inhibitor JNJ-61803534. Sci. Rep. 2021, 11, 11066. [Google Scholar] [CrossRef] [PubMed]
- Boshtam, M.; Asgary, S.; Kouhpayeh, S.; Shariati, L.; Khanahmad, H. Aptamers Against Pro- and Anti-Inflammatory Cytokines: A Review. Inflammation 2017, 40, 340–349. [Google Scholar] [CrossRef]
- Ishiguro, A.; Akiyama, T.; Adachi, H.; Inoue, J.I.; Nakamura, Y. Therapeutic potential of anti-interleukin-17A aptamer: Suppression of interleukin-17A signaling and attenuation of autoimmunity in two mouse models. Arthritis Rheum. 2011, 63, 455–466. [Google Scholar] [CrossRef]
- Adachi, H.; Ishiguro, A.; Hamada, M.; Sakota, E.; Asai, K.; Nakamura, Y. Antagonistic RNA aptamer specific to a heterodimeric form of human interleukin-17A/F. Biochimie 2011, 93, 1081–1088. [Google Scholar] [CrossRef]
- Shobeiri, S.S.; Rezaee, M.A.; Pordel, S.; Haghnnavaz, N.; Dashti, M.; Moghadam, M.; Sankian, M. Anti-IL-17A ssDNA aptamer ameliorated psoriasis skin lesions in the imiquimod-induced psoriasis mouse model. Int. Immunopharmacol. 2022, 110, 108963. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.Q.; Zhong, J.; Wu, X.L.; Chen, Q.; Peng, H.; Liu, S.Q. IL-17RA aptamer-mediated repression of IL-6 inhibits synovium inflammation in a murine model of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 711–718. [Google Scholar] [CrossRef]
- Liu, H.; Kang, R.S.; Bagnowski, K.; Yu, J.M.; Radecki, S.; Daniel, W.L.; Anderson, B.R.; Nallagatla, S.; Schook, A.; Agarwal, R.; et al. Targeting the IL-17 Receptor Using Liposomal Spherical Nucleic Acids as Topical Therapy for Psoriasis. J. Investig. Dermatol. 2020, 140, 435–444.e4. [Google Scholar] [CrossRef]
- Song, P.; Chou, Y.K.; Zhang, X.; Meza-Romero, R.; Yomogida, K.; Benedek, G.; Chu, C.-Q. CD4 aptamer-RORγt shRNA Chimera Inhibits IL-17 Synthesis by Human CD4+ T cells. Biochem. Biophys Res. Commun. 2014, 452, 1040–1045. [Google Scholar] [CrossRef]
- Shi, X.; Song, P.; Tao, S.; Zhang, X.; Chu, C.Q. Silencing RORγt in Human CD4+ T cells with CD30 aptamer-RORγt shRNA Chimera. Sci. Rep. 2019, 9, 10375. [Google Scholar] [CrossRef] [PubMed]
- Doble, R.; McDermott, M.F.; Cesur, Ö.; Stonehouse, N.J.; Wittmann, M. IL-17A RNA aptamer: Possible therapeutic potential in some cells, more than we bargained for in others. J. Investig. Dermatol. 2014, 134, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Hekmatimoghaddam, S.; Iman, M.; Shahdadi Sardo, H.; Jebali, A. Gelatin hydrogel containing cerium oxide nanoparticles covered by interleukin-17 aptamar as an anti- inflammatory agent for brain inflammation. J. Neuroimmunol. 2019, 326, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Freeley, M.; Long, A. Advances in siRNA delivery to T-cells: Potential clinical applications for inflammatory disease, cancer and infection. Biochem. J. 2013, 455, 133–147. [Google Scholar] [CrossRef]
- Liu, S.; Desharnais, J.; Sahasrabudhe, P.V.; Jin, P.; Li, W.; Oates, B.D.; Shanker, S.; Banker, M.E.; Chrunyk, B.A.; Song, X.; et al. Inhibiting complex IL-17A and IL-17RA interactions with a linear peptide. Sci. Rep. 2016, 6, 26071. [Google Scholar] [CrossRef]
- Dallenbach, K.; Maurer, P.; Röhn, T.; Zabel, F.; Kopf, M.; Bachmann, M.F. Protective effect of a germline, IL-17-neutralizing antibody in murine models of autoimmune inflammatory disease. Eur. J. Immunol. 2015, 45, 1238–1247. [Google Scholar] [CrossRef]
- Behrens, F.; Taylor, P.C.; Wetzel, D.; Brun, N.C.; Brandt-Juergens, J.; Drescher, E.; Dokoupilova, E.; Rowińska-Osuch9, A.; Martin Abdel-Kader, N.; Vlam, K. de Izokibep (ABY-035) in patients with active psoriatic arthritis—16-week results from A phase 2 study. Ann. Rheum. Dis. 2022, 81, 170–171. [Google Scholar] [CrossRef]
