miRNAs’ Cross-Involvement in Skin Allergies: A New Horizon for the Pathogenesis, Diagnosis and Therapy of Atopic Dermatitis, Allergic Contact Dermatitis and Chronic Spontaneous Urticaria
Abstract
1. Introduction
1.1. Pathogenesis of Atopic Dermatitis
1.2. Pathogenesis of Allergic Contact Dermatitis
1.3. Pathogenesis of Chronic Spontaneous Urticaria
1.4. MicroRNAs
2. miRNA in AD, ACD and CSU: Pathogenetic Role and Therapeutic Strategies
2.1. Pro-Inflammatory and Anti-Inflammatory miRNA in AD
2.2. Pro-Inflammatory and Anti-Inflammatory miRNA in ACD
2.3. Pro-Inflammatory and Anti-Inflammatory miRNA in CSU
3. Therapeutic Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic Dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Nettis, E.; Patella, V.; Brancaccio, R.; Detoraki, C.; Di Leo, E.; Incorvaia, C.; Macchia, L.; Pellacani, G.; Bonzano, L. Efficacy of Dupilumab in Concomitant Atopic Dermatitis and Chronic Rhinosinusitis With Nasal Polyps: A Preliminary Study. Allergy Asthma Immunol. Res. 2021, 13, 347–349. [Google Scholar] [CrossRef]
- Megna, M.; Patruno, C.; Balato, A.; Rongioletti, F.; Stingeni, L.; Balato, N.; Ayala, F.; Brambilla, L.; Congedo, M.; Corazza, M.; et al. An Italian Multicentre Study on Adult Atopic Dermatitis: Persistent versus Adult-Onset Disease. Arch. Derm. Res. 2017, 309, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Worm, M. Dupilumab in the Treatment of Moderate-to-Severe Atopic Dermatitis. Expert Rev. Clin. Immunol. 2017, 13, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Feldman, S.R. Management of Atopic Dermatitis. Adherence in Atopic Dermatitis. Introduction. Adv. Exp. Med. Biol. 2017, 1027, 139–159. [Google Scholar] [CrossRef]
- Elias, P.M.; Steinhoff, M. “Outside-to-inside” (and Now Back to “Outside”) Pathogenic Mechanisms in Atopic Dermatitis. J. Investig. Dermatol. 2008, 128, 1067–1070. [Google Scholar] [CrossRef]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative Stress and Atopic Dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Reszka, E.; Gutowska-Owsiak, D.; Trzeciak, M.; Lange, M.; Jarczak, J.; Niedoszytko, M.; Jablonska, E.; Romantowski, J.; Strapagiel, D.; et al. Genetic and Epigenetic Aspects of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 6484. [Google Scholar] [CrossRef]
- Zaniboni, M.C.; Samorano, L.P.; Orfali, R.L.; Aoki, V. Skin Barrier in Atopic Dermatitis: Beyond Filaggrin. Bras Derm. 2016, 91, 472–478. [Google Scholar] [CrossRef]
- Katsunuma, T.; Kawahara, H.; Yuki, K.; Akasawa, A.; Saito, H. Impaired Interferon-Gamma Production in a Subset Population of Severe Atopic Dermatitis. Int. Arch. Allergy Immunol. 2004, 134, 240–247. [Google Scholar] [CrossRef]
- Malik, K.; Heitmiller, K.D.; Czarnowicki, T. An Update on the Pathophysiology of Atopic Dermatitis. Derm. Clin. 2017, 35, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common Loss-of-Function Variants of the Epidermal Barrier Protein Filaggrin Are a Major Predisposing Factor for Atopic Dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.J.; et al. A Role for IL-25 and IL-33-Driven Type-2 Innate Lymphoid Cells in Atopic Dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef]
- Ito, T.; Wang, Y.H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.F.; Yao, Z.; Cao, W.; Liu, Y.J. TSLP-Activated Dendritic Cells Induce an Inflammatory T Helper Type 2 Cell Response through OX40 Ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.H.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting Key Proximal Drivers of Type 2 Inflammation in Disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.A.; Luster, A.D. T Cell Homing to Epithelial Barriers in Allergic Disease. Nat. Med. 2012, 18, 705–715. [Google Scholar] [CrossRef]
- Liu, B.; Tai, Y.; Achanta, S.; Kaelberer, M.M.; Caceres, A.I.; Shao, X.; Fang, J.; Jordt, S.E. IL-33/ST2 Signaling Excites Sensory Neurons and Mediates Itch Response in a Mouse Model of Poison Ivy Contact Allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E7572–E7579. [Google Scholar] [CrossRef]
- Nassau, S.; Fonacier, L. Allergic Contact Dermatitis. Med. Clin. N. Am. 2020, 104, 61–76. [Google Scholar] [CrossRef]
- Zack, B.; Arrandale, V.H.; Holness, D.L. Preventing Occupational Skin Disease: A Review of Training Programs. Dermatitis 2017, 28, 169–182. [Google Scholar] [CrossRef]
- Rustemeyer, T.; Van Hoogstraten, I.M.W.; Von Blomberg, B.M.E.; Gibbs, S.; Scheper, R.J. Mechanisms of Irritant and Allergic Contact Dermatitis. Contact Dermat. 2011, 43–90. [Google Scholar] [CrossRef]
- Adelman, D.C.; Casale, T.B.; Corren, J.; Ovid Technologies, Inc. Manual of Allergy and Immunology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; p. 494. [Google Scholar]
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The International EAACI/GA2LEN/EuroGuiDerm/APAAACI Guideline for the Definition, Classification, Diagnosis, and Management of Urticaria. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 734–766. [Google Scholar] [CrossRef]
- Nettis, E.; Cegolon, L.; Di Leo, E.; Lodi Rizzini, F.; Detoraki, A.; Canonica, G.W. Omalizumab in Chronic Spontaneous Urticaria: Efficacy, Safety, Predictors of Treatment Outcome, and Time to Response. Ann. Allergy Asthma Immunol. 2018, 121, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, C.; Toubi, E.; Maurer, M.; Triggiani, M.; Ballmer-Weber, B.; Marsland, A.; Ferrer, M.; Knulst, A.; Giménez-Arnau, A. Treatment of Chronic Spontaneous Urticaria with an Inadequate Response to H1-Antihistamines: An Expert Opinion. Eur. J. Dermatol. 2017, 27, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bracken, S.J.; Abraham, S.; MacLeod, A.S. Autoimmune Theories of Chronic Spontaneous Urticaria. Front. Immunol. 2019, 10, 627. [Google Scholar] [CrossRef]
- Grattan, C.E.H.; Dawn, G.; Gibbs, S.; Francis, D.M. Blood Basophil Numbers in Chronic Ordinary Urticaria and Healthy Controls: Diurnal Variation, Influence of Loratadine and Prednisolone and Relationship to Disease Activity. Clin. Exp. Allergy 2003, 33, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Kern, F.; Lichtenstein, L.M. Defective Histamine Release in Chronic Urticaria. J. Clin. Investig. 1976, 57, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.S.; Sui, J.F.; Chen, X.H.; Ran, X.Z.; Yang, Z.F.; Guan, W.D.; Yang, T. Detection of CD4+ CD25+ FOXP3+ Regulatory T Cells in Peripheral Blood of Patients with Chronic Autoimmune Urticaria. Australas J. Dermatol. 2011, 52, e15–e18. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Ming, B.; Wu, X.; Chen, Y.; Dong, L. Regulatory T-Cell Levels in Systemic Lupus Erythematosus Patients: A Meta-Analysis. Lupus 2019, 28, 445–454. [Google Scholar] [CrossRef]