- Scala, E.; Cacciapuoti, S.; Garzorz-Stark, N.; Megna, M.; Marasca, C.; Seiringer, P.; Volz, T.; Eyerich, K.; Fabbrocini, G. Review hidradenitis suppurativa: Where we are and where we are going. Cells 2021, 10, 2094. [Google Scholar] [CrossRef]
- Scala, E.; Di Caprio, R.; Cacciapuoti, S.; Caiazzo, G.; Fusco, A.; Tortorella, E.; Fabbrocini, G.; Balato, A. A new T helper 17 cytokine in hidradenitis suppurativa: Antimicrobial and proinflammatory role of interleukin-26. Br. J. Dermatol. 2019, 181, 1038–1045. [Google Scholar] [CrossRef]
- Kashetsky, N.; Mufti, A.; Alabdulrazzaq, S.; Lytvyn, Y.; Sachdeva, M.; Rahat, A.; Yeung, J. Treatment Outcomes of IL-17 Inhibitors in Hidradenitis Suppurativa: A Systematic Review. J. Cutan. Med. Surg. 2022, 26, 79–86. [Google Scholar] [CrossRef]
- Alpsoy, E. Behçet’s disease: A comprehensive review with a focus on epidemiology, etiology and clinical features, and management of mucocutaneous lesions. J. Dermatol. 2016, 43, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Di Scala, G.; Bettiol, A.; Cojan, R.D.; Finocchi, M.; Silvestri, E.; Emmi, G. Efficacy of the anti-IL 17 secukinumab in refractory Behçet’s syndrome: A preliminary study. J. Autoimmun. 2019, 97, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Su, G.; Kijlstra, A.; Yang, P. Activation of the interleukin-23/interleukin-17 signalling pathway in autoinflammatory and autoimmune uveitis. Prog. Retin. Eye Res. 2021, 80, 100866. [Google Scholar] [CrossRef]
- Bullens, D.M.A.; Decraene, A.; Seys, S.; Dupont, L.J. IL-17A in Human Respiratory Diseases: Innate or Adaptive Immunity? Clinical Implications. Clin. Dev. Immunol. 2013, 2013, 840315. [Google Scholar] [CrossRef]
- Ritzmann, F.; Lunding, L.P.; Bals, R.; Wegmann, M.; Beisswenger, C. IL-17 Cytokines and Chronic Lung Diseases. Cells 2022, 11, 2132. [Google Scholar] [CrossRef]
- Liu, M.; Wu, K.; Lin, J.; Xie, Q.; Liu, Y.; Huang, Y.; Zeng, J.; Yang, Z.; Wang, Y.; Dong, S.; et al. Emerging Biological Functions of IL-17A: A New Target in Chronic Obstructive Pulmonary Disease? Front. Pharmacol. 2021, 12, 695957. [Google Scholar] [CrossRef]
| IL-17 Family Members | Physiological Effects | Pathological Effects |
|---|---|---|
| IL-17A | Bone remodeling (stimulation of osteoclastogenesis) [45] Recruitment of myeloid cells to the site of infection [46] Participation in immune responses against extracellular fungal [47] and bacterial agents [48] Maintenance of intestinal microbiota homeostasis [49] Maintenance of the epithelial barrier in the gut [50] Promotes tissue healing by activation of proliferation [51] Induces the production of bradykinin in the epithelial cells of the tubules of the kidneys in acute injury [52] Participation in the regulation of glucose and lipid metabolism [53] | Development of inflammatory arthritis [35] Possible link between depression and increased levels of IL-17 [54] Key role in the pathogenesis of spondyloarthritis [55] Injurious role in ischemic stroke [56] Involvement in the progression of neurocognitive disorders [57] The key link in the pathogenesis of psoriasis [58] The role in the development of liver fibrosis is being studied [59] |
| IL-17B | Blocks IL-25 signaling [60] TNF-α production induction [61] Participates in the process of embryonic development of bone tissue [62] Participates in the healing of bone fractures [63] The role in the regeneration of liver cells is being studied [64] | Key role in the progression of tumors: gastric [65], pancreas, lungs and breast [66], incl. increases the risk of metastasis [67] Activation and maintenance of chronic inflammation [68] Involved in the development of inflammatory arthritis [69] The role in the pathogenesis of systemic lupus erythematosus is being studied [70] |