- Morita, T.; Shima, Y.; Wing, J.B.; Sakaguchi, S.; Ogata, A.; Kumanogoh, A. The Proportion of Regulatory T Cells in Patients with Rheumatoid Arthritis: A Meta-Analysis. PLoS ONE 2016, 11, e0162306. [Google Scholar] [CrossRef]
- Vasagar, K.; Vonakis, B.M.; Gober, L.M.; Viksman, A.; Gibbons, S.P.; Saini, S.S. Evidence of in Vivo Basophil Activation in Chronic Idiopathic Urticaria. Clin. Exp. Allergy 2006, 36, 770–776. [Google Scholar] [CrossRef]
- Ulambayar, B.; Chen, Y.H.; Ban, G.Y.; Lee, J.H.; Jung, C.G.; Yang, E.M.; Park, H.S.; Ye, Y.M. Detection of Circulating IgG Autoantibody to FcεRIα in Sera from Chronic Spontaneous Urticaria Patients. J. Microbiol. Immunol. Infect. 2020, 53, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qi, R.; Yang, Y.; Gao, X.; Chen, H.; Xiao, T. Serum MiR-125a-5p and CCL17 Upregulated in Chronic Spontaneous Urticaria and Correlated with Treatment Response. Acta Derm. Venereol. 2019, 99, 571–578. [Google Scholar] [CrossRef]
- Ambros, V.; Lee, R.C.; Lavanway, A.; Williams, P.T.; Jewell, D. MicroRNAs and Other Tiny Endogenous RNAs in C. Elegans. Curr. Biol. 2003, 13, 807–818. [Google Scholar] [CrossRef]
- Setoyama, T.; Ling, H.; Natsugoe, S.; Calin, G.A. Non-Coding RNAs for Medical Practice in Oncology. Keio J. Med. 2011, 60, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Borchert, G.M.; Lanier, W.; Davidson, B.L. RNA Polymerase III Transcribes Human MicroRNAs. Nat. Struct Mol. Biol. 2006, 13, 1097–1101. [Google Scholar] [CrossRef]
- Pasquinelli, A.E.; Reinhart, B.J.; Slack, F.; Martindale, M.Q.; Kuroda, M.I.; Maller, B.; Hayward, D.C.; Ball, E.E.; Degnan, B.; Müller, P.; et al. Conservation of the Sequence and Temporal Expression of Let-7 Heterochronic Regulatory RNA. Nature 2000, 408, 86–89. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Akiyama, Y.; Yuasa, Y. Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS ONE 2013, 8, e62589. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. MiRBase: Annotating High Confidence MicroRNAs Using Deep Sequencing Data. Nucleic. Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef]
- Sayed, D.; Abdellatif, M. MicroRNAs in Development and Disease. Physiol. Rev. 2011, 91, 827–887. [Google Scholar] [CrossRef]
- Hutvágner, G.; Zamore, P.D. A MicroRNA in a Multiple-Turnover RNAi Enzyme Complex. Science 2002, 297, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates That Thousands of Human Genes Are MicroRNA Targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Hu, H.Y.; Yan, Z.; Xu, Y.; Hu, H.; Menzel, C.; Zhou, Y.H.; Chen, W.; Khaitovich, P. Sequence Features Associated with MicroRNA Strand Selection in Humans and Flies. BMC Genom. 2009, 10, 413. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of MRNA Translation and Stability by MicroRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 14, 172. [Google Scholar] [CrossRef]
- Tong, K.L.; Tan, K.E.; Lim, Y.Y.; Tien, X.Y.; Wong, P.F. CircRNA-miRNA interactions in atherogenesis. Mol. Cell. Biochem. 2022, 477, 2703–2733. [Google Scholar] [CrossRef]
- Huntzinger, E.; Izaurralde, E. Gene Silencing by MicroRNAs: Contributions of Translational Repression and MRNA Decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef]
- Ambros, V. The Functions of Animal MicroRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, G.; Chiantore, M.V.; Mangino, G.; Percario, Z.A.; Affabris, E.; Romeo, G. Cancer Regulator MicroRNA: Potential Relevance in Diagnosis, Prognosis and Treatment of Cancer. Curr. Med. Chem. 2012, 19, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, C.P.; Ryan, J.J. The Role of Th2 Cytokines in Mast Cell Homeostasis. Immunol. Rev. 2001, 179, 82–93. [Google Scholar] [CrossRef]
- Banerjee, J.; Sen, C.K. MicroRNAs in skin and wound healing. Methods Mol. Biol. 2013, 936, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Frankel, H.C.; Qureshi, A.A. Comparative Effectiveness of Topical Calcineurin Inhibitors in Adult Patients with Atopic Dermatitis. Am. J. Clin. Dermatol. 2012, 13, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Janson, P.; Majuri, M.L.; Savinko, T.; Fyhrquist, N.; Eidsmo, L.; Xu, N.; Meisgen, F.; Wei, T.; Bradley, M.; et al. MiR-155 Is Overexpressed in Patients with Atopic Dermatitis and Modulates T-Cell Proliferative Responses by Targeting Cytotoxic T Lymphocyte-Associated Antigen 4. J. Allergy Clin. Immunol. 2010, 126, 581–589.e20. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Pivarcsi, A. Advances in MicroRNAs: Implications for Immunity and Inflammatory Diseases. J. Cell Mol. Med. 2009, 13, 24–38. [Google Scholar] [CrossRef]
- Rodriguez, A.; Vigorito, E.; Clare, S.; Warren, M.V.; Couttet, P.; Soond, D.R.; Van Dongen, S.; Grocock, R.J.; Das, P.P.; Miska, E.A.; et al. Requirement of Bic/MicroRNA-155 for Normal Immune Function. Science 2007, 316, 608–611. [Google Scholar] [CrossRef]
- Thai, T.H.; Calado, D.P.; Casola, S.; Ansel, K.M.; Xiao, C.; Xue, Y.; Murphy, A.; Frendewey, D.; Valenzuela, D.; Kutok, J.L.; et al. Regulation of the Germinal Center Response by MicroRNA-155. Science 2007, 316, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Rebane, A.; Runnel, T.; Aab, A.; Maslovskaja, J.; Rückert, B.; Zimmermann, M.; Plaas, M.; Kärner, J.; Treis, A.; Pihlap, M.; et al. MicroRNA-146a Alleviates Chronic Skin Inflammation in Atopic Dermatitis through Suppression of Innate Immune Responses in Keratinocytes. J. Allergy Clin. Immunol. 2014, 134, 836–847.e11. [Google Scholar] [CrossRef]
- West, C.; McDermott, M.F. Effects of MicroRNA-146a on the Proliferation and Apoptosis of Human Osteochondrocytes by Targeting TRAF6 through the NF- ΚB Signalling Pathway. Biosci. Rep. 2017, 37, 180. [Google Scholar] [CrossRef]
- Park, H.; Huang, X.; Lu, C.; Cairo, M.S.; Zhou, X. MicroRNA-146a and MicroRNA-146b Regulate Human Dendritic Cell Apoptosis and Cytokine Production by Targeting TRAF6 and IRAK1 Proteins. J. Biol. Chem. 2015, 290, 2831–2841. [Google Scholar] [CrossRef]
- Lindner, J.M.; Kayo, H.; Hedlund, S.; Fukuda, Y.; Fukao, T.; Nielsen, P.J. Cutting Edge: The Transcription Factor Bob1 Counteracts B Cell Activation and Regulates MiR-146a in B Cells. J. Immunol. 2014, 192, 4483–4486. [Google Scholar] [CrossRef]
- Williams, A.E.; Perry, M.M.; Moschos, S.A.; Larner-Svensson, H.M.; Lindsay, M.A. Role of MiRNA-146a in the Regulation of the Innate Immune Response and Cancer. Biochem. Soc. Trans. 2008, 36, 1211–1215. [Google Scholar] [CrossRef]
- Chen, X.F.; Zhang, L.J.; Zhang, J.; Dou, X.; Shao, Y.; Jia, X.J.; Zhang, W.; Yu, B. MiR-151a Is Involved in the Pathogenesis of Atopic Dermatitis by Regulating Interleukin-12 Receptor Β2. Exp. Dermatol. 2018, 27, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, C.D.; Lee, A.Y. Administration of Poly(I:C) Improved Dermatophagoides Farinae-Induced Atopic Dermatitis-like Skin Lesions in NC/Nga Mice by the Regulation of Th1/Th2 Balance. Vaccine 2012, 30, 2405–2410. [Google Scholar] [CrossRef]