| IL-17C | Participation in the regulation of the innate immune response in epithelial cells [71] Protection of the peripheral nervous system during the activation of the herpes virus [72] | Development of psoriatic skin lesions [73] Participation in the development of skin lesions in atopic dermatitis [74] Aggravation of the course of autoimmune encephalitis [75] The role in the development of kidney damage in SLE is being studied [76] |
| IL-17D | Antitumor immune response [77] Probably involved in local immune reactions, inhibition of hematopoiesis [78] Antiviral immune response in cytomegalovirus infection [79] Regulation of homeostasis in the intestine, probably anti-inflammatory effect in colitis [80] Possible role in the development of the immune response in bacterial infection [81] | Possibly involved in the development of severe sepsis [82] |
| IL-17E (IL-25) | Participation in the immune response to parasitic invasion [83,84,85] Anti-inflammatory effects in the colonic mucosa [86], while there is evidence of pro-inflammatory activity in the colonic mucosa [60] Anti-inflammatory activity in the central nervous system [87] Participation in the development of thymus cells [88] | Participation in the development of psoriasis, however, no convincing evidence for its role in the development of articular syndrome has been obtained [89] Induction of inflammatory reactions of the allergic type [90] Exacerbation of bronchial asthma [91] |
| IL-17F | Immune reactions in mucous membranes, including antifungal immune response [92] Combined effects with IL-17A. | The role in the development of psoriasis and psoriatic arthritis is being studied [93] Activation of mucin hypersecretion, participation in inflammation in bronchial asthma [94] |
| Monoclonal Antibody | IL-17 Family Member | Therapeutic Indications | State Registration |
|---|---|---|---|
| Secukinumab | IL-17A | Plaque psoriasis Psoriatic arthritis Radiographic axial spondyloarthritis Non-radiographic axial spondyloarthritis Enthesitis-related arthritis | Food and Drug Administration USA (FDA US)—2015 European Medicines Agency (EMA)—2015 State Register of Medical Remedies Russian Federation (SRMR RF)—2016 |
| Ixekizumab | IL-17A | Plaque psoriasis Psoriatic arthritis Radiographic axial spondyloarthritis Non-radiographic axial spondyloarthritis | FDA US—2016 EMA—2016 SRMR RF—2018 |
| Netakimab | IL-17A | Plaque psoriasis Radiographic axial spondyloarthritis Psoriatic arthritis | SRMR RF—2019 |
| Bimekizumab | IL-17A IL-17F | Plaque psoriasis | EMA—2021 |
| Brodalumab | IL-17A-receptor | Plaque psoriasis | FDA US—2017 EMA—2017 |
| JAK Inhibitor | Main Selectivity to JAK Isoform | Therapeutic Indications | Trials in SpA | State Registration |
|---|---|---|---|---|
| Approved for spondyloarthritis | ||||
| Tofacitinib | JAK1, JAK2, JAK3 | Rheumatoid arthritis Psoriatic arthritis Ulcerative colitis Radiographic-axial spondyloarthritis Active polyarticular juvenile idiopathic arthritis Juvenile psoriatic arthritis in patients 2 years of age and older Plaque psoriasis | Efficacy and safety of Tofacitinib in subjects with active rx-axSpA: phase III (NCT03502616) [200] Efficacy and safety of Tofacitinib in psoriatic arthritis: comparator study OPAL BROADEN: phase III (NCT01877668) [201] Tofacitinib in psoriatic arthritis subjects with inadequate response to TNF Inhibitors OPAL BEYOND: phase III (NCT01882439) [202] | FDA US—2012 EMA—2017 SRMR RF—2013 |