- Guo, H.W.; Yun, C.X.; Hou, G.H.; Du, J.; Huang, X.; Lu, Y.; Keller, E.T.; Zhang, J.; Deng, J.G. Mangiferin Attenuates TH1/TH2 Cytokine Imbalance in an Ovalbumin-Induced Asthmatic Mouse Model. PLoS ONE 2014, 9, e100394. [Google Scholar] [CrossRef]
- Jia, H.Z.; Liu, S.L.; Zou, Y.F.; Chen, X.F.; Yu, L.; Wan, J.; Zhang, H.Y.; Chen, Q.; Xiong, Y.; Yu, B.; et al. MicroRNA-223 Is Involved in the Pathogenesis of Atopic Dermatitis by Affecting Histamine-N-Methyltransferase. Cell Mol. Biol. 2018, 64, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Allantaz, F.; Cheng, D.T.; Bergauer, T.; Ravindran, P.; Rossier, M.F.; Ebeling, M.; Badi, L.; Reis, B.; Bitter, H.; D’Asaro, M.; et al. Expression Profiling of Human Immune Cell Subsets Identifies MiRNA-MRNA Regulatory Relationships Correlated with Cell Type Specific Expression. PLoS ONE 2012, 7, e29979. [Google Scholar] [CrossRef] [PubMed]
- Herberth, G.; Bauer, M.; Gasch, M.; Hinz, D.; Röder, S.; Olek, S.; Kohajda, T.; Rolle-Kampczyk, U.; Von Bergen, M.; Sack, U.; et al. Maternal and Cord Blood MiR-223 Expression Associates with Prenatal Tobacco Smoke Exposure and Low Regulatory T-Cell Numbers. J. Allergy Clin. Immunol. 2014, 133, 543–550.e4. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Qi, R.; Xu, J.; Di, Z.; Zheng, H.; Huo, W.; Zhang, L.; Chen, H.; Gao, X. Profiling of Serum and Urinary MicroRNAs in Children with Atopic Dermatitis. PLoS ONE 2014, 9, e115448. [Google Scholar] [CrossRef] [PubMed]
- Freire De Carvalho, J.; Machado Ribeiro, F. The Potential Role of MicroRNAs as Biomarkers in Atopic Dermatitis: A Systematic Review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11801–11803. [Google Scholar] [CrossRef]
- Vaher, H.; Runnel, T.; Urgard, E.; Aab, A.; Carreras Badosa, G.; Maslovskaja, J.; Abram, K.; Raam, L.; Kaldvee, B.; Annilo, T.; et al. MiR-10a-5p Is Increased in Atopic Dermatitis and Has Capacity to Inhibit Keratinocyte Proliferation. Allergy 2019, 74, 2146–2156. [Google Scholar] [CrossRef]
- Chaoying Gu; Yin Li; Jinfeng Wu; Jinhua Xu IFN-γ-Induced MicroRNA-29b up-Regulation Contributes Tokeratinocyte Apoptosis in Atopic Dermatitis through Inhibiting Bcl2L2—PubMed. Int. J. Clin. Exp. Pathol. 2017, 10, 10117–10126.
- Ralfkiaer, U.; Lindahl, L.M.; Litman, T.; Gjerdrum, L.M.; Ahler, C.B.; Gniadecki, R.; Marstrand, T.; Fredholm, S.; Iversen, L.; Wasik, M.A.; et al. MicroRNA Expression in Early Mycosis Fungoides Is Distinctly Different from Atopic Dermatitis and Advanced Cutaneous T-Cell Lymphoma. Anticancer. Res. 2014, 34, 7207–7217. [Google Scholar] [PubMed]
- Sonkoly, E.; Wei, T.; Janson, P.C.J.; Sääf, A.; Lundeberg, L.; Tengvall-Linder, M.; Norstedt, G.; Alenius, H.; Homey, B.; Scheynius, A.; et al. MicroRNAs: Novel Regulators Involved in the Pathogenesis of Psoriasis? PLoS ONE 2007, 2, e610. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.P.; Nguyen, G.H.; Jin, H.Z. MicroRNA-143 Inhibits IL-13-Induced Dysregulation of the Epidermal Barrier-Related Proteins in Skin Keratinocytes via Targeting to IL-13Rα1. Mol. Cell Biochem. 2016, 416, 63–70. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, J.; Nam, J.; Sun, H.; Lee, Y.H.; Lee, T.J.; Aguiar, R.C.T.; Kim, S.W. MicroRNA-124 Links P53 to the NF-ΚB Pathway in B-Cell Lymphomas. Leukemia 2015, 29, 1868–1874. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, B.; Wang, C.; Wang, H.; Huang, P.; Pan, Y. MicroRNA-124 Alleviates Chronic Skin Inflammation in Atopic Eczema via Suppressing Innate Immune Responses in Keratinocytes. Cell Immunol. 2017, 319, 53–60. [Google Scholar] [CrossRef]
- Vennegaard, M.T.; Bonefeld, C.M.; Hagedorn, P.H.; Bangsgaard, N.; Løvendorf, M.B.; Odum, N.; Woetmann, A.; Geisler, C.; Skov, L. Allergic Contact Dermatitis Induces Upregulation of Identical MicroRNAs in Humans and Mice. Contact Dermat. 2012, 67, 298–305. [Google Scholar] [CrossRef]
- Gulati, N.; Løvendorf, M.B.; Zibert, J.R.; Akat, K.M.; Renwick, N.; Tuschl, T.; Krueger, J.G. Unique MicroRNAs Appear at Different Times during the Course of a Delayed-Type Hypersensitivity Reaction in Human Skin. Exp. Dermatol. 2015, 24, 953–957. [Google Scholar] [CrossRef]
- Anderson, S.E.; Beezhold, K.; Lukomska, E.; Richardson, J.; Long, C.; Anderson, K.; Franko, J.; Meade, B.J.; Beezhold, D.H. Expression Kinetics of MiRNA Involved in Dermal Toluene 2,4-Diisocyanate Sensitization. J. Immunotoxicol. 2014, 11, 250–259. [Google Scholar] [CrossRef]
- Werner, P.; Wisgrill, L.; Riskumäki, M.; Jalonen, E.; Vendelin, J.; Suomela, S.; Lauerma, A.; Alenius, H.; Fyhrquist, N. Identification of Novel MiRNA-MRNA Regulatory Networks in Contact Dermatitis by Integrated Microarray Analysis. Allergy 2021, 76, 1257–1261. [Google Scholar] [CrossRef]
- Lin, C.K.E.; Kaptein, J.S.; Sheikh, J. Differential Expression of MicroRNAs and Their Possible Roles in Patients with Chronic Idiopathic Urticaria and Active Hives. Allergy Rhinol. 2017, 8, e67–e80. [Google Scholar] [CrossRef]
- Yoon, W.S.; Lee, S.S.; Chae, Y.S.; Park, Y.K. Therapeutic Effects of Recombinant Salmonella Typhimurium Harboring CCL22 MiRNA on Atopic Dermatitis-like Skin in Mice. Exp. Mol. Med. 2011, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, T.; Zhang, S.; Tang, Q.; Yan, Y.; Feng, H. Integrated Bioinformatics and Validation Reveal IL1B and Its Related Molecules as Potential Biomarkers in Chronic Spontaneous Urticaria. Front. Immunol. 2022, 13, 850993. [Google Scholar] [CrossRef] [PubMed]
- Innao, V.; Allegra, A.; Pulvirenti, N.; Allegra, A.G.; Musolino, C. Therapeutic Potential of AntagomiRs in Haematological and Oncological Neoplasms. Eur. J. Cancer Care 2020, 29, e13208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lian, Y.; Xie, N.; Cheng, X.; Chen, C.; Xu, H.; Zheng, Y. Antagomirs Targeting MiR-142-5p Attenuate Pilocarpine-Induced Status Epilepticus in Mice. Exp. Cell Res. 2020, 393, 112089. [Google Scholar] [CrossRef]
- Reschke, C.R.; Silva, L.F.A.; Vangoor, V.R.; Rosso, M.; David, B.; Cavanagh, B.L.; Connolly, N.M.C.; Brennan, G.P.; Sanz-Rodriguez, A.; Mooney, C.; et al. Systemic Delivery of Antagomirs during Blood-Brain Barrier Disruption Is Disease-Modifying in Experimental Epilepsy. Mol. Ther. 2021, 29, 2041–2052. [Google Scholar] [CrossRef]
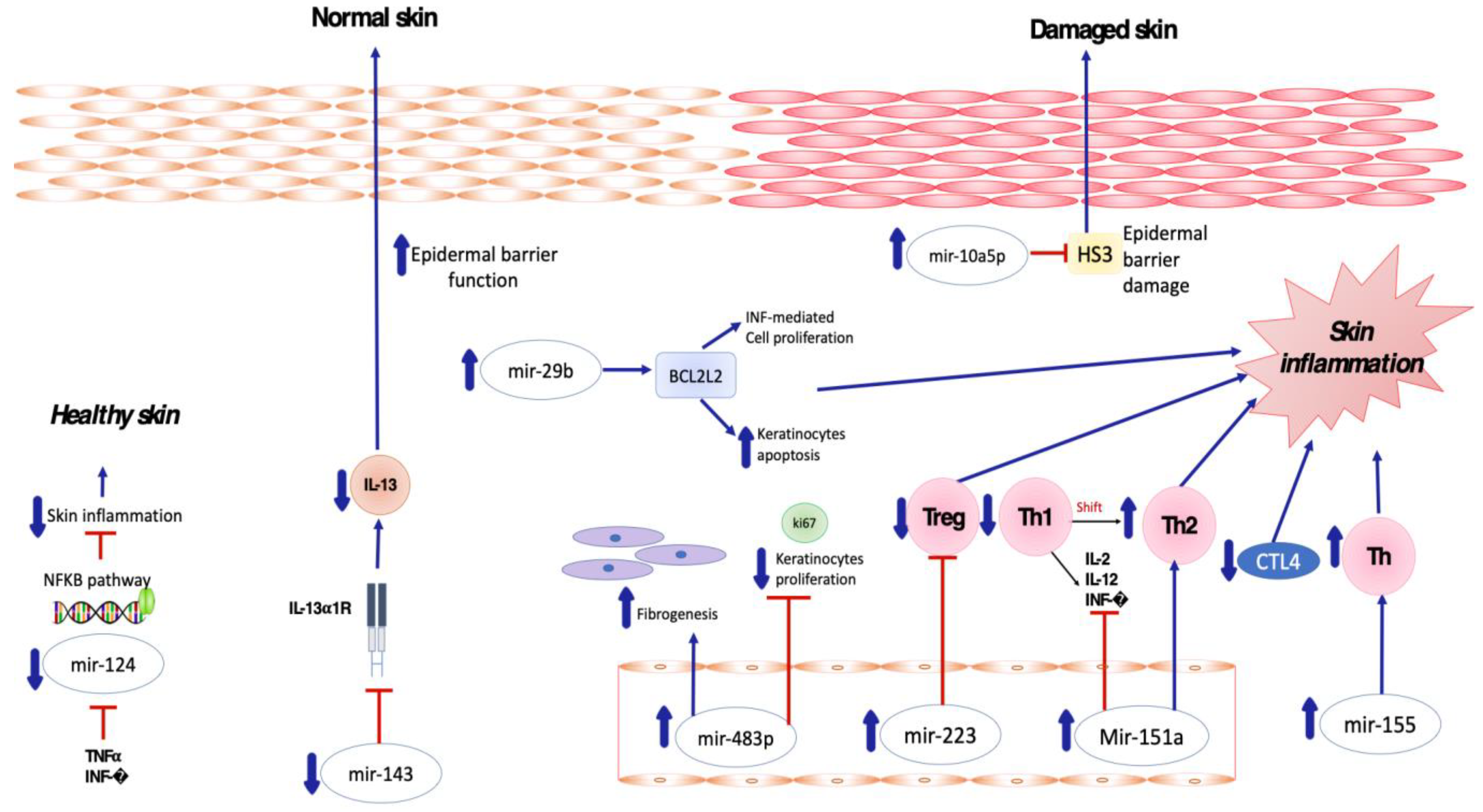
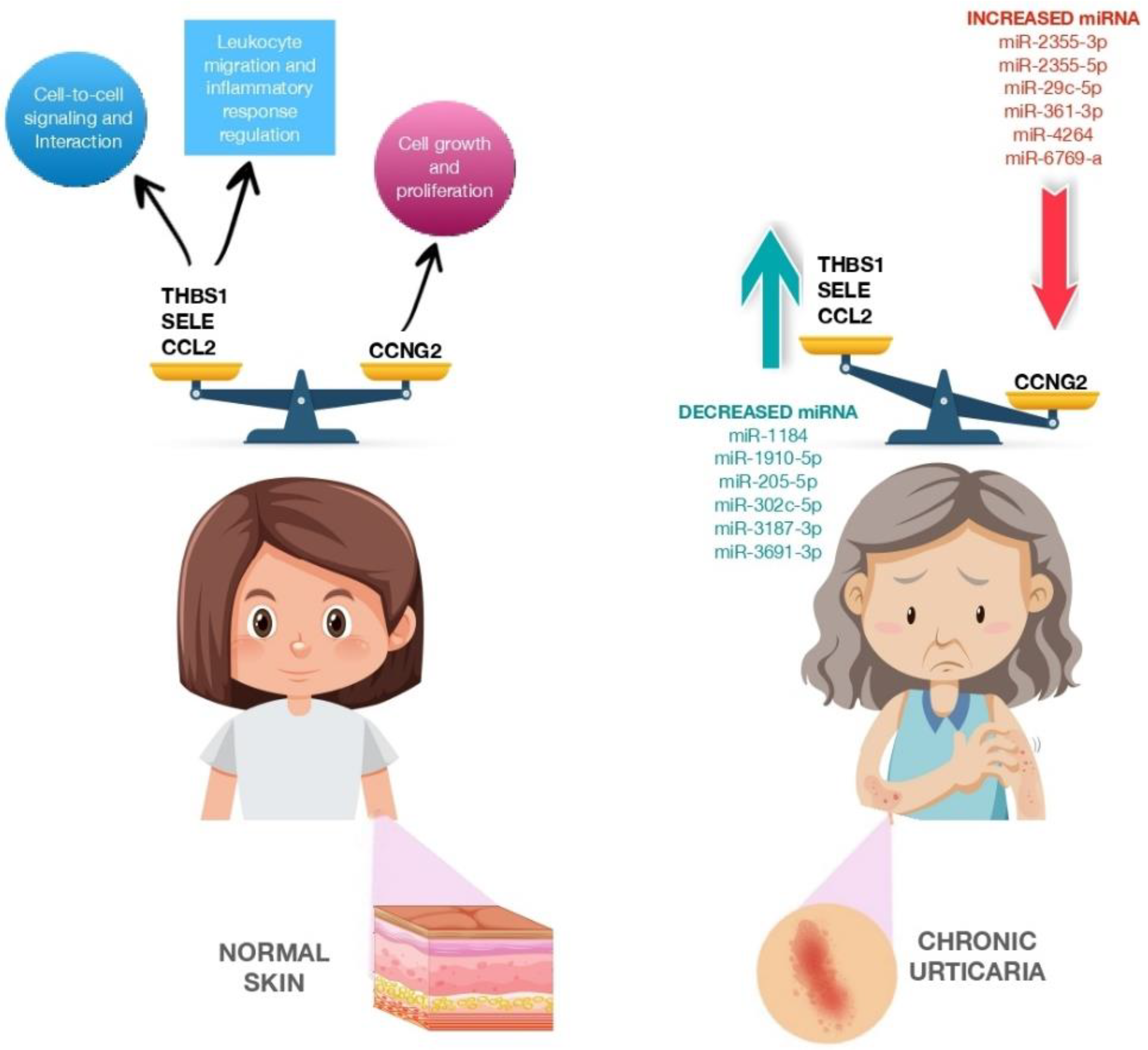
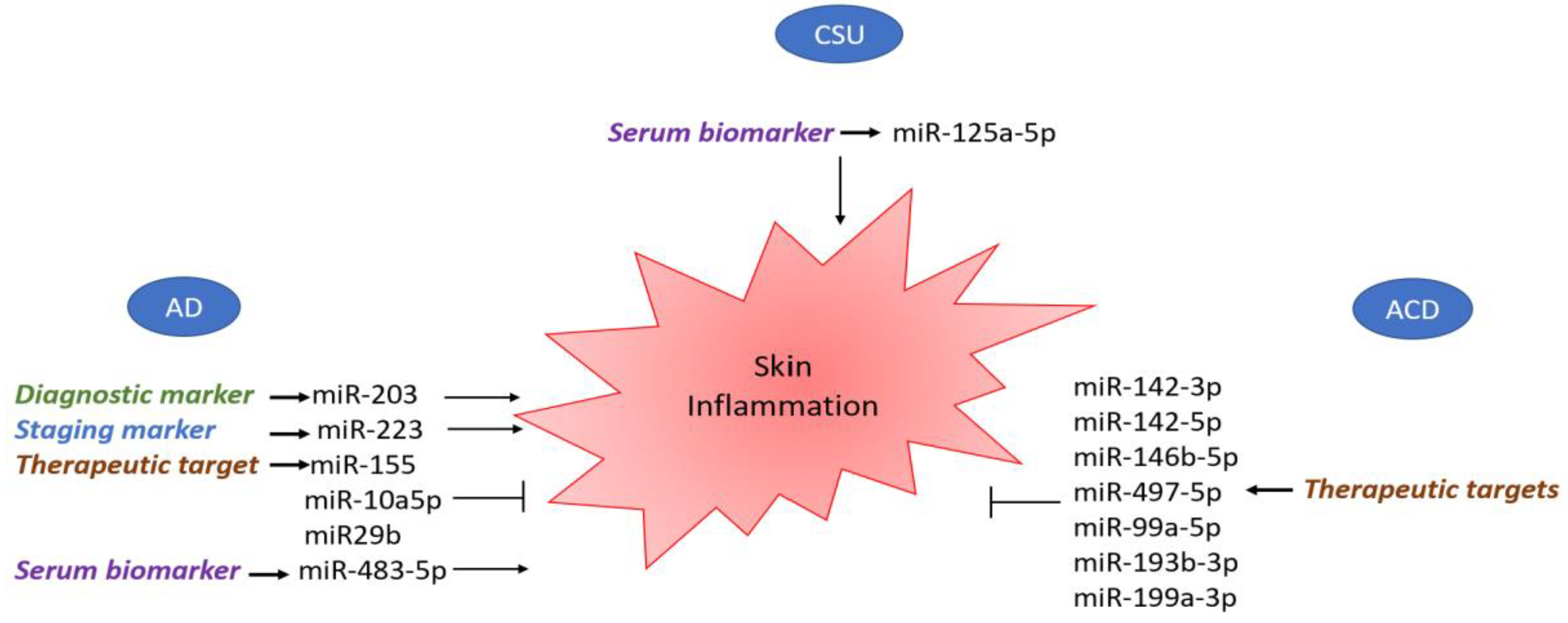
| Study | miRNA | Study Population | Samples | Results | Molecular Pathways/Mechanism of Action |
|---|---|---|---|---|---|
| Sonkoly, E., et al. [54] 2010 | miR-155 | Humans (18) | Serum | Upregulated | Overexpression of miR-155 decreases CTLA-4 levels and increases proliferation in T helper cells, promoting chronic skin inflammation. |