| Upadacitinib | JAK1 | Rheumatoid arthritis Psoriatic arthritis Ulcerative colitis Radiographic-axial spondyloarthritis Nonradiographic-axial spondyloarthritis | A study evaluating the safety and efficacy of upadacitinib in adults with active rx-axSpA SELECT-AXIS 1: phase II/III (NCT03178487) [203] A study to evaluate efficacy and safety of upadacitinib in adults with axSpA SELECT AXIS 2: phase III (NCT04169373) [196] A study comparing upadacitinib to placebo in participants with active psoriatic arthritis who have a history of inadequate response to at least one bDMARD SELECT-PsA 2 (NCT03104374) [204] | EMA—2019 FDA—2019 SRMR RF—2019 |
| Baricitinib | JAK1, JAK2 | Rheumatoid arthritis Atopic dermatitis Alopecia areata COVID-19 | A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis [205], but clinical development of baricitinib for the treatment of PsA has been halted [206] | EMA—2016 FDA—2018 SRMR RF—2018 |
| Filogotinib | JAK1 | Rheumatoid arthritis Ulcerative colitis | A study to assess efficacy and safety of filgotinib in rx-axSpA TORTUGA: phase II (NCT03117270) [207] An open-label, long-term extension study with filgotinib in active psoriatic arthritis: phase II (NCT03320876) | EMA—2020 |
| Not approved for rheumatological applications | ||||
| Deucravacitinib | TYK2 | Plaque psoriasis | Efficacy and safety of BMS-986165 compared with placebo in participants with active psoriatic arthritis: phase II (NCT03881059) [208] | FDA—2022 EMA—2023 |
| Target | Therapeutic Nucleic Acid | Experimental Model | Ref. |
|---|---|---|---|
| IL-17A | 2’-F-RNA aptamers Apt21-2 and Apt3-4 | mouse model of multiple sclerosis and inflammatory arthritis | [216] |
| 2’-F-RNA aptamer AptAF42dope1 | primary human foreskin fibroblast BJ cells | [217] | |
| DNA aptamers M2 and M7 | imiquimod induced psoriasis mouse model | [218] | |
| IL-17RA | DNA aptamer RA10-6 | mouse model of osteoarthritis | [219] |
| Liposomes + antisense oligonucleotide | imiquimod induced psoriasis mouse model human cytokine-induced psoriasis skin model | [220] | |
| Th17 cells | CD4 aptamer + RORγt shRNA | CD4+ cells | [221] |
| CD30 aptamer + RORγt shRNA | CD30+ and CD4+ cells | [222] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davydova, A.; Kurochkina, Y.; Goncharova, V.; Vorobyeva, M.; Korolev, M. The Interleukine-17 Cytokine Family: Role in Development and Progression of Spondyloarthritis, Current and Potential Therapeutic Inhibitors. Biomedicines 2023, 11, 1328. https://doi.org/10.3390/biomedicines11051328
Davydova A, Kurochkina Y, Goncharova V, Vorobyeva M, Korolev M. The Interleukine-17 Cytokine Family: Role in Development and Progression of Spondyloarthritis, Current and Potential Therapeutic Inhibitors. Biomedicines. 2023; 11(5):1328. https://doi.org/10.3390/biomedicines11051328
Chicago/Turabian StyleDavydova, Anna, Yuliya Kurochkina, Veronika Goncharova, Mariya Vorobyeva, and Maksim Korolev. 2023. "The Interleukine-17 Cytokine Family: Role in Development and Progression of Spondyloarthritis, Current and Potential Therapeutic Inhibitors" Biomedicines 11, no. 5: 1328. https://doi.org/10.3390/biomedicines11051328
APA StyleDavydova, A., Kurochkina, Y., Goncharova, V., Vorobyeva, M., & Korolev, M. (2023). The Interleukine-17 Cytokine Family: Role in Development and Progression of Spondyloarthritis, Current and Potential Therapeutic Inhibitors. Biomedicines, 11(5), 1328. https://doi.org/10.3390/biomedicines11051328