| Rebane, A., et al. [58] 2014 | miR-146a | Humans/mice | Skin | Upregulated | miR-146a decreases the expression of IFN-γ-inducible genes CCL5, CCL8 and ubiquitin D (UBD) in keratinocytes and in a mouse model of AD by targeting the upstream mediators of NF-κB signaling—IRAK1 and CARD10. |
| Chen, X.F., et al. [63] 2014 | miR-151a | Humans (500) | Plasma | Overexpressed | miR-151a targets the IL-12 receptor β2 (IL12RB2), a subunit of the IL-12 receptor. |
| Jia, H.Z., et al. [66] 2018 | miR-223 | N/A | Serum | Upregulated | miR-223 expression is correlated with lower Treg cell numbers, a decreased number of which at birth correlates with an increased risk of AD. |
| Lv, Y., et al. [49] 2014 | miR-483-5p | Humans (30) | Serum and urine | Upregulated | miR-483-5p modulates fibrogenesis through the regulation of collagen homeostasis. |
| Vaher, H., et al. [51] 2019 | miR-10a-5p | Humans (10) | Skin (lesional and non-lesional) | Upregulated | miR-10a-5p is a direct target of HAS3, a damage-associated positive regulator of keratinocyte proliferation and migration. Upregulation of miR-10a-5p affects keratinocyte proliferation, thus impairing normal skin barrier function. |
| Gu, C., et al. [52] 2017 | miR-29b | Humans (21) | Lesional skin and serum | Upregulated | miR-29b triggers IFN-γ-mediated apoptosis of keratinocytes by targeting BCL2L. |
| Lv, Y., et al. [69] 2014 | miR-203 | Humans (30) | Serum | Upregulated | The miR-203 target gene is the regulator of cytokine production SOCS-3 (suppressor of cytokine signaling 3). |
| Skin | Upregulated | ||||
| Urine | Downregulated | ||||
| Zeng, Y.P., et al. [75] 2018 | miR-143 | N/A | Skin | Downregulated | miRNA-143 decreases IL-13 activity and inflammatory reaction by inhibiting IL-13 receptor-alpha1 (IL-13Ra1) in epidermal keratinocytes. |
| Yang, Z., et al. [77] 2017 | miR-124 | Humans (37) | Serum | Downregulated | miR-124 inhibits the p65 subunit of NF-kB and downregulates CCL5 and CCL8, thereby regulating inflammatory responses of keratinocytes and chronic skin inflammation in AD. |
| Study | miRNA | Study Population | Samples | Results | Molecular Pathways/Mechanism of Action |
|---|---|---|---|---|---|
| Werner et al. [81] 2020 | miR-142-3p, miR-142-5p, miR-146b-5p, miR-155-5p | Humans (nickel sulfate, epoxy resin (EP) and methylochloroisothia zolinone (MCI); n = 5 for each), irritants (sodium lauryl sulfate (SLS, n = 9) and nonanoic acid (NO, n = 5)) and from non-affected skin (baseline, n = 5). | Skin | Upregulated | miR-155-5p: enrichment of biological processes for axon guidance, smooth muscle cell migration and leukocyte/T cell apoptotic process. |
| Werner et al. [81] 2020 | miR-497-5p | Humans (nickel sulfate, epoxy resin (EP) and methylochloroisothia zolinone (MCI); n = 5 for each), irritants (sodium lauryl sulfate (SLS, n = 9) and nonanoic acid (NO, n = 5)) and from non-affected skin (baseline, n = 5). | Skin (patch tests with MCI) | Upregulated | T cell activation, cell–cell adhesion, cytokine and chemokine regulation pathways and a role in TGF-β-pathways via the regulation of SMAD3. |
| Werner et al. [81] 2020 | miR-23b-3p, miR-99a-5p, miR-193b-3p, miR-199a-3p | Humans (nickel sulfate, epoxy resin (EP) and methylochloroisothia zolinone (MCI); n = 5 for each), irritants (sodium lauryl sulfate (SLS, n = 9) and nonanoic acid (NO, n = 5)) and from non-affected skin (baseline, n = 5). | Skin (Patch Tests with MCI) | Upregulated | miR23b-3p and miR-99a-5p: skin homeostasis and development in vitro via TGIF1 and IGFR1. miR-193b-3p and miR-199a-3: leukocyte proliferation and keratinocyte/epidermis differentiation. |
| Vennegaard et al. [78] 2012 | miR-21, miR-223, miR-142-3p, miR-142-5p | Humans (nickel sulfate, epoxy resin (EP) and methylochloroisothia zolinone (MCI); n = 5 for each), irritants (sodium lauryl sulfate (SLS, n = 9) and nonanoic acid (NO, n = 5)) and from non-affected skin (baseline, n = 5). | Skin | Upregulated | T cells, T cell activation and skin inflammation. |
| Gulati et al. [79] 2015 | miR-21, miR-223, miR-142-3p, miR-142-5p | Humans (7) (DPCP at day 3, day 14 and day 120) | Skin | Upregulated | T cells, T cell activation and skin inflammation. |
| Anderson et al. [80] 2014 | miR-21, miR-22, miR-155, miR-126, miR-27b, miR-210, miR-31, miR-301a | Murine (toluene 2,4-diisocyanate (TDI)). | Skin | Upregulated | T cells, T cell activation and skin inflammation. |
| Study | miRNA | Study Population | Samples | Results | Molecular Pathways/Mechanism of Action |
|---|---|---|---|---|---|
| Lin et al. [82] 2017 | miR-2355-3p miR-2355-5p miR-4264 miR-29c-5p miR-361-3p miR-6769a-5p | Humans (12) | Serum | Upregulated | Cell growth and proliferation |
| Lin et al. [82] 2017 | miR-1184 miR-1910-5p miR-205-5p miR-302c-5p miR-3187-3p miR-3691-3p miR-4649-5p miR-4733-5p miR-6799-3p miR-6800-3p | Humans (12) | Serum | Downregulated | Cell-to-cell signaling and interaction, cellular movement, regulation of leukocyte migration, tissue development immune cell trafficking, regulation of inflammatory response |
| Zhang et al. [33] 2019 | miR-125a-5p | Humans (20 active CIU patients and 20 healthy controls) | Serum | Upregulated | BLC2, STAT3, TGF-β and CCL17 |
| Key Points |
|---|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brancaccio, R.; Murdaca, G.; Casella, R.; Loverre, T.; Bonzano, L.; Nettis, E.; Gangemi, S. miRNAs’ Cross-Involvement in Skin Allergies: A New Horizon for the Pathogenesis, Diagnosis and Therapy of Atopic Dermatitis, Allergic Contact Dermatitis and Chronic Spontaneous Urticaria. Biomedicines 2023, 11, 1266. https://doi.org/10.3390/biomedicines11051266
Brancaccio R, Murdaca G, Casella R, Loverre T, Bonzano L, Nettis E, Gangemi S. miRNAs’ Cross-Involvement in Skin Allergies: A New Horizon for the Pathogenesis, Diagnosis and Therapy of Atopic Dermatitis, Allergic Contact Dermatitis and Chronic Spontaneous Urticaria. Biomedicines. 2023; 11(5):1266. https://doi.org/10.3390/biomedicines11051266
Chicago/Turabian StyleBrancaccio, Raffaele, Giuseppe Murdaca, Rossella Casella, Teresa Loverre, Laura Bonzano, Eustachio Nettis, and Sebastiano Gangemi. 2023. "miRNAs’ Cross-Involvement in Skin Allergies: A New Horizon for the Pathogenesis, Diagnosis and Therapy of Atopic Dermatitis, Allergic Contact Dermatitis and Chronic Spontaneous Urticaria" Biomedicines 11, no. 5: 1266. https://doi.org/10.3390/biomedicines11051266
APA StyleBrancaccio, R., Murdaca, G., Casella, R., Loverre, T., Bonzano, L., Nettis, E., & Gangemi, S. (2023). miRNAs’ Cross-Involvement in Skin Allergies: A New Horizon for the Pathogenesis, Diagnosis and Therapy of Atopic Dermatitis, Allergic Contact Dermatitis and Chronic Spontaneous Urticaria. Biomedicines, 11(5), 1266. https://doi.org/10.3390/biomedicines11051266







